Abstract
Timely identification of endangered populations is vital to save them from extirpation. Here we tested whether six commonly used early-warning metrics are useful predictors of impending extirpation in laboratory rotifer (Brachionus calyciflorus) populations. To this end, we cultured nine rotifer clones in a constant laboratory environment, in which the rotifer populations were known to grow well, and in a deteriorating environment, in which the populations eventually perished. We monitored population densities in both environments until the populations in the deteriorating environment had gone extinct. We then used the population-density time series to compute the early-warning metrics and the temporal trends in these metrics. We found true positives (i.e. correct signals) in only two metrics, the standard deviation and the coefficient of variation, but the standard deviation also generated a false positive. Moreover, the signal produced by the coefficient of variation appeared when the populations in the deteriorating environment were about to cross the critical threshold and began to decline. As such, it cannot be regarded as an early-warning signal. Together, these findings support the growing evidence that density-based generic early-warning metrics—against their intended use—might not be universally suited to identify populations that are about to collapse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forecasting the fate of natural populations is of increasing importance for wildlife managers, as the rate of species loss has accelerated in the wake of the industrial revolution and will probably continue to accelerate (Ceballos et al., 2015; De Vos et al., 2015). Anticipating population decline to prevent extirpation has therefore become a major goal in conservation biology (Collen et al., 2011). However, accurate prediction of local extinction events is notoriously difficult due to uncertainty in parameter estimates, incomplete knowledge about driving processes, and environmental stochasticity (Ludwig, 1999; Clark et al., 2001; Regan et al., 2002). These difficulties raise the question of whether impending extinctions can be anticipated early enough to be averted (Biggs et al., 2009).
Recent studies on laboratory systems (Drake & Griffen, 2010; Dai et al., 2012; Veraart et al., 2012; Dai et al., 2013; Clements & Ozgul, 2016) and natural populations (Hefley et al., 2013) suggest that population collapses might be anticipated well before they actually happen. In these studies, predictions were based on systematic temporal changes of certain metrics—calculated from population-size time series—that are jointly known as generic early-warning signals (Scheffer et al., 2009; Dakos et al., 2012). These signals are used to measure a system-inherent process termed critical slowing down, which refers to the return time to equilibrium upon small perturbations (critical return time sensu Wissel, 1984). This return time increases if a gradual change in an underlying parameter (e.g. the intrinsic rate of increase r) leads to gradual loss of resilience, bringing the system closer to a tipping point (Veraart et al., 2012). At such a point, termed local bifurcation, the system changes equilibrium state (Strogatz, 2014). As a consequence of the increasing return time prior to the bifurcation, the autocorrelation and the variability of fluctuations in the system increase (Scheffer et al., 2009; Ditlevsen & Johnsen, 2010). These two latter phenomena, rising autocorrelation and variability, are the primary processes underlying the generic early-warning signals (Dakos et al., 2012).
The link from bifurcation theory to population dynamics is straightforward: a population with an intrinsic rate of increase r ≥ 0 will persist, while a population with r < 0 will go extinct; that is, the bifurcation point is characterised by r = 0 (Hefley et al., 2013; Krkošek & Drake, 2014; Strogatz, 2014). Moreover, with abundance time-series data at hand, bifurcation points can be inferred directly from the realised population growth, that is, from changes in the slope of the population growth trajectories (Burthe et al., 2016). If this slope changes from positive to negative values, populations are assumed to have crossed a bifurcation point.
Monogonont rotifers are particularly interesting organisms for testing the potential of generic early-warning metrics. This is because they occupy an important intermediate position in the trophic chain in aquatic habitats as grazers of phytoplankton and prey of invertebrates and fish (Wallace et al., 2006). Moreover, because of their fast life history, they are considered ideal model organisms for eco-evolutionary studies (Declerck & Papakostas, 2016). In this context, rotifers from Lake Orta, a historically perturbed lake in northern Italy, represent a particularly promising model system (Sommer et al., 2016). This is because of the exceptionally detailed documentation of Lake Orta’s pollution history (Bonacina & Baudo, 2001) and the possibility to resurrect decades-old populations from resting stages preserved in the lake’s sediments (Piscia et al., 2012).
The growth rate of laboratory rotifer populations can easily be manipulated by altering the conditions for survival and reproduction. In gradually deteriorating environments, for example, growth rate will decline, and populations will eventually go extinct. We applied such a methodology to test the reliability of six generic early-warning metrics—autocorrelation at-lag-1 (AC), return rate (RR), standard deviation (SD), coefficient of variation (CV), skewness (SK), and kurtosis (KU)—as predictors of population collapse in the planktonic freshwater rotifer Brachionus calyciflorus Pallas. According to theory (e.g. Dakos et al., 2012), AC, CV, SD, and KU are predicted to increase when a system approaches a bifurcation, while RR is predicted to decrease. SK, on the other hand, may increase or decrease depending on whether the alternative equilibrium state beyond the bifurcation point is larger or smaller than the initial equilibrium state (Dakos et al., 2012). In our rotifer system, the alternative state (extirpation of the active population) is smaller than the initial state (some population size larger than zero). Following Dakos et al. (2012), we therefore expected SK to decrease—that is, to be negative and to become more negative—in populations approaching a bifurcation.
Materials and methods
Study organism
The life cycle of B. calyciflorus is characterised by cyclical parthenogenesis, which consists of interrelated amictic (asexual) and mictic (sexual) reproductive phases (Wallace et al., 2006). The dominant mode of reproduction is the asexual phase, in which amictic females develop from unfertilised, diploid eggs by clonal reproduction. Under certain conditions (e.g. crowding), amictic females may produce mictic daughters that produce haploid oocytes, which will develop into males, if the mictic daughters are not inseminated (Gilbert, 2003). Alternatively, if mictic females are inseminated, the oocytes develop into so-called resting eggs (actually diapausing embryos: Boschetti et al., 2011) that are shed by the female and remain dormant for a few days to several months before they hatch (Becks & Agrawal, 2013; Martínez-Ruiz & García-Roger, 2015). Hatching success in B. calyciflorus is affected by temperature and exposure to light (Pourriot & Snell, 1983; Schröder, 2005). For the purpose of this study, we focused on the dynamics of the active population.
General culturing methods
We used soft, artificial freshwater as culture medium for the rotifers. We prepared this medium by dissolving 48 mg NaHCO3, 30 mg CaSO4·2H2O, 61.43 mg MgSO4·7H2O, and 2 mg KCl in one litre of deionised water (U.S. Environmental Protection Agency, 2002). Hereafter, we refer to this medium as EPAs.
First, we extracted B. calyciflorus resting eggs from Lake Orta sediments that were collected in 2012. We immediately incubated the extracted eggs at room temperature (~23°C) under a daily 16-h photoperiod (5000–6000 lx), and we used emerging hatchlings to establish clonal stock cultures (details on the egg extraction and hatching methods can be found in Sommer et al. (2016)). We kept the stock cultures in 50-mL screw-cap polypropylene tubes (Sarstedt AG & Co., Nümbrecht, Germany), which we stored at 20°C in complete darkness in an incubator (Panasonic SGMIR-154).
The single-celled green alga Chlorella vulgaris Beijerinck served as food source for the rotifers. C. vulgaris was cultured at room temperature (~23°C) in an illuminated (~2500 lx) and aerated 0.5 L glass bottle filled with 400 mL of modified WC medium (Guillard & Lorenzen, 1972; for the modifications, see Sommer et al., 2016). This medium has previously been used to maintain C. vulgaris cultures (Massie et al., 2010). We removed about half of the algal culture every Monday, Wednesday, and Friday and refilled the culture bottle with new growth medium. In doing so, we kept the C. vulgaris culture in the logarithmic growth phase. Prior to feeding the rotifers, we centrifuged the removed algae and resuspended the algal pellet in EPAs (for details, see Sommer et al. (2016)).
We refreshed the rotifer stock cultures each Monday, Wednesday, and Friday by transferring about 50–100 individuals to new culture tubes containing fresh EPAs and algae. Initially, we fed the rotifers algae at a density of 2 × 106 cells mL−1. This density was increased to about 5 × 106 C. vulgaris cells mL−1 three days prior to the experiment in order to increase the probability that adult females would be carrying eggs and, as such, were identifiable as mictic or amictic rotifers at the start of the experiment. At this point, the rotifer cultures were between 15 and 34 days old.
Experimental procedures
We tested nine B. calyciflorus clones under two conditions, a constant and a deteriorating environment, which resulted in 18 monoclonal experimental populations. Each of these populations was started with ten randomly selected females carrying, on average, two amictic eggs (range: 1–3 eggs per female); food, temperature, and light conditions were the same as described above. In these experimental populations, we monitored female densities only (see below), because males neither contribute to immediate population growth nor do they affect food levels; B. calyciflorus males are small (body length is ~100 µm compared to ~300 µm in females) and short-lived (1–2 days), and they lack digestive organs (Wallace et al., 2006). Females, on the other hand, may live for 10–11 days when cultured at 20°C (Halbach, 1970).
We refreshed the experimental populations every second day by sieving the cultures through a 30-µm mesh, which retained all females, including neonates, but not the males. We immediately washed the retained females into a petri dish and removed dead individuals, dropped resting eggs, and clumps of digested and excreted algae. We then transferred all live females to fresh medium containing algae and, in the case of the deteriorating environment, the appropriate pollution level (see below). We used fresh medium to avoid deterioration of the unpolluted environment and to control pollution levels in the increasingly copper-polluted environment.
We let the pre-experimental populations grow for ten days before we started with the pollution treatment (hereafter day 0 of the experiment), because B. calyciflorus populations are expected to reach a stable juvenile-to-adult ratio after this period of time when started from a single female (Halbach & Halbach-Keup, 1974). The pollution treatment consisted of increasing concentrations of copper (Cu2+), which we added as copper sulphate pentahydrate to the rotifer culture medium. On day 0, we added 2 µg Cu2+ L−1 to the tubes containing the deteriorating environment and increased the pollution level by another 2 µg Cu2+ L−1 every second day during medium renewal.
We used increasing concentrations of copper in the culture medium to gradually push the populations to extinction, because copper constituted the most dominant contaminant in Lake Orta’s pollution history, along with ammonium sulphate (e.g. Camusso et al., 1991; Calderoni et al., 1992; Bonacina, 2001). Moreover, copper is a frequently used—and therefore well-studied—contaminant in ecotoxicological assays with rotifers (Snell & Janssen, 1995). It is known, for example, to impair the swimming and feeding behaviour of B. calyciflorus and to negatively affect demographic parameters such as life expectancy at hatching, survival rate, generation time, and population growth rate (Snell et al., 1991; Snell & Moffat, 1992; Ferrando et al., 1993; Janssen et al., 1993, 1994; Preston & Snell 2001; Gama-Flores et al., 2007). As such, increasing copper contamination seemed to be an adequate choice as an environmental driver of population decline of our laboratory system.
Population sampling began four days prior to the start of the pollution treatment and was performed every second day before medium renewal. Prior to sampling, we gently shook the experimental tubes in order to homogenise the rotifer cultures. We then removed two 1-mL samples (4% of the culture volume) from each tube and pooled the two samples by transferring them to round, capped plastic boxes (22 mm × 13 mm, diameter × height). Using a stereo-microscope (Nikon SMZ18), we immediately removed all dead rotifers from the samples and added 105 µL of tissue fixative (20× HistoChoice®) to each box in order to preserve the samples for later analyses. For a given clone, sampling of the unpolluted population stopped when the corresponding polluted population had gone extinct. We counted the rotifers in each 2-mL sample within three days of preservation and converted the count data to densities (females mL−1) for further analyses.
Data analyses
To identify potential bifurcation points, we first fitted a generalised additive model (GAM) with a Gaussian error distribution to the population-density time series using treatment (constant or deteriorating environment) as a covariate. We then calculated the slope of the GAM-fitted population growth trajectories in each environment as the first-order derivative with respect to time (Burthe et al., 2016), using finite differences with Δt = 0.001 days. We assumed that the rotifer populations had crossed a bifurcation point when this slope dropped and stayed below zero (Drake & Griffen, 2010; Clements & Ozgul, 2016). To account for uncertainty in the estimates, we calculated the 95% confidence intervals (95% CIs) of the slopes by sampling 1000 sets of model coefficients from a multivariate normal distribution. This distribution was parameterised with the average estimates of the parameters and their variance–covariance matrix.
Next, we investigated temporal trends in the six early-warning metrics. To this end, we first computed all metrics at each population census across the nine clonal populations per environment; this is the preferred method when replicates (here clones) are available (Boettiger & Hastings, 2012). We did not investigate any metrics related to spectral properties, because these metrics are calculated within—rather than across—individual time series (Dakos et al., 2012). Autocorrelation at-lag-1 (AC) measures the similarity between population densities at successive time steps and was calculated as the Pearson correlation coefficient between these densities, while return rate (RR) is the inverse of that similarity. The standard deviation (SD), the coefficient of variation (CV = SD/mean), skewness (SK, the standardised third moment), and kurtosis (KU, the standardised fourth moment) were calculated using the standard formulae for these statistics (e.g. Dakos et al., 2012). We then fitted GAMs to the time series of each metric and calculated the derivatives and their 95% CIs (cf. methods above). We considered a change in a given metric to be significant if the 95% CI of the slope of the metric did not include zero. Regarding populations in the deteriorating environment, we distinguished between true positives—indicated by significant slopes in the expected direction (positive for AC, SD, CV, and KU; negative for RR and SK) prior to the bifurcation—and false negatives (non-significant slopes or slopes in the opposite direction). If a slope of a metric was significant and in the expected direction for the populations kept in the constant environment (i.e. in the absence of a bifurcation), we considered this slope to represent a false positive (Burthe et al., 2016).
Results
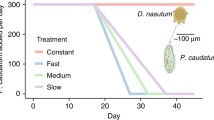
Population growth trajectories in the two treatments were rather similar during the first month of the experiment, but they diverged later on (Fig. 1, left panel). In the constant environment, average population growth was positive throughout the experiment. In the deteriorating environment, on the other hand, it was positive up to day 30, but became negative afterwards (Fig. 1, right panel). The change from positive to negative population growth on day 30 (day 26–33, 95% CI as obtained from the GAM) suggests that the populations in the deteriorating environment crossed the bifurcation point around that day. These populations eventually went extinct.
Growth trajectories (left panel) and growth rates (right panel) of B. calyciflorus populations cultured under unpolluted (blue lines) and increasingly copper-polluted (red lines) conditions. Heavy lines are GAM-fitted curves; shaded areas represent the corresponding 95% confidence intervals. Thin lines in the left panel represent individual clones (n = 9 for each environment). Hatched lines at day 30 mark the estimated bifurcation point in the deteriorating environment
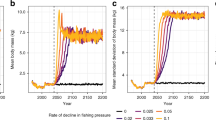
Together, the six early-warning metrics produced only two true positives in the deteriorating environment and one false positive in the constant environment. In detail, the individual performances of the metrics were as follows: AC neither generated a true positive nor a false positive. It was generally high, but it did not change significantly before the bifurcation (Fig. 2a). Likewise, RR was fairly stable and did not produce any signal (Fig. 2b). In contrast, SD increased steeply during the first week of the experiment, then remained fairly stable until about day 45, but declined rapidly afterwards (Fig. 2c). This pattern was observed in both treatments. Hence, it produced a true and a false positive at the very beginning of the experiment. CV, on the other hand, gradually declined in both environments during the first month of the experiment (Fig. 2d). While it continued to decline in the constant environment, it began increasing around day 29 (day 18–32, 95% CI as obtained from the GAM) in the deteriorating environment. That is, CV produced no false positive, yet it generated a true positive around the time of the bifurcation. Furthermore, SK decreased from positive values to zero during the first two weeks (Fig. 2e). While it remained close to zero in the constant environment till the end of the experiment, it began increasing around day 40 (i.e. after the bifurcation) in the deteriorating environment. Hence, it neither produced a true nor a false positive (note that we expected SK to be negative—and to become more negative—prior to the bifurcation). Finally, KU remained fairly stable throughout the experimental period in both environments (Fig. 2f). As such, it did not produce a signal (true or false) either.
Temporal trends (left panels) and slope of temporal trends (right panels) of six early-warning metrics calculated from population-density time series of nine B. calyciflorus clones cultured in an unpolluted (blue) and an increasingly copper-polluted (red) environment. a Autocorrelation at-lag-1, AC; b return rate, RR; c standard deviation, SD; d coefficient of variation, CV; e skewness, SK; and f kurtosis, KU. Symbols in the left panels are individual estimates based on data collected on census days. Heavy lines and shaded areas are GAM-fitted curves and corresponding 95% confidence intervals, respectively. Hatched lines at day 30 indicate the estimated bifurcation in the populations cultured under increasingly copper-polluted conditions
Discussion
We tested the potential of six generic early-warning metrics as indicators of impending collapses in laboratory rotifer populations and found that none of the metrics predicted extirpation of the active populations reliably. Four of the metrics—AC, RR, SK, and KU—generated neither a true nor a false positive. Moreover, although SD produced a true positive in the deteriorating environment, this signal was unreliable, because SD also produced a false positive in the constant environment at the same time. In addition, both signals appeared only briefly at the very beginning of the experiment and most likely resulted from transient population dynamics rather than from critical slowing down (cf. Drake & Griffen, 2010). CV, on the other hand, generated only a true positive. This signal, however, appeared late—around the time the populations in the deteriorating environment were about to cross the bifurcation point—and therefore cannot be regarded as an early-warning signal. Moreover, the rise in CV was largely caused by declining population densities rather than increasing variation in the time-series data, as indicated by the fairly stable SD values at that time. As such, the signal does not seem to result from critical slowing down.
Although collapsing populations in the deteriorating environment may have escaped true extirpation by surviving in the diapausing stage (i.e. via the production of resting eggs), this characteristic of the Brachionus life cycle—which itself can be a response to environmental stress (Piscia et al., 2016)—should not have affected the presence of potential early-warning signals. This is because the signals are expected to build up whenever a system approaches a bifurcation point (Scheffer et al., 2009); that is, they should be present irrespective of the state of the population beyond that point. Since we were interested in investigating the predictive potential of the metrics regarding extirpation of the active population, the nature of that alternative state—true extirpation or persistence in the resting stage—was not investigated quantitatively. However, this alternative state—or more precisely, the nature of the transition across the bifurcation point—could have affected the detectability of potential early-warning signals. For a given birth rate of amictic females, for example, gradually decreasing survival rate (in the case of true extirpation) or increasing proportion of mictic females (in the case of persistence in the resting stage) both lower the growth rate of the active populations (Montero-Pau et al., 2014). However, the dynamics between these two processes likely differ, which, in turn, might affect the detectability of the signals they produce.
Usually, generic early-warning signals tend to work reliably for long (>100 data points) time series as obtained, for example, from simulated data (e.g. Dakos et al., 2012; Clements et al., 2015). This reliability could be due to the way the data are generated and/or the length of time series produced by simulations. Regarding our study, however, it seems unlikely that the comparatively low number of data points obtained up to the estimated bifurcation was the reason for the lack of signals. In an experimental study comparable to the one presented here, Drake & Griffen (2010) cultured Daphnia populations in a deteriorating environment (declining food density) and monitored population sizes weekly until the populations went extinct. From the abundance data, they estimated that the populations had crossed the bifurcation point about 17 weeks after the onset of food decline. In other words, Drake & Griffen (2010) had a similar number of data points per time series available for their analyses; yet, they found correct signals in all the metrics they investigated.
One factor that might explain the discrepancy between the results of Drake & Griffen (2010) and the work presented here is the extent of spatial sampling. A recent analysis of both simulated time-series data and the data of Drake & Griffen (2010) revealed that temporal as well as spatial subsampling can impair the detectability of early-warning signals (Clements et al., 2015). While Drake & Griffen (2010) censused the entire populations, we estimated densities from subsamples, a constraint imposed by the impossibility to non-destructively count all the individuals in the experimental cultures. However, because the rotifer populations in our study reached much higher densities than the Daphnia populations investigated by Drake & Griffen (2010), potential negative effects of partial sampling on the detectability of the early-warning signals in our study were probably less severe than the analyses of Clements et al. (2015) might suggest.
Theoretically, generic early-warning signals are expected to be emitted by a wide range of bifurcation dynamics (Kéfi et al., 2013), including the one potentially generated by our experimental approach (transcritical bifurcation; e.g. Strogatz, 2014; Scheffer et al., 2015). However, in a simulation of predator/prey dynamics, Boerlijst et al. (2013) showed that generic early-warning signals might only be found in the dynamics of specific life-history stages of an organism. Yet, identifying which stage will eventually produce a signal requires information about the underlying mathematics—the dominant eigenvector—of the impending bifurcation (Boerlijst et al., 2013). Moreover, individual heterogeneity in life-history traits may obscure early-warning signals (Krkošek & Drake, 2014), although more recent work suggests that including such traits as additional statistics in the form of composite demographic and phenotypic (e.g. body size) metrics can improve the performance of the early-warning signals (Clements & Ozgul, 2016). In any case, lacking such crucial information about our study system, monitoring the dynamics of egg, juvenile, and adult stages separately, and collecting body-size data of these stages, seems worth a try in future studies. Unfortunately, such a detailed analysis was not possible here due to poor preservation of the collected samples.
Finally, the possibility remains that the rotifer populations in our study system did just not produce any early-warning signal. This could have been the case if, for example, the populations in the deteriorating environment were tracking a declining carrying capacity and, in doing so, never crossed a bifurcation point. Alternatively, copper pollution might have increased too fast in order to allow the rotifer populations to generate a warning signal (Dakos et al., 2012). This alternative interpretation seems to be supported by the observation that population growth in the deteriorating environment was impaired only at concentrations larger than 25 µg Cu2+ L−1; that is, the rotifers did not suffer much before day 25. Such a late response to the copper treatment means that the populations in the deteriorating environment had less than a week to build up early-warning signals, a potential constraint that might have impaired the predictive power of the metrics. In future studies, such temporal constraints could be avoided using slower rates of environmental forcing.
Conclusions
None of the generic early-warning metrics tested here indicated extirpation of laboratory rotifer populations early and reliably; only CV produced a true, but no false, positive. This result is in line with experiments on other laboratory systems (e.g. Daphnia: Drake & Griffen, 2010; yeast: Dai et al., 2012; protists: Clements & Ozgul, 2016) that found the strongest signal in CV compared to other metrics. However, in our study, the signal was generated too late to serve as a useful early-warning signal. All other tested metrics produced either no signal at all (AC, RR, SK, and KU) or both true and false positives (SD). Further, while it has been shown that combining several metrics into composite indices can amplify the strength of signals (Drake & Griffen, 2010; Clements & Ozgul, 2016), we did not apply such a methodology here, because we did not find any reliable early-warning signal in the first place. Although this lack of reliable signals might have partly been due to incomplete population censusing—a methodological constraint that can impair the detectability of early-warning signals—monitoring the size of natural populations is often based on some sort of subsampling as well (Clements et al., 2015). Taken together, the results of our laboratory experiment support the growing number of studies (e.g. Hastings & Wysham, 2010; Perretti & Munch, 2012; Boerlijst et al., 2013; Krkošek & Drake, 2014; Clements et al., 2015; Dakos et al., 2015; Burthe et al., 2016) that challenge the usefulness of single metrics as indicators of approaching shifts in system dynamics.
References
Becks, L. & A. F. Agrawal, 2013. Higher rates of sex evolve under K-selection. Journal of Evolutionary Biology 26: 900–905.
Biggs, R., S. R. Carpenter & W. A. Brock, 2009. Turning back from the brink: detecting an impending regime shift in time to avert it. Proceedings of the National Academy of Sciences of the United States of America 106: 826–831.
Boerlijst, M. C., T. Oudman & A. M. de Roos, 2013. Catastrophic collapse can occur without early warning: examples of silent catastrophes in structured ecological models. PLoS ONE 8: e62033.
Boettiger, C. & A. Hastings, 2012. Quantifying limits to detection of early warning for critical transitions. Journal of the Royal Society Interface 9: 2527–2539.
Bonacina, C., 2001. Lake Orta: the undermining of an ecosystem. Journal of Limnology 60: 53–59.
Bonacina, C. & R. Baudo, 2001. Lake Orta: a case study (Part 1). Journal of Limnology 60: 50–52.
Boschetti, C., F. Leasi & C. Ricci, 2011. Developmental stages in diapausing eggs: an investigation across monogonont rotifer species. Hydrobiologia 662: 149–155.
Burthe, S. J., P. A. Henrys, E. B. Mackay, B. M. Spears, R. Campbell, L. Carvalho, B. Dudley, I. D. M. Gunn, D. G. Johns, S. C. Maberly, L. May, M. A. Newell, S. Wanless, I. J. Winfield, S. J. Thackeray & F. Daunt, 2016. Do early warning indicators consistently predict nonlinear change in long-term ecological data? Journal of Applied Ecology 53: 666–676.
Calderoni, A., R. Mosello & D. Ruggiu, 1992. Sixty years of limnology on Lago d’Orta: a case history of recovery from heavy pollution. Memorie dell’Istituto Italiano di Idrobiologia 50: 201–223.
Camusso, M., G. Tartari & A. Zirino, 1991. Measurement and prediction of copper ion activity in Lake Orta, Italy. Environmental Science & Technology 25: 678–683.
Clark, J. S., S. R. Carpenter, M. Barber, S. Collins, A. Dobson, J. A. Foley, D. M. Lodge, M. Pascual, R. Pielke Jr., W. Pizer, C. Pringle, W. V. Reid, K. A. Rose, O. Sala, W. H. Schlesinger, D. H. Wall & D. Wear, 2001. Ecological forecasts: an emerging imperative. Science 293: 657–660.
Ceballos, G., P. R. Ehrlich, A. D. Barnosky, A. García, R. M. Pringle & T. M. Palmer, 2015. Accelerated modern human-induced species losses: entering the sixth mass extinction. Science Advances 1: e1400253.
Clements, C. F., J. M. Drake, J. I. Griffiths & A. Ozgul, 2015. Factors influencing the detectability of early warning signals of population collapse. The American Naturalist 186: 50–58.
Clements, C. F. & A. Ozgul, 2016. Including trait-based early warning signals helps predict population collapse. Nature Communications 7: 10984.
Collen, B., L. McRae, S. Deinet, A. De Palma, T. Carranza, N. Cooper, J. Loh & J. E. M. Baillie, 2011. Predicting how populations decline to extinction. Philosophical Transactions of the Royal Society B 366: 2577–2586.
Dai, L., K. S. Korolev & J. Gore, 2013. Slower recovery in space before collapse of connected populations. Nature 496: 355–358.
Dai, L., D. Vorselen, K. S. Korolev & J. Gore, 2012. Generic indicators for loss of resilience before a tipping point leading to population collapse. Science 336: 1175–1177.
Dakos, V., S. R. Carpenter, W. A. Brock, A. M. Ellison, V. Guttal, A. R. Ives, S. Kéfi, V. Livina, D. A. Seekell, E. H. van Nes & M. Scheffer, 2012. Methods for detecting early warnings of critical transitions in time series illustrated using simulated ecological data. PLoS ONE 7: e41010.
Dakos, V., S. R. Carpenter, E. H. van Nes & M. Scheffer, 2015. Resilience indicators: prospects and limitations for early warnings of regime shifts. Philosophical Transactions of the Royal Society B 370: 20130263.
Declerck, S. A. J. & S. Papakostas, 2016. Monogonont rotifers as model systems for the study of micro-evolutionary adaptation and its eco-evolutionary implications. Hydrobiologia. doi:10.1007/s10750-016-2782-y.
De Vos, J. M., L. N. Joppa, J. L. Gittleman, P. R. Stephens & S. L. Pimm, 2015. Estimating the normal background rate of species extinction. Conservation Biology 29: 452–462.
Ditlevsen, P. D. & S. J. Johnsen, 2010. Tipping points: early warning and wishful thinking. Geophysical Research Letters 37: L19703.
Drake, J. M. & B. D. Griffen, 2010. Early warning signals of extinction in deteriorating environments. Nature 467: 456–459.
Ferrando, M. D., C. R. Janssen, E. Andreu & G. Persoone, 1993. Ecotoxicological studies with the freshwater rotifer Brachionus calyciflorus III. The effects of chemicals on the feeding behavior. Ecotoxicology and Environmental Safety 26: 1–9.
Gama-Flores, J. L., M. E. Castellanos-Paez, S. S. S. Sarma & S. Nandini, 2007. Effect of pulsed exposure to heavy metals (copper and cadmium) on some population variables of Brachionus calyciflorus Pallas (Rotifera: Brachionidae: Monogononta). Hydrobiologia 593: 201–208.
Gilbert, J. J., 2003. Environmental and endogenous control of sexuality in a rotifer life cycle: developmental and population biology. Evolution & Development 5: 19–24.
Guillard, R. R. L. & C. J. Lorenzen, 1972. Yellow-green algae with chlorophyllide c. Journal of Phycology 8: 10–14.
Halbach, U., 1970. Einfluss der Temperatur auf die Populationsdynamik des planktischen Rädertieres Brachionus calyciflorus Pallas. Oecologia 4: 176–207.
Halbach, U. & G. Halbach-Keup, 1974. Quantitative Beziehungen zwischen Phytoplankton und der Populationsdynamik des Rotators Brachionus calyciflorus Pallas. Befunde aus Laboratoriumsexperimenten und Freilanduntersuchungen. Archiv für Hydrobiologie 73: 273–309.
Hastings, A. & D. B. Wysham, 2010. Regime shifts in ecological systems can occur with no warning. Ecology Letters 13: 464–472.
Hefley, T. J., A. J. Tyre & E. E. Blankenship, 2013. Statistical indicators and state-space population models predict extinction in a population of bobwhite quail. Theoretical Ecology 6: 319–331.
Janssen, C. R., G. Persoone & T. W. Snell, 1994. Cyst-based toxicity tests. VIII. Short-chronic toxicity tests with the freshwater rotifer Brachionus calyciflorus. Aquatic Toxicology 28: 243–258.
Janssen, C. R., M. D. Ferrando Rodrigo & G. Persoone, 1993. Ecotoxicological studies with the freshwater rotifer Brachionus calyciflorus I. Conceptual framework and applications. Hydrobiologia 255/256: 21–32.
Kéfi, S., V. Dakos, M. Scheffer, E. H. van Nes & M. Rietkerk, 2013. Early warning signals also precede non-catastrophic transitions. Oikos 122: 641–648.
Krkošek, M. & J. M. Drake, 2014. On signals of phase transitions in salmon population dynamics. Proceedings of the Royal Society B 281: 20133221.
Ludwig, D., 1999. Is it meaningful to estimate a probability of extinction? Ecology 80: 298–310.
Martínez-Ruiz, C. & E. M. García-Roger, 2015. Being first increases the probability of long diapause in rotifer resting eggs. Hydrobiologia 745: 111–121.
Massie, T. M., B. Blasius, G. Weithoff, U. Gaedke & G. F. Fussmann, 2010. Cycles, phase synchronization, and entrainment in single-species phytoplankton populations. Proceedings of the National Academy of Sciences of the United States of America 107: 4236–4241.
Montero-Pau, J., C. Gabaldón, M. J. Carmona & M. Serra, 2014. Measuring the potential for growth in populations investing in diapause. Ecological Modelling 272: 76–83.
Perretti, C. T. & S. B. Munch, 2012. Regime shift indicators fail under noise levels commonly observed in ecological systems. Ecological Applications 22: 1772–1779.
Piscia, R., P. Guilizzoni, D. Fontaneto, D. A. L. Vignati, P. G. Appleby & M. Manca, 2012. Dynamics of rotifer and cladoceran resting stages during copper pollution and recovery in a subalpine lake. Annales de Limnologie - International Journal of Limnology 48: 151–160.
Piscia, R., S. Tabozzi, R. Bettinetti, L. Nevalainen & M. M. Manca, 2016. Unexpected increases in rotifer resting egg abundances during the period of contamination of Lake Orta. Journal of Limnology 75(s2): 76–85.
Pourriot, R. & T. W. Snell, 1983. Resting eggs in rotifers. Hydrobiologia 104: 213–224.
Preston, B. L. & T. W. Snell, 2001. Full life-cycle toxicity assessment using rotifer resting egg production: implications for ecological risk assessment. Environmental Pollution 114: 399–406.
Regan, H. M., M. Colyvan & M. A. Burgman, 2002. A taxonomy and treatment of uncertainty for ecology and conservation biology. Ecological Applications 12: 618–628.
Scheffer, M., J. Bascompte, W. A. Brock, V. Brovkin, S. R. Carpenter, V. Dakos, H. Held, E. H. van Nes, M. Rietkerk & G. Sugihara, 2009. Early-warning signals for critical transitions. Nature 461: 53–59.
Scheffer, M., S. R. Carpenter, V. Dakos & E. H. van Nes, 2015. Generic indicators of ecological resilience: inferring the chance of a critical transition. Annual Review of Ecology, Evolution, and Systematics 46: 145–167.
Schröder, T., 2005. Diapause in monogonont rotifers. Hydrobiologia 546: 291–306.
Snell, T. W. & B. D. Moffat, 1992. A 2-d life cycle test with the rotifer Brachionus calyciflorus. Environmental Toxicology and Chemistry 11: 1249–1257.
Snell, T. W. & C. R. Janssen, 1995. Rotifers in ecotoxicology: a review. Hydrobiologia 313/314: 231–247.
Snell, T. W., B. D. Moffat, C. Janssen & G. Persoone, 1991. Acute toxicity tests using rotifers IV. Effects of cyst age, temperature, and salinity on the sensitivity of Brachionus calyciflorus. Ecotoxicology and Environmental Safety 21: 308–317.
Sommer, S., S. Nandini, S. S. S. Sarma, A. Ozgul & D. Fontaneto, 2016. Rotifers in Lake Orta: a potential ecological and evolutionary model system. Journal of Limnology 75(s2): 67–75.
Strogatz, S. H., 2014. Nonlinear Dynamics and Chaos. With Applications to Physics, Biology, Chemistry, and Engineering, 2nd ed. Westview Press, Boulder.
U.S. Environmental Protection Agency, 2002. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, 5th ed. Office of Water, Washington, DC.
Veraart, A. J., E. J. Faassen, V. Dakos, E. H. van Nes, M. Lürling & M. Scheffer, 2012. Recovery rates reflect distance to a tipping point in a living system. Nature 481: 357–359.
Wallace, R. L., T. W. Snell, C. Ricci & T. Nogrady, 2006. Rotifera: Biology, Ecology and Systematics, Vol. 1, 2nd ed. Backhuys Publishers, Leiden.
Wissel, C., 1984. A universal law of the characteristic return time near thresholds. Oecologia 65: 101–107.
Acknowledgements
Lake Orta sediments were collected and processed by Andrea Lami, Piero Guilizzoni, and Stefano Gerli (Institute of Ecosystem Study, Verbania Pallanza, Italy). We thank Chris Clements and two anonymous reviewers for helpful comments on the manuscript. This study was supported by a European Research Council Starting Grant to A. O. (Grant No. 337785).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: M. Devetter, D. Fontaneto, C. D. Jersabek, D. B. Mark Welch, L. May & E. J. Walsh / Evolving rotifers, evolving science
Rights and permissions
About this article
Cite this article
Sommer, S., van Benthem, K.J., Fontaneto, D. et al. Are generic early-warning signals reliable indicators of population collapse in rotifers?. Hydrobiologia 796, 111–120 (2017). https://doi.org/10.1007/s10750-016-2948-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2948-7