Abstract
The parasite communities of Scyliorhinus canicula and Galeus melastomus are studied for the first time in the Mediterranean. Their seasonal and geographical variations, and their relationship with environmental and fish biological data were tested. The parasite communities of both sharks were characterized by low richness and diversity, and high dominance. Infracommunity structure and composition differed between both species probably due to the consumption of different prey associated with their different bathymetric distributions. For G. melastomus, parasite infracommunity structure and the abundance of some parasites differed across seasons and/or localities due to different dynamics of intermediate hosts populations, in turn linked to different environmental conditions. While Ditrachybothridium macrocephalum was more abundant in juvenile specimens of G. melastomus as a result of ontogenic diet shifts, Grillotia sp. accumulated in adult hosts. The abundance of Proleptus obtusus was significantly higher in S. canicula, likely due to its shallower distribution coupled with higher consumption of reptantian decapods with respect to G. melastomus. Monogenean parasites were associated to high turbidity and temperature levels, which are known to enhance monogenean infection and reproductive success. Cestodes of G. melastomus were linked to high turbidity and O2 levels, which increase zooplankton biomass, favouring the transmission of heteroxenous parasites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elasmobranchs are of great relevance for the human being from an ecological, commercial, economic, conservationist and cultural point of view. Sharks and their relatives play a significant role as top predators in marine food webs, in which they are key components, regulating trophic interactions and community composition (Stevens et al., 2000; Ruiz et al., 2016). Unfortunately, they are the main target of the fishing industry: the global market of shark products (which includes meat, fins, and other shark-derived commercial goods, such as liver oil, cartilage or skin) declares nearly 1 billion USD per year (Dent & Clarke, 2015). Such a high commercial importance has led to the overexploitation of these interesting animals, raising concerns about their vulnerability, conservation status and population decline (Stevens et al., 2000; Gross, 2014; Dent & Clarke, 2015). Of the approximately one thousand elasmobranch species included in the IUCN Red List of threatened species, nearly 20% hold one of the three endangered categories (IUCN, 2016), and approximately 44% are categorized as “data deficient”, which suggests that the real percentage of threatened species of this group can be actually higher. Among the factors that make elasmobranchs particularly vulnerable to population mining, their life-cycle strategy, characterized by slow growth, late sexual maturity and low fecundity, is of major relevance (Stevens et al., 2000).
However, in spite of their relevancy and justified research interest, there is still little knowledge on many aspects of sharks biology and ecology. A good example is the composition and dynamics of shark parasite communities, which have been largely neglected (the following list covers virtually all the existing literature on shark parasite communities: Henderson & Dunne, 1998; Moore, 2001; Henderson et al., 2002; Klimpel et al., 2003; Chambers, 2008; Isbert et al., 2015). In addition, these studies have been conducted in northeastern Atlantic waters in almost all cases, and are completely absent from the Mediterranean Sea.
The spotted dogfish Scyliorhinus canicula (L., 1758) (Carcharhiniformes: Scyliorhinidae) and the blackmouth catshark Galeus melastomus Rafinesque, 1810 (Carcharhiniformes: Scyliorhinidae) are the two most abundant sharks in the Balearic Sea (NW Mediterranean) (Massutí & Moranta, 2003). These small demersal sharks are an important by-catch in northwestern Mediterranean waters (Carbonell et al., 2003) and both have shown to be negatively affected by trawling activity (Carbonell et al., 2003; Dimech et al., 2012).
Galeus melastomus shows a very wide bathymetric range, being the most important shark in the upper and middle slopes (i.e., 400–800 and 800–1400 m, approximately) in terms of abundance and biomass (Carrassón et al., 1992; Massutí & Moranta, 2003; D’Onghia et al., 2004). In contrast, S. canicula reaches its maximum abundance around 100 m depth, dominating the elasmobranch fauna of the continental shelf, although it can be found in the upper slope down to 500 m (Massutí & Moranta, 2003).
Regarding their feeding biology, both species are generalistic predators and occasional scavengers that consume mainly benthic and benthopelagic prey (Carrassón et al., 1992; Valls et al., 2011; Mnrasi et al., 2012).
The metazoan parasite community of S. canicula was already described in the northeastern Atlantic Ocean by Henderson & Dunne (1998) and Moore (2001). In the case of G. melastomus, although several records of different parasites exist (Pintner, 1899, 1930; Sproston, 1946; Brinkmann, 1988; Euzet et al., 1993; Raibaut et al., 1998; Dallarés et al., 2015; among others), these mainly come from northern Atlantic waters.
Population dynamics of trophically transmitted parasites are mostly influenced by host feeding habits (Campbell et al., 1980), which largely depend on prey availability, in turn linked to specific environmental conditions (Dallarés et al., 2016). Host population dynamics (e.g., abundance or density) and biological features (e.g., diet shifts, age or body size) also drive parasite abundance and, therefore, community composition and structure (Sasal et al., 1997; Bagge et al., 2004). This responsiveness of parasites to environmental factors and to host biology and ecology makes them suitable indicators of trophic interactions, host stocks and environmental health, among others (Willams et al., 1992; Pérez-del-Olmo et al., 2009; Dallarés et al., 2016).
Given the high importance of S. canicula and G. melastomus in the NW Mediterranean marine ecosystems, the foremost aim of present work is to study the parasite communities of both species in this area. Differences on the parasite community composition and structure between both sharks are assessed and discussed. Seasonal variability throughout the year (and geographical differentiation between two localities in the case of G. melastomus) is tested for parasite community descriptors and for the abundance of the most frequent parasites. The influence of environmental variables (namely temperature, salinity, turbidity, O2 content of water masses and phytoplankton concentration) on the abundance patterns of individual parasites is further addressed. Finally, the impact of parasite infection on host health is assessed by analysing the response of fish general condition indices to parasite loads.
We hypothesize that parasite communities of both sharks will differ as a consequence of their different trophic habits, which should be importantly linked to the different availability of intermediate hosts in their respective bathymetric distributions. However, a fairly high degree of resemblance is expected in the general features of such communities as a result of the similarities found between both sharks in terms of feeding ecology. We also expect seasonal variations in abundances of heteroxenous parasites (i.e., those using more than one host species during their life cycle), which should be linked to the temporal dynamics of their intermediate hosts [especially of those experiencing high turnovers along the year, such as zooplankton (Sardou et al., 1996)]. For monoxenous parasites (i.e., those using a single host species to complete their life cycle), fluctuations of environmental variables should significantly contribute to their abundance dynamics instead, since these parasites do not rely on trophic interactions for transmission. Although parasites occur naturally and they do not necessarily imply a risk for host health, a certain decrease of fish condition indices is expected in heavily parasitized hosts or in those infected by especially harmful parasites.
Materials and methods
Sampling area and specimen collection
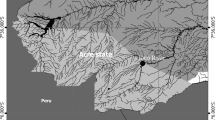
A total of 41 specimens of S. canicula and 159 of G. melastomus was collected during 2007 at the continental slope of the Balearic Sea off the mouth of the River Besòs (Barcelona) (seasonally) and off Vilanova (only in summer) (Fig. 1). The samples were obtained using a semi-balloon otter trawl (OTSB) and a commercial fishing gear (BOU) at depths comprised between 53 and 68 m for S. canicula and between 549 and 809 m for G. melastomus (Table 1). On board, fish were freshly frozen at –20°C for further parasitological examination.
Map of the study area in the Balearic Sea showing locations of sampling hauls. Times symbol Besós winter; filled circle Besós spring; open circle Besós summer; open diamond Besós autumn; filled square Vilanova summer. Hauls located in the continental shelf (≤200 m depth) correspond to samples of Scyliorhinus canicula, while those located in the continental slope (>200 m depth) correspond to samples of Galeus melastomus
Simultaneously to sampling hauls, measures of temperature (T) in °C, salinity (S) in psu, O2 content of water masses in ml/l, turbidity (voltage) and phytoplankton pigment concentration (Chla) were obtained at 5 m above the bottom by deployment of a CTD profiler (Table 1).
Parasitological study
In the laboratory and prior to dissection, total length (TL) in mm and total weight (TW) in g were obtained for each fish. Subsequently, external surfaces and gills were examined for the presence of ectoparasites. All internal organs were dissected out and examined separately for endoparasites under stereomicroscope. In the case of S. canicula, body musculature was also examined under stereomicroscope by compression between two glass plates. All metazoan parasites collected were preserved in 70% ethanol. Monogeneans, digeneans and cestodes were stained with iron acetocarmine and examined as permanent mounts in Canada balsam. Nematode larvae and crustaceans were observed as temporary mounts in saline solution. All parasites were identified to the lowest possible taxonomic level.
Data analyses
For G. melastomus, two size-based groups of hosts were defined, corresponding to immature (TL < 34 cm for males and TL < 40 cm for females) and mature specimens (TL ≥ 34 cm for males and TL ≥ 40 cm for females) (Capapé & Zaouali, 1977).
Ecological terms used for parasite populations and communities follow Bush et al. (1997): prevalence (P%) was calculated as the proportion of hosts in each sample infected by a given parasite and mean abundance (MA) as the total number of individuals of a given parasite taxa found in a particular host species divided by the total number of hosts examined of such species. For both species, parasite species displaying a P% > 10% in at least one seasonal/geographical group were considered not accidental and are henceforth called common (see indications in Table 2). Parasite infrapopulations and infracommunities (i.e., all parasites of a given species in an individual fish and all infrapopulations in an individual fish, respectively) were used as replicate samples in the analyses. Infracommunity richness, abundance, diversity and dominance were calculated, the two latter based on parasite abundance, using Brillouin’s Index (PRIMER v6; Anderson et al., 2008) and Berger–Parker dominance index (calculated as the number of individuals of the most abundant parasite species in a given host divided by the total number of parasites found in such host), respectively. Fish condition was assessed by the condition factor (K, calculated as TW × 100/TL3), and the hepatosomatic index (HSI, calculated as liver weight (g) × 100/TW).
Spearman’s rank correlation (r s) tests were applied in order to assess the association of parasite infracommunity parameters (i.e., richness, abundance, diversity and dominance) with host TL, and condition parameters (i.e., K and HSI). The same tests were used to test the relationship between the abundance of common parasites of both hosts and the same fish biological factors.
Kruskal–Wallis tests and generalized linear model (GZM) analyses were performed to test the differences on parasite infracommunity parameters and on the prevalence and abundance of common parasites (using fish TL as covariate), respectively, among seasons. Similarly, general linear models (GLM) followed by post hoc tests using Bonferroni correction were carried out to assess differences among seasons on fish TL and condition parameters for both species. The same analyses were repeated using matched seasonal samples (i.e., summer) of G. melastomus collected in Besós and Vilanova in order to test geographical variability in parasite infracommunity parameters, the prevalence and abundance of common parasites and in fish TL and condition parameters.
Permutational multivariate analyses of variance (PERMANOVA) were carried out using abundance data of parasite infracommunities to test differences in the parasite community structure among the four seasons sampled off Besós for the two species of sharks, and between matched seasonal samples from off Besós and Vilanova for G. melastomus. Such analyses were applied using PERMANOVA+ for PRIMERv6 (Anderson et al., 2008) on Bray–Curtis similarity matrices generated from the logarithmically transformed (log (X + 1)) abundance data, and permutation P-values were obtained under unrestricted permutation of raw data (9999 perms).
Canonical correspondence analyses (CCA) were used to relate the abundance of the common parasites found in the two species of sharks with environmental variables (Ter Braak, 1986). In CCA plots, arrows represent explanatory variables and are proportional in length to their importance on the explained variable.
A non-parametric Multi-Dimensional Scaling (MDS) was applied on infracommunity data of S. canicula and G. melastomus in order to visualize the ordination of parasite infracommunities of the two distinct hosts. Then, using the samples collected from off Besós, t Student tests (assuming non-equal variances) were applied to test differences on infracommunity richness, diversity and dominance between S. canicula and G. melastomus, and GZM were used to test differences on infracommunity abundance and on the overall abundance of the shared parasites between both hosts.
Results
The parasite community of Scyliorhinus canicula
The parasite community of S. canicula included a total of five parasite species comprising three nematodes, one monogenean and one cestode (Table 2). The nematode Hysterothylacium aduncum (Rudolphi, 1802) constitutes a new host record for this species. Additional parasites recovered were the monogenean Hexabothrium appendiculatum (Kuhn, 1829), the cestode Nybelinia lingualis Cuvier, 1817 and the nematodes Anisakis sp. ascribed to morphotype Anisakis Type II sensu Berland (1961) and Proleptus obtusus Dujardin, 1845. All sharks examined were infected by at least one parasite.
No relationship was detected between any of the infracommunity parameters or the abundance of common parasites and fish TL, K or HSI (P > 0.05 in all cases). Kruskal–Wallis tests and GZMs revealed no seasonal differences for infracommunity parameters or for the abundance or prevalence of common parasites (P > 0.05 in all cases) (Tables 2, 3). Regarding fish biological factors, only K showed significant seasonal variations (F (3, 7) = 6.293, P = 0.001) being higher in summer and autumn than in the other two seasons (Table 3).
The PERMANOVA applied on parasite infracommunities showed no seasonal effect on the structure of such communities (P (perm) > 0.05). The CCA relating common parasites of S. canicula and environmental variables accumulated 99.8% of the total variance (Fig. 2). The abundance of the monogenean H. appendiculatum was strongly associated to high near-bottom turbidity and, to a lesser extent, temperature and O2 concentration, while the nematode H. aduncum was associated to high salinity levels.
Canonical correspondence analysis (CCA) showing associations between the common parasites of Scyliorhinus canicula and environmental variables. Abbreviations for parasite names: Ansp, Anisakis Type II; Heap, Hexabothrium appendiculatum; Hyad, Hysterothylacium aduncum; Nyli, Nybelinia lingualis; Prob, Proleptus obtusus. Abbreviations for locality-season groups: Ba Besós autumn, Bsp Besós spring, Bsu Besós summer, Bw Besós winter. Abbreviations for environmental variables: Chla chlorophyll a concentration, O 2 oxygen concentration, S salinity, T temperature, Turb turbidity
The parasite community of Galeus melastomus
A total of 13 parasite species comprising five nematodes, three cestodes, two monogeneans, two digeneans and one copepod were recovered from the specimens of G. melastomus examined (Table 4). Of these, ten species constitute new host records for this host: the monogeneans Erpocotyle sp. and Leptocotyle minor (Monticelli, 1888); the digeneans Otodistomum cestoides (Van Beneden, 1871) Odhner, 1911 and Accacoelidae gen. sp.; the cestode Grillotia sp.; and the nematodes H. aduncum, Anisakis sp. ascribed to morphotype Anisakis Type II sensu Berland (1961), P. obtusus, Piscicapillaria baylisi Moravec, 1987 and Cucullanus sp. The cestodes Ditrachybothridium macrocephalum Rees, (1959) and Sphyriocephalus viridis (Wagener, 1854) and the copepod Eudactylina sp. were also recovered from this shark. Overall prevalence of infection in G. melastomus was 46%.
Infracommunity richness and the abundance of the cestodes D. macrocephalum and Grillotia sp. were significantly correlated with fish TL (r s = 0.17, P = 0.04; r s = −0.16, P = 0.04 and r s = 0.27, P = 0.001, respectively), with the abundance of D. macrocephalum being higher in juvenile sharks and infracommunity richness and the abundance of Grillotia sp. reaching higher values in adult hosts. Furthermore, the abundance of D. macrocephalum and P. obtusus showed significant positive and negative relationships, respectively, with K (r s = 0.19, P = 0.019 and r s = −0.23, P = 0.004, respectively), and the abundance of D. macrocephalum and Grillotia sp. were negatively and positively related, respectively, to HSI (r s = −0.28, P = 0.004 and r s = 0.20, P = 0.042, respectively). Parasite infracommunity descriptors were not significantly related to K or HSI (P > 0.05 in all cases).
The abundance of Grillotia sp. showed significant differences among seasons, being maximum in summer and autumn (χ 2 = 10.643, P = 0.014). In the cases of infracommunity mean abundance and abundance of D. macrocephalum, interactions were found between the factor season and fish TL (χ 2 = 16.478, P = 0.001 and χ 2 = 11.386, P = 0.003, respectively), and the analyses were thus repeated considering the two size groups of hosts separately. Infracommunity mean abundance and abundance of D. macrocephalum displayed significant seasonal variability in juvenile sharks, (χ 2 = 21.737, P < 0.001 and χ 2 = 22.870, P < 0.001, respectively), both being highest in spring than in the rest of seasons (Tables 3, 4).
No seasonal differences were detected for infracommunity richness, diversity or dominance, or for the prevalence of common parasites (P > 0.05 in all cases).
Concerning geographical variability, infracommunity richness and abundance were significantly higher in samples from off Besós than in those from off Vilanova in summer (χ 2 = 6.031, P = 0.014 and χ 2 = 5.679, P = 0.017, respectively) (Table 3). No significant differences were detected between localities for abundance or prevalence values of common parasites, although Erpocotyle sp., and P. obtusus were absent from the samples from off Vilanova (Table 4).
Fish TL and HSI were significantly lower in autumn samples than in the rest of seasons (F (3,132) = 10.937, P < 0.001 and F (3,96) = 5.446, P = 0.002, respectively), while fish K reached minimum values in summer (F (3,130) = 3.861, P = 0.011). Among fish biological descriptors, only K showed significant differences between localities (F (1,54) = 6.845, P = 0.012), being higher in fishes from off Besós than in those from off Vilanova (Table 3).
The PERMANOVA analyses applied on infracommunity abundance data revealed significant differences in the structure of parasite infracommunities among the four seasons sampled off Besós (Pseudo-F (3,67) = 3.1495, P (perm) < 0.001, unique perms = 9928). Post hoc pairwise comparisons separated winter and spring samples, which grouped together, from summer and autumn ones, which grouped together as well. In contrast, no geographical differences between infracommunities collected off Besós and Vilanova in summer were detected (P (perm) > 0.05).
The CCA relating common parasites of G. melastomus and environmental variables accumulated 95.0% of the total variance (Fig. 3). The abundance of the cestode D. macrocephalum was associated to high near-bottom turbidity coinciding with some hauls from off Besós in winter and spring. The parasites Erpocotyle sp. and Grillotia sp. were linked to high O2 concentration, partly associated to hauls from off Besós in autumn. Finally, the nematode P. obtusus was associated to high levels of salinity and temperature, in this case associated to hauls from off Besós, although of any particular season.
Canonical correspondence analysis (CCA) showing associations between the common parasites of Galeus melastomus and environmental variables. Abbreviations for parasite names: Dima, Ditrachybothridium macrocephalum; Ersp, Erpocotyle sp.; Grsp, Grillotia sp.; Prob, Proleptus obtusus. Abbreviations for locality-season groups: Ba Besós autumn, Bsp Besós spring, Bsu Besós summer, Bw Besós winter. Abbreviations for environmental variables: Chla chlorophyll a concentration, O 2 oxygen concentration, S salinity, T temperature, Turb turbidity
Comparison between the two sharks addressed
The MDS providing an ordination of parasite infracommunities of both hosts evidenced a clear differentiation between samples of S. canicula and G. melastomus (Stress = 0.04, Fig. 4).
Overall parasite infracommunity richness and dominance were significantly higher in S. canicula than in G. melastomus (t = −5.540, P < 0.001 and t = −3.851, P < 0.001, respectively), as also were total infracommunity abundance and the abundance of the nematode P. obtusus (χ 2 = 365.441, P < 0.001 and χ 2 = 305.229, P < 0.001, respectively, see Table 3). Infracommunity diversity and the abundance of the other two shared parasites (i.e., H. aduncum and Anisakis Type II) showed no significantly different values between hosts (P > 0.05 in both cases).
Discussion
The present study addresses the complete parasite communities of shark species in the Mediterranean Sea for the first time. In the NW Mediterranean, S. canicula is characterized by an impoverished parasite community, with low richness and diversity and strongly dominated by a single species (i.e., the nematode P. obtusus). Galeus melastomus, for which the parasite community is described herein for the first time, shows in comparison markedly higher total parasite richness (13 vs. 5 parasite taxa), although parasite infracommunities are still depauperate, with lower mean richness and similar diversity and dominance values than S. canicula.
These coincidences are in accordance with what was hypothesized in the introduction section and are likely due to the similarities that both sharks show regarding biological and ecological features. Both are small-sized species which live close to the bottom and mainly feed on benthic and benthopelagic organisms, among which euphausiid crustaceans, cephalopods, natantian decapods and teleosts are shared prey (Valls et al., 2011). The similar proportion of larval forms of trophically transmitted parasites in both sharks (70% in G. melastomus and 75% in S. canicula) further suggests a similar position in trophic food webs, as has also been deduced from stable nitrogen isotopes (Polunin et al., 2001).
In spite of the observed similarities in the general descriptors of the parasite faunas of G. melastomus and S. canicula, composition and structure of infracommunities differ between both species, as evidenced by their distinct ordination in the MDS and as also formerly hypothesized. A different dietary composition (Carrassón et al., 1992; Valls et al., 2011; Mnrasi et al., 2012), largely explained by the distinct availability of benthopelagic prey assemblages in the different depths inhabited by S. canicula and G. melastomus (the continental shelf and the upper slope, respectively), can account for this pattern. Variations in benthopelagic faunal assemblages occur along depth gradients in response to different environmental conditions (Cartes et al., 2006, 2013) and lead to different transmission dynamics for trophically transmitted parasites and, therefore, to different composition of parasite communities.
The low mean richness and diversity, and high dominance of infracommunities observed for S. canicula and, to a lesser extent, for G. melastomus, have also been reported from other small-sized sharks from different areas. According to Isbert et al. (2015), infracommunities of Etmopterus spinax (L.) from the NE Atlantic were also characterized by low mean richness and high dominance values. Moore (2001) and Henderson et al. (2002) also reported depauperate infracommunities in S. canicula and Squalus acanthias L., respectively, from the NE Atlantic as well.
As far as we are concerned, available studies on shark parasite communities have focused on small-sized sharks from North-Atlantic waters, in which the total number of parasite taxa is usually low. Henderson & Dunne (1998) found five different parasites in S. canicula, Chambers (2008) reported eight parasites from Centroscyllium fabricii (Reinhardt), Henderson et al. (2002) recovered 10 parasites from S. acanthias and Moore (2001) and Isbert et al. (2015) found 11 different taxa in S. canicula and E. spinax, respectively. In larger, although still small-sized sharks, Palm & Schröeder (2001) reported six parasites from Heptranchias perlo (Bonnaterre), three from Deania calcea (Lowe), seven from Deania profundorum (Smith & Radcliffe) and nine from Deania histricosa Garman. Isbert et al. (2015) suggested that poor parasite faunas, with low richness and diversity and high dominance values, could represent features of small sharks. Although this would be to some extent supported by present results, there are few data available for comparison and the suggested trends could be a characteristic pattern of sharks in general, regardless of their size.
Different studies on teleost parasite communities have repeatedly reported lower parasite richness in Mediterranean with respect to Atlantic populations (e.g., Pérez-del-Olmo et al., 2009; Mattiucci et al., 2014; Constenla et al., 2015). A smaller fish size, lower food consumption and lower biomass and abundance of animal communities in the Mediterranean have been suggested as possible explanations for such pattern (see Constenla et al., 2015 and references therein). Actually, maximum size of S. canicula in the Mediterranean is lower than elsewhere (Compagno, 1984), and a similar pattern has been observed for Mediterranean specimens of G. melastomus with respect to their Atlantic counterparts (Compagno, 1984; Carrassón et al., 1992; present results). However, results available to date are far from sufficient to conclude whether elasmobranch parasite communities are more diverse and abundant in Atlantic than in Mediterranean waters. Parasitological data for S. canicula are scarce (only Henderson & Dunne (1998) and Moore (2001) have analysed the parasite community of this shark in the Atlantic, and present results constitute the first report from the Mediterranean), and in the case of G. melastomus present results represent the first description of its parasite community, and comparative data are thus completely absent. Undoubtedly, additional parasitological studies in sharks from Mediterranean and Atlantic waters will clarify if the same trend observed in teleosts is applicable to shark parasite communities.
Fish condition indices vary in response to multiple factors and clear relationships between them and parasite loads are usually difficult to determine (Heins & Baker, 2008). Fishes with reduced health condition may show higher susceptibility to parasite infections (Dallarés et al., 2016). It is also possible that parasite infections do not reach the threshold needed to affect condition indices or even that healthier fishes harbour more abundant and rich parasite communities, as suggested by Dallarés et al. (2014). In any case, unless the impact of a given parasite on host fitness is strong (i.e., Heins & Baker, 2008 detected heavily reduced reproductive fitness in the three-spine stickleback Gasterosteus aculeatus infected with the cestode Schistocephalus sp.), studies assessing the effects of parasite burden on general fish condition indices have yielded inconsistent results which must therefore be carefully considered.
In this sense, the contrary associations detected in G. melastomus between the parasites D. macrocephalum and P. obtusus and fish condition factor, or between the cestodes D. macrocephalum and Grillotia sp. and fish hepatosomatic index, do not allow stating any general trend. Such relationships, observed for a few individual parasites only, may reflect variations in parasite loads in response to specific biological aspects of their host (i.e., dietary trends) or environmental patterns that are coupled with variations in fish condition parameters, instead of an impact of parasites on fish health.
These observations, alongside with the absence of noticeable effects of the parasite load on S. canicula general condition, point to a negligible repercussion of the parasite burden in general fish condition indices in the two sharks addressed, contrary as hypothesized.
The absence of significant differences among seasons in the case of S. canicula, either for the abundance or prevalence of the different parasites recovered or for infracommunity descriptors and structure might be attributed to the low number of hosts available for each seasonal group. Further studies with increased number of specimens are needed in order to confirm the observed lack of seasonal patterns.
The much lower infracommunity richness and abundance observed in Vilanova with respect to Besós for samples of G. melastomus could be possibly explained by the vicinity of the Besós submarine canyon to the latter locality. Submarine canyons, formed as a result of river discharge (the Besós River in this case), favour aggregation of zooplankton and more complex invertebrate communities (Macquart-Moulin & Patriti, 1996; Rumolo et al., 2015), which can presumably enhance parasite transmission. A similar pattern has been observed in the case of the parasite communities of the teleost Phycis blennoides (Brünnich) in the same area, where the more abundant, rich and diverse parasite composition of samples collected off the mainland versus the insular slope in the Balearic basin are partly explained by the higher availability of benthic prey linked to submarine canyons in samples off the mainland slope (Dallarés et al., 2016).
Parasites with direct life cycles, such as monogeneans, are subjected to environmental rather than to biotic factors, in contrast with trophically transmitted parasites. Effects of higher temperature and/or turbidity levels in increasing monogenean infection success have been demonstrated (Skinner, 1982; Brazenor & Hutson, 2015) and are consistent with the association observed between H. appendiculatum and these environmental parameters in S. canicula. While higher temperatures can enhance monogenean hatching success and reduce time to maturity (Brazenor & Hutson, 2015), aquatic environments with increased turbidity due to high levels of suspended materials can provoke irritation and inflammation of gill filaments accompanied by an inhibition of fish defense mechanisms, increasing susceptibility to infection by gill parasites, such as monogeneans (Skinner, 1982; Madi & Ueta, 2009). Few studies have addressed the response of these parasites to O2 levels (Monni & Cognetti-Varriale, 2002; Raymond et al., 2006) and this relationship is not well understood yet, although an enhancement of monogenean reproduction and infection success in more oxygenated environments, as documented for other invertebrates (Cheung et al., 2008) could be suggested. Apart from the direct effects of environmental variables on monogenean biology, an additional explanation based on indirect effects should be considered. Higher water turbidity and O2 have been linked to increased biomass of zooplankton, which enhances suprabenthos biomass and favours fish aggregation due to higher prey availability (Cartes et al., 2013). Ultimately, the increase in shark population size at a local scale could stimulate the transmission and increase the abundance of monogeneans, as has been reported by Bagge et al. (2004).
Ditrachybothridium macrocephalum seems to be exclusively distributed in the deep sea (Rees, 1959; Bray & Olson, 2004; Dallarés et al., 2015), which can explain its absence from S. canicula and its high prevalence and abundance values in G. melastomus, its definitive host (Dallarés et al., 2015). It is believed that these cestodes use two invertebrate intermediate hosts (a filter-feeding crustacean as first and a shrimp or crab as second) before reaching the elasmobranch final host where they will develop into adults (Tyler, 2006). Among the known prey of G. melastomus (Carrassón et al., 1992), amphipods and different decapods have been found to host larval stages of diphyllideans (see Bray & Olson, 2004 and references therein). As already highlighted by Dallarés et al. (2015), the higher abundance of D. macrocephalum in juvenile compared to adult sharks is in all likelihood related to the ontogenetic diet shift undergone by this host (Carrassón et al., 1992). According to Carrassón et al. (1992), adults consume larger prey such as cephalopods, teleosts and large crustaceans, than juveniles, for which smaller crustaceans like mysids, amphipods or euphausiids are the main target. Furthermore and according to the same authors, adults of G. melastomus are generally distributed in deeper grounds than juvenile specimens. In this sense, the decapod Calocaris macandreae is an important prey in the sampled area, and its presence in guts decreases with age and depth (Carrassón et al., 1992). The rest of decapods increase in importance with age and amphipods are only relevant below 1000 m. Therefore, C. macandreae could be a transmitter for this parasite in present samples.
The aggregation of benthopelagic fish in response to high near-bottom turbidity levels (explained above) probably stimulates parasite transmission and can explain the link between this environmental variable and the abundance of D. macrocephalum in the CCA. The close association observed between water turbidity and Besós winter samples (further corroborated by Rumolo et al., 2015 with the environmental data recorded in the present study) suggests that the transmission of larval stages of D. macrocephalum in their invertebrate hosts is enhanced in winter. The parasites must reach their final host with some temporal delay, which can explain the maximum abundance levels attained by this parasite in spring samples of G. melastomus.
Grillotia sp. is one of the most frequent genera recovered from fishes within the cestode order Trypanorhyncha (Beveridge & Campbell, 2007). Furthermore, representatives of this genus have been frequently reported in the deep sea (Palm, 2004; Klimpel et al., 2009; Costa et al., 2016), and specifically in deep-dwelling fishes from the sampled area (Dallarés et al., 2016, unpublished results of the ANTROMARE project), as is the case of present samples of G. melastomus. Although copepods act as first intermediate hosts for trypanorhynchs (Palm, 2004), these are not abundant in the diet of G. melastomus (Carrassón et al., 1992). Other prey, such as cephalopods or teleosts, in turn preying on copepods, could be the transmitters of this parasite to G. melastomus. Its higher abundance in adult sharks responds to the accumulation of the larval forms of the parasite in host’s tissues until the latter is consumed by a larger predator, where plerocerci excyst and develop into adults. The kitefin shark Dalatias licha (Bonnaterre), which is known to prey on G. melastomus in the NW Mediterranean Sea (Matallanas, 1982), or Hexanchus griseus (Bonnaterre), which can also prey on smaller sharks (Ebert, 1994), could be potential final hosts for the specimens of Grillotia sp. recovered.
The association observed between the abundance of Grillotia sp. and high O2 levels is in accordance with what is known about the life cycle of this parasite. As explained above, high O2 levels enhance copepod biomass (Moon et al., 2006; Cartes et al., 2013), which likely favours the transmission of trypanorhynch cestodes to their subsequent hosts, with G. melastomus among them. Oxygen levels off Besós at ca. 700 m depth increase in winter and spring according to Cartes et al. (2011), and maximum abundance values of Grillotia sp. in G. melastomus were observed in summer and autumn, which might be explained by the time needed by the parasite to reach higher trophic levels, similarly as suggested for D. macrocephalum.
The physalopterid nematode P. obtusus, the dominant parasite in infracommunities of S. canicula, seems to display a shallow-water distribution, being able to infect different elasmobranchs from the continental shelves as final hosts (Moravec et al., 2002; Morris et al., 2016). Although very few information is available about the life cycle of physalopterids, P. obtusus is known to have a two-host life cycle, with sharks being final and crustaceans intermediate hosts. In the marine environment, larval forms of this nematode have been recovered from the shore crabs Carcinus maenas, Pagurus bernhardus, Pachygrapsus marmoratus and Hyas araneus (Moravec, 2007 and references therein). Accordingly, Valls et al. (2011) reported reptantian decapods as the most important prey of S. canicula on the continental shelf off the slope of the Balearic Islands (NW Mediterranean Sea) (36% IRI). The great importance of shallow-water reptantian decapods in the diet of S. canicula is thus in accordance with the high prevalence and abundance of P. obtusus found in this shark, in the present and in previous studies (Henderson & Dunne, 1998; Moore, 2001). In the case of the deeper-distributed G. melastomus, Carrassón et al. (1992) found that the decapod C. macandreae, a possible candidate as intermediate host of P. obtusus, can be important in juveniles but is only a casual prey in adult sharks at 371–667 m off the continental slope of the Balearic Sea. Valls et al. (2011) also reported that reptantian decapods seem to be of minor importance at depths between 500 and 750 m in the slope of the Balearic Islands. Hence, the low prevalence and abundance values attained by P. obtusus in G. melastomus are likely explained by the shallower distribution of the former coupled with the low presence of decapods in the diet of the latter.
In a similar way as P. obtusus in G. melastomus, the raphidascaridid nematode H. aduncum appeared also linked to high water salinities in present samples of S. canicula. This trend was also observed in the teleost P. blennoides off the same waters (Dallarés et al., 2016). In spite of these coincidences, the abundance of H. aduncum in S. canicula was low and more consistent results should be obtained before any generalizations are made. The presence of this nematode in both shark species is indicative of its broad bathymetric distribution, which extends from the continental shelf to the abyssal depths (Klimpel et al., 2009). Actually, the importance in terms of prevalence and mean abundance of H. aduncum seems to increase with depth, as has been confirmed in different studies on deep-sea fish from the sampled area (Mateu et al., 2014; Pérez-i-García et al., 2015; unpublished results from the ANTROMARE project).
In conclusion, the present study highlights the importance of different ecological variables in shaping parasite community composition and structure. Depth is found to be a determinant factor, with parasite abundance and prevalence patterns responding to bathymetric gradients, probably as a result of variations in the composition of benthopelagic prey assemblages. In this sense, links between parasite loads and available data on shark trophic relationships can be established and used to elucidate possible parasite transmission patterns. Individual environmental variables, which vary in response to seasonal, geographical and bathymetric gradients, may have an important role in determining faunal aggregation and distribution in the one hand, and in influencing parasite biology in the other. For instance, high near-bottom turbidity and temperature levels seem to enhance infection and reproductive success of monogeneans, while high O2 and turbidity levels are known to enhance zooplankton biomass, and thus favour parasite transmission of heteroxenous parasites. Finally, the presence of submarine canyons, which channel organic matter through the slopes, is an additional factor associated to more abundant and diverse faunal assemblages and, therefore, to higher parasite richness and abundance.
References
Anderson, M. J., R. N. Gorley & K. R. Clarke, 2008. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. PRIMER-E, Plymouth.
Bagge, A. M., R. Poulin & E. T. Valtonen, 2004. Fish population size, and not density, as the determining factor of parasite infection: a case study. Parasitology 128: 305–313.
Beveridge, I. & R. A. Campbell, 2007. Revision of the Grillotia erinaceus (van Beneden, 1858) species complex (Cestoda: Trypanorhyncha), with the description of G. brayi n. sp. Systematic Parasitology 68: 1–31.
Bray, R. A. & P. D. Olson, 2004. The plerocercus of Ditrachybothridium macrocephalum Rees, 1959 from two deep-sea elasmobranchs, with a molecular analysis of its position within the order Diphyllidea and a checklist of the hosts of larval diphyllideans. Systematic Parasitology 59: 159–167.
Brazenor, A. K. & K. S. Hutson, 2015. Effects of temperature and salinity on the life cycle of Neobenedenia sp. (Monogenea: Capsalidae) infecting farmed barramundi (Lates calcarifer). Parasitology Research 114: 1875–1886.
Brinkmann Jr., A., 1988. Presence of Otodistomum sp. metacercariae in Norwegian marine fishes. Sarsia 73: 79–82.
Bush, A. O., K. D. Lafferty, J. M. Lotz, A. W. Shostak, et al., 1997. Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology 83: 575–583.
Campbell, R. A., R. L. Haedrich & T. A. Munroe, 1980. Parasitism and ecological relationships among deep-sea benthic fishes. Marine Biology 57: 301–313.
Capapé, C. & J. Zaouali, 1977. Contribution a la biologie des Scyliorhinidae des cótes Tunisiennes. VI: Galeus melastomus Rafinesque, 1810 Répartition géographique et bathymetrique, sexualité, reproduction, fécondité. Cahiers de Biologie Marine 18: 449–463.
Carbonell, A., F. Alemany, P. Merella, A. Quetglas & E. Román, 2003. The by-catch of sharks in the western Mediterranean (Balearic Islands) trawl fishery. Fisheries Research 61: 7–18.
Carrassón, M., C. Stefanescu & J. E. Cartes, 1992. Diets and bathymetric distributions of two bathyal sharks of the Catalan Deep sea (Western Mediterranean). Marine Ecology Progress Series 82: 21–30.
Cartes, J. E., T. Madurell, E. Fanelli & J. L. López-Jurado, 2006. Dynamics of suprabenthos-zooplankton communities around the Balearic Islands (western Mediterranean): influence of environmental variables and effects on the biological cycle of Aristeus antennatus. Journal of Marine Systems 71: 316–335.
Cartes, J. E., F. Maynou, P. Abelló, M. Emelianov, L. Gil de Sola & M. Solé, 2011. Long-term changes in the abundance and deepening of the deep-sea shrimp Aristaeomorpha foliacea in the Balearic Basin: relationships with hydrographic changes at the Levantine Intermediate Water. Journal of Marine Systems 88: 516–525.
Cartes, J. E., E. Fanelli, C. López-Pérez & M. Lebrato, 2013. Deep-sea macroplankton distribution (at 400 to 2300 m) in the northwestern Mediterranean in relation to environmental factors. Journal of Marine Systems 113–114: 75–87.
Chambers, C., 2008. Determining deep-sea fish community structure in the Arctic: using species assemblages, stomach contents, parasite infracommunities and stable isotopes to evaluate trophic interactions. PhD Thesis. University of Manitoba, Winnipeg.
Cheung, S. G., H. Y. Chan, C. C. Liu & P. K. Shin, 2008. Effect of prolonged hypoxia on food consumption, respiration, growth and reproduction in marine scavenging gastropod Nassarius festivus. Marine Pollution Bulletin 57: 280–286.
Compagno, L. J. V., 1984. FAO species catalogue. Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Part 1. Hexanchiformes to Lamniformes, Vol. 4. Food and Agriculture Organization of the United Nations, Rome.
Constenla, M., F. E. Montero, F. Padrós, J. E. Cartes, V. Papiol & M. Carrassón, 2015. Annual variation of parasite communities of deep-sea macrourid fishes from the western Mediterranean Sea and their relationship with fish diet and histopathological alterations. Deep-Sea Research I 104: 106–121.
Costa, G., M. Khadem, T. Dellinger, M. Biscoito & E. Melo-Moreira, 2016. Larval cestodes infecting the deep-water fish, Cataetyx laticeps (Pisces: Bythidae) from Madeira Archipelago, Atlantic Ocean. Acta Parasitologica 61: 187–190.
Dallarés, S., M. Constenla, F. Padrós, J. E. Cartes, M. Solé & M. Carrassón, 2014. Parasites of the deep-sea fish Mora moro (Risso, 1810) from the NW Mediterranean Sea and relationship with fish diet and enzymatic biomarkers. Deep-Sea Research I 92: 115–126.
Dallarés, S., A. Pérez-del-Olmo, M. Carrassón & R. Kuchta, 2015. Morphological and molecular characterisation of Ditrachybothridium macrocephalum Rees, 1959 (Cestoda: Diphyllidea) from Galeus melastomus Rafinesque in the Western Mediterranean. Systematic Parasitology 92: 45–55.
Dallarés, S., C. M. Moyà-Alcover, F. Padrós, J. E. Cartes, M. Solé, C. Castañeda & M. Carrassón, 2016. The parasite community of Phycis blennoides (Brünnich, 1768) from the Balearic Sea in relation to diet, biochemical markers, histopathology and environmental variables. Deep-Sea Research I 118: 84–100.
Dent, F. & S. Clarke, 2015. State of the global market for shark products. FAO Fisheries and Aquaculture Technical Paper 590.
Dimech, M., M. J. Kaiser, S. Ragonese & P. J. Schembri, 2012. Ecosystem effects of fishing on the continental slope in the Central Mediterranean Sea. Marine Ecology Progress Series 449: 41–54.
D’Onghia, G., C.-Y. Politou, A. Bozzano, D. Lloris, G. Rotllant, L. Sion & F. Mastrototaro, 2004. Deep-water fish assemblages in the Mediterranean Sea. Scientia Marina 68: 87–99.
Ebert, D. A., 1994. Diet of the sixgill shark Hexanchus griseus off southern Africa. South African Journal of Marine Science 14: 213–218.
Euzet, L., C. Combes & A. Caro, 1993. A Checklist of Monogenea and Mediterranean fish. Second international Symposium on Monogenea.
Gross, M., 2014. Learning to live with sharks. Current Biology 24: R341–R344.
Heins, D. C. & J. A. Baker, 2008. The stickleback-Schistocephalus host-parasite system as a model for understanding the effect of a macroparasite on host reproduction. Behaviour 145: 625–645.
Henderson, A. C. & J. J. Dunne, 1998. The metazoan parasites of the lesser-spotted dogfish Scyliorhinus canicula (L.) from the Galway Bay area. The Irish Naturalists’ Journal 26: 104–107.
Henderson, A. C., K. Flannery & J. Dunne, 2002. An investigation into the metazoan parasites of the spiny dogfish (Squalus acanthias L.), off the west coast of Ireland. Journal of Natural History 36: 1747–1760.
Isbert, W., C. Rodríguez-Cabello, I. Frutos, I. Preciado, F. E. Montero & A. Pérez-del-Olmo, 2015. Metazoan parasite communities and diet of the velvet belly lantern shark Etmopterus spinax (Squaliformes: Etmopteridae): a comparison of two deep-sea ecosystems. Journal of Fish Biology 86: 687–706.
IUCN, 2016. IUCN Red List of Threatened Species. Version 2016.2 [available on internet at www.iucnredlist.org]. Downloaded on 13 October 2016.
Klimpel, S., H. W. Palm & A. Seehagen, 2003. Metazoan parasites and food composition of juvenile Etmopterus spinax (L., 1758) (Dalatiidae, Squaliformes) from the Norwegian Deep. Parasitology Research 89: 245–251.
Klimpel, S., M. W. Busch, E. Kellermanns, S. Kleinertz & H. W. Palm, 2009. Metazoan Deep-Sea Fish Parasites. Verlag Natur & Wissenschaft, Solingen.
Macquart-Moulin, C. & G. Patriti, 1996. Accumulation of migratory micronekton crustaceans over the upper slope and submarine canyons of the northwestern Mediterranean. Deep-Sea Research I 43: 579–601.
Madi, R. R. & M. T. Ueta, 2009. O papel de Ancyrocephalinae (Monogenea: Dactylogyridae), parasito de Geophagus brasiliensis (Pisces: Cichlidae), como indicador ambiental. Revista Brasileira de Parasitologia Veterinaria 18: 38–41.
Massutí, E. & J. Moranta, 2003. Demersal assemblages and depth distribution of elasmobranchs from the continental shelf and slope off the Balearic Islands (Western Mediterranean). ICES Journal of Marine Science 60: 753–766.
Matallanas, J., 1982. Feeding-habits of Scymnorhinus licha in catalan waters. Journal of Fish Biology 20: 155–163.
Mateu, P., F. E. Montero & M. Carrassón, 2014. Geographical variation in metazoan parasites of the deep-sea fish Bathypterois mediterraneus Bauchot, 1962 (Osteichthyes: Ipnopidae) from the Western Mediterranean. Deep-Sea Research I 87: 24–29.
Mattiucci, S., A. Garcia, P. Cipriani, M. Neves Santos, G. Nascetti & R. Cimmaruta, 2014. Metazoan parasite infection in the swordfish, Xiphias gladius, from the Mediterranean Sea and comparison with Atlantic populations: implications for its stock characterization. Parasite 21: 35.
Mnrasi, N., O. el Kamel, M. Boumaïza, C. Reynaud & C. Capapé, 2012. Food and feeding habits of the small-spotted catshark, Scyliorhinus canicula (Chontrichthyes: Scyliorhinidae) from the northern coast of Tunisia (central Mediterranean). Cahiers de Biologie Marine 53: 139–150.
Moon, S. Y., H. S. Yoon, H. Y. Soh & S. D. Choi, 2006. Environmental factors and variation characteristics of zooplankton communities in Gamak Bay. Ocean Polar Research 28: 79–94.
Moore, A. B. M., 2001. Metazoan parasites of the lesser-spotted dogfish Scyliorhinus canicula and their potential as stock discrimination tools. Journal of the Marine Biology Association of the UK 81: 1009–1013.
Monni, G. & A. M. Cognetti-Varriale, 2002. Antigenicity of Pseudodactylogyrus anguillae and P-bini (Monogenea) in the European eel (Anguilla anguilla, L.) under different oxygenation conditions. Fish and Shellfish Immunology 13: 125–131.
Moravec, F., Jo G. Van As & I. Dyková, 2002. Proleptus obtusus Dujardin, 1845 (Nematoda: Physalopteridae) from the puffader shyshark Haploblepharus edwardsii (Scyliorhynidae) from off South Africa. Systematic Parasitology 53: 169–173.
Moravec, F., 2007. Some aspects of the taxonomy and biology of adult spirurine nematodes parasitic in fishes: a review. Folia Parasitologica 54: 239–257.
Morris, T., A. Avenant-Oldewage, S. Lamberth & C. Reed, 2016. Shark parasites as bio-indicators of metals in two South African embayments. Marine Pollution Bulletin 104: 221–228.
Palm, H. W., 2004. The Trypanorhyncha Diesing, 1863. PKSPL-IPB Press, Bogor.
Palm, H. W. & P. Schröder, 2001. Cestode parasites from the elasmobranchs Heptranchias perlo and Deania from the Great Meteor Bank, central East Atlantic. Aquatic Living Resources 14: 137–144.
Pérez-del-Olmo, A., M. Fernández, J. A. Raga, A. Kostadinova & S. Morand, 2009. Not everything is everywhere: the distance decay of similarity in a marine host-parasite system. Journal of Biogeography 36: 200–209.
Pérez-i-García, D., M. Constenla, F. Padrós, A. Soler-Membrives, M. Solé & M. Carrassón, 2015. Parasite communities of the deep-sea fish Alepocephalus rostratus Risso, 1820 in the Balearic Sea (NW Mediterranean) along the slope and relationships with enzymatic biomarkers and health indicators. Deep-Sea Research I 99: 65–74.
Pintner, T., 1899. Die Rhynchodäaldrüsen der Tetrarhynchen. Arbeiten aus den Zoologischen Instituten Universität Wien Tom. XII, Hft 1: 1–24.
Pintner, T., 1930. Wenigbekanntes und Unbekanntes von Rüsselband würmern. Sitzungsberichten der Kaiserlichen Akademie der Wissenschaften in Wien, Mathematisch-Naturwissenschaftiche Klasse 139: 445–537.
Polunin, N. V. C., B. Morales-Nin, W. E. Pawsey, J. E. Cartes, J. K. Pinnegar & J. Moranta, 2001. Feeding relationships in Mediterranean bathyal assemblages elucidated by stable nitrogen and carbon isotope data. Marine Ecology Progress Series 220: 13–23.
Raibaut, A., C. Combes & F. Benoit, 1998. Analysis of the parasitic copepod species richness among Mediterranean fish. Journal of Marine Systems 15: 185–206.
Raymond, K. M. N., L. L. Chapman & C. A. Lanciani, 2006. Host, macrohabitat, and microhabitat specificity in the gill parasite Afrodiplozoon polycotyleus (Monogenea). Journal of Parasitology 92: 1211–1217.
Rees, G., 1959. Ditrachybothridium macrocephalum gen. nov., sp. nov., a cestode from some elasmobranch fishes. Parasitology 49: 191–209.
Ruiz, D. J., S. Banks & M. Wolff, 2016. Elucidating fishing effects in a large-predator dominated system: the case of Darwin and Wolf Islands (Galápagos). Journal of Sea Research 107: 1–11.
Rumolo, P., J. E. Cartes, E. Fanelli, V. Papiol, M. Sprovieri, S. Mirto, S. Gherardi & A. Bonanno, 2015. Seasonal variations in the source of sea bottom organic matter off Catalonia coasts (Western Mediterranean): links with hydrography and biological response. Journal of Oceanography 71: 325–343.
Sardou, J., M. Etienne & V. Andersen, 1996. Seasonal abundance and vertical distributions of macroplankton and micronekton in the Northwestern Mediterranean Sea. Oceanologica Acta 19: 645–656.
Sasal, P., S. Morand & J.-F. Guégan, 1997. Determinants of parasite species richness in Mediterranean marine fishes. Marine Ecology Progress Series 149: 61–71.
Skinner, R. H., 1982. The interrelation of water quality, gill parasites, and gill pathology of some fishes from south Biscayne Bay, Florida. Fishery Bulletin 80: 269–280.
Sproston, N., 1946. A synopsis of the monogenetic trematodes. Transactions of the Zoological Society of London 25: 185–600.
Stevens, J. D., R. Bonfil, N. K. Dulvy & P. A. Walker, 2000. The effects of fishing on sharks, rays and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES Journal of Marine Science 57: 476–494.
Ter Braak, C. J. F., 1986. Canonical correspondence analyses a new eigenvector technique for multivariate direct gradient analysis. Ecology 67: 1167–1179.
Tyler, G. A., 2006. Tapeworms of elasmobranchs (Part II). A monograph on the Diphyllidea (Platyhelminthes, Cestoda). Bulletin of the University of Nebraska State Museum 20: 1–142.
Valls, M., A. Quetglas, F. Ordines & J. Moranta, 2011. Feeding ecology of demersal elasmobranchs from the shelf and slope off the Balearic Sea (Western Mediterranean). Scientia Marina 75: 633–639.
Willams, H. H., K. MacKenzie & A. M. McCarthy, 1992. Parasites as biological indicators of the population biology, migrations, diet and phylogenetics of fish. Reviews in Fish Biology and Fisheries 2: 144–176.
Acknowledgements
We thank two anonymous reviewers for their useful comments on the manuscript. We are grateful to Mar Costa for her collaboration in the dissection of some specimens of S. canicula and in the identification of their parasites. This study was supported by the Spanish Ministry of Science and Innovation (MICYT) project BIOMARE (CTM2006–13508–C02–01MAR). S. Dallarés benefits of a PIF Ph.D. student Grant from the Universitat Autònoma de Barcelona (UAB) (Grant Number PIF2012–53296).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Vasilis Valavanis
Sara Dallarés and Ana Pérez-del-Olmo contributed equally towards this study.
Rights and permissions
About this article
Cite this article
Dallarés, S., Pérez-del-Olmo, A., Montero, F.E. et al. Composition and seasonal dynamics of the parasite communities of Scyliorhinus canicula (L., 1758) and Galeus melastomus Rafinesque, 1810 (Elasmobranchii) from the NW Mediterranean Sea in relation to host biology and ecological features. Hydrobiologia 799, 275–291 (2017). https://doi.org/10.1007/s10750-017-3226-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3226-z