Abstract
Monogonont rotifers typically undergo diapause to overpass adverse periods and often show within-population variation in diapause duration. Whether such variation corresponds to phenotypic plasticity, genetic polymorphism, or a bet-hedging strategy to cope with environmental unpredictability still remains unanswered. While bet hedging in diapause duration is often invoked in the rotifer literature, empirical evidence is scant and the description of a proximate mechanism responsible of the within-genotype variation required for bet hedging is still pending. We experimentally explored the role of maternal effects as responsible for the variability observed in the timing of resting egg hatching. By tracking the offspring of controlled crosses in clonal lineages of Brachionus plicatilis, we tested for differences in resting egg diapause duration due to (1) clone effect (controlling for genetic variability), (2) mother effect within clones, and (3) mother age and laying order (as candidate proximate factors explaining variability). We found a significant effect of laying order: the first eggs produced exhibited longer diapauses than eggs produced later. Finally, we critically discussed the idea that if rotifer mothers within a clone cannot anticipate the environment their offspring will experience, then they may produce resting eggs that vary in their timing of hatching via this maternal effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monogonont rotifers are cyclical parthenogens, many of them reproducing parthenogenetically at low population densities, but inducing sexual reproduction in a quorum-sensing fashion as a response to a chemical stimulus indicative of crowding (Stelzer & Snell, 2003; Schröder & Gilbert, 2004; Snell et al., 2006). The output of sexual reproduction is the so-called resting egg, an encysted embryo that undergoes diapause and allows temporary populations to withstand with adverse periods (e.g., pond desiccation or presence of antagonists). Typically, after the end of adverse conditions, not all resting eggs hatch in the planktonic growing season following to their production, but exhibit variability in diapause duration, with some of these eggs remaining viable for decades or even centuries (Marcus et al., 1994; Kotani et al., 2001; García-Roger et al., 2006a). Several processes may account to explain this variability, such as predictive phenotypic plasticity or genetic polymorphism (Schröder, 2005; see also Evans & Dennehy, 2005). However, in uncertain environments—those in which the cues predicting environmental variation at the intergenerational scale are not reliable—it has been proposed that variability in diapause duration may be an instance of diversified bet hedging, a strategy by which a genotype could reduce variance in reproductive success by simultaneously investing in different phenotypes in advance of future unpredictable conditions (Simons & Johnston, 2006; Simons, 2009, 2011; Childs et al., 2010). In the case of rotifers, habitat unpredictability is characterized by season-to-season fluctuations in the length of the planktonic growing season (García-Roger et al., 2014). Therefore, habitats may vary from highly predictable (when the length of the growing season extends regularly among seasons) to unpredictable (when the length of the growing season greatly fluctuates among seasons). Schröder (2005) suggested that producing resting eggs with more or less prolonged diapauses might be a case of diversified bet hedging if ‘Early’ (resting eggs being ready to hatch almost immediately after production) and ‘Late’ (resting eggs remaining in longer diapauses in the egg bank) hatchlings are derived from the same clone. Because resting eggs are the only link between one growing season and the next, such a diversification would involve risk spreading within the whole sexual offspring of a rotifer clone, thus maximizing the long-term growth rate of the clone in habitats experiencing unpredictable events of zero fitness (e.g., resting egg hatching at the beginning of a new growing season could occasionally be followed by a failure to produce a new cohort of resting eggs if season length is too short; see García-Roger et al., 2014).
Although bet hedging has been frequently invoked in the rotifer literature, the scarce empirical work on rotifer diapause has neither yet discriminated it against predictive phenotypic plasticity or genetic polymorphism (García-Roger et al., 2014). Furthermore, the description of a proximate mechanism responsible of the within-genotype variation in rotifer diapause duration required for bet hedging is still pending. Hence, diversified bet hedging in this trait could be generated either by ‘adaptive coin-flipping plasticity’ resulting from developmental instability (e.g., random phenotypic variation in shell thickness or permeability; for review in other organisms see Evans & Dennehy, 2005) or by non-genetic maternal effects (i.e., those effects which occur when the phenotype of the mother or the environment she experiences influence the phenotype of her offspring over and above the direct effect of the transmitted genes; Marshall & Uller, 2007), such as effects of the physiological status of the mothers (Philippi & Seger, 1989; Menu & Desouhant, 2002; Crean & Marshall, 2009). Nonetheless, whatever the mechanism underlying variability, a condition for bet hedging in resting egg diapause is that an ‘Early’ or ‘Late’ phenotype must be expressed in response to environmental factors without predictive value for the next growing season (see Menu & Desouhant, 2002; Evans & Dennehy, 2005; Crean & Marshall, 2009).
Here, we investigated and discussed whether any maternal effect can provide the within-genotype variation required for bet hedging in the timing of resting egg hatching by focusing on the well-described monogonont rotifer Brachionus plicatilis. Previous studies on this rotifer species have shown that females undergo physiological changes with age (King, 1969; King & Miracle, 1980; Snell & Childress, 1987; Carmona et al., 1994). More recently, Kim & Hagiwara (2011) have shown that female age has also effects on the morphology and viability of resting eggs in B. plicatilis. Hence, we hypothesized that the aging of rotifer females could affect the duration of diapause in resting eggs. The reproductive effort of producing several resting eggs per female was also hypothesized to have an effect on the fate of rotifer resting eggs. Such an effect was summarized in terms of resting egg laying order. Therefore, by tracking the offspring of controlled crosses in clonal lineages of B. plicatilis, we tested for differences in the hatching phenotype (‘Early’ vs. ‘Late’) due to: (1) ‘Clone’ effect, which accounted for genetic variability, (2) ‘Mother’ effect within clones, and (3) ‘Mother age’ and ‘Resting egg laying order’, as candidate explanatory variables controlling for maternal effects.
Materials and methods
Origin and description of the studied clones
Ten clones of the rotifer B. plicatilis were founded by parthenogenetic proliferation from the hatchlings of resting eggs collected in 2010 from the superficial sediments of Salobrejo lake, a shallow temporary pond in Eastern Spain (for location details, see García-Roger et al., 2006b). This pond was chosen on the basis of our previous knowledge on its variable hydroperiod regime and the existence of intermediate resting egg-hatching rates (García-Roger et al., 2006a, b, 2014). The studied clones were originally identified with molecular markers by Gabaldón et al. (2013) and then cultured individually in f/2-enriched medium (Guillard, 1975) prepared with 12 ppt artificial seawater (Instant Ocean®, Aquarium Systems), at 25°C and constant illumination (150 μmol quanta m−2 s−1) until the start-up of the experiments. During this time, rotifers were fed with the algae Tetraselims suecica at a constant concentration of 2 × 105 cells ml−1. The culture medium was renewed weekly by removing a fraction of medium including rotifers (dilution rate = 0.7 week−1). This procedure was implemented in order to keep the clones under constant growth conditions and prevent the induction of premature sexual reproduction through the periodic reduction of rotifer population density.
Fertilization assays for within clone resting egg production
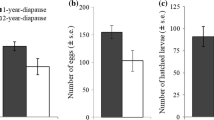
Resting eggs for experiments were produced after intraclonal fertilization of virgin sexual females following the procedure described by Tortajada et al. (2009). Note that because resting eggs are the result of meiosis and syngamy, intraclonal mating in monogonont rotifers is equivalent to selfing in hermaphrodite organisms, which could amplify the expression of genetic variability with respect to clonal propagation. Briefly, 100 asexual females of each clone were initially transferred to individual Erlenmeyer flasks with 150 ml of culture medium (see above), and kept under constant illumination (150–170 μmol quanta m2 s−1) and 20°C. In these conditions, high abundance of males and females can be obtained for mating (Tortajada et al., 2009). Flasks were monitored daily to calculate female density and mictic ratio (i.e., the proportion of ovigerous females that were sexual) until a density of 100 females ml−1, which ensured the induction of sexual reproduction, was reached. If necessary, an inoculum of highly-concentrated fresh medium was added in order to keep algae density over 2 × 105 cells ml−1 during this growth period. Next, 10 ml aliquots of the grown cultures were taken in vials and shaken in order to detach female and male eggs from mothers. Only eggs exhibiting embryo movement (i.e., embryos in an advanced stage of development) were picked up since they usually hatch in less than 4–5 h (Tortajada et al., 2009). For each clone, 100 female eggs and 100 male eggs were pipetted into a well with 1 ml of culture medium. This small volume facilitates male–female encounters, but individuals can still swim freely and perform random mating. In order to maximize the chance of fertilization, mating wells were stored at 18°C for 3 days. After this period, females, which at this point could not be differentiated as sexual or asexual, were isolated individually in 96-multiwell plates (Nunc™) with 150 μl of culture medium and let to reproduce until death following a dynamic life-table approach. Females were then classified according to the type of eggs and offspring they produced as: (1) asexual females (those producing female offspring), (2) unfertilized sexual females (those producing male offspring), or (3) fertilized sexual females (those producing resting eggs). A complete description of the B. plicatilis life cycle and female types can be found in Tortajada et al. (2009). From this point, only fertilized females were followed and monitored every 24 h until all died. Daily, the survival and number of resting eggs produced per female were recorded, and females were transferred to fresh culture medium so as to ensure a constant food-rich environment for resting egg production. From the data in our life-table, we obtained the lifespan and net fecundity (expressed as total number of resting eggs produced per female) of the cohort of fertilized females for each clone.
Hatching experiments
Once resting eggs were produced, they were individually transferred into new 96-multiwell plates (Nunc™) to be directly subjected to standard hatching conditions (25°C, 6 ppt artificial seawater, and constant illumination; see García-Roger et al., 2005, 2006a). Contrary to previous beliefs, some recent studies and our own preliminary observations confirmed that a fraction of newly produced resting eggs is able to hatch a few (1–5) days after production with no need for longer refractory periods (Becks & Agrawal, 2012; Scheuerl & Stelzer, 2013). Each resting egg was labeled by: (1) clone, (2) mother from which the egg proceeded (within clone), (3) laying order, and (4) mother age.
Resting eggs were checked daily for hatching or deterioration until no hatchlings were observed for a 3-day period in a given clone (i.e., saturation of cumulative hatching curves, as observed in a preliminary experiment). Curves of cumulative hatchlings of clones reached asymptote after 20–40 days, but a fraction of resting eggs remained still unhatched in all clones. Those resting eggs that hatched during this 20- to 40-day period were classified as ‘Early’ hatchers, whereas resting eggs that did not hatch during this period were conveniently classified as ‘Deteriorated’- when exhibiting abnormal shape or color indicative of dead embryos (García-Roger et al., 2005; Kim & Hagiwara, 2011)—or ‘Late’ hatchers—if still looking healthy and potentially viable (García-Roger et al., 2005). The viability of ‘Late’ hatchers eggs was confirmed after keeping them in storage conditions (60 ppt, 4°C, no light) for an additional 30-day period and then being re-exposed to the same hatching conditions.
Data analyses
We tested for differences among clones and, where allowed by replication, among individual fertilized females within clones on the following life-history traits obtained from the life-table and hatching experiments: (1) lifespan, (2) net fecundity, (3) resting egg deterioration (i.e., the counts of ‘Deteriorated’ vs. ‘Not deteriorated’ resting eggs), and (4) hatching phenotype (i.e., the counts of ‘Early’ vs. ‘Late’ hatchers), by means of Generalized Linear Mixed Models (GLMMs) using restricted maximum likelihood (REML) with different error distributions and link functions (see “Results” section). Both ‘Clone’ (C) and ‘Mother’, which was nested within ‘Clone’ (M(C)), were treated as random effects and their significance was tested by fitting nested sub-models including only one of the two factors, and then performing likelihood ratio tests against the saturated model (i.e., that including both factors; Pinheiro & Bates, 2000).
The relationship between the extent of resting egg deterioration and mother age was analyzed using mixed-effects binomial regression with ‘Clone’ as blocking factor. We observed that the production of abnormal or dead embryos mostly occurred at advanced ages. Thus, for further analyses on resting egg laying order, we considered only resting eggs that looked healthy at the moment of their production. In order to test the significance of maternal effects on the duration of diapause in resting eggs, we used GLMMs on binary data of the counts of ‘Early’ and ‘Late’ hatchers using ‘Clone’ (C) and ‘Mother within Clone’ (M(C)) as random factors, ‘Resting egg laying order’ (LO) as a categorical predictor, and ‘Mother age’ (MA) as covariate. Then, we explored a set of models of reducing complexity, varying from complete dependence to complete independence among factors and covariates (Logan, 2010). In all models, we fitted a binomial distribution of data and logit as link function. Model validations were assessed graphically using diagnostic plots. To simplify the structure of fixed effects, we performed maximum likelihood (ML) ratio tests hierarchically between pairs of the nested models, what allowed us to select a best model (Johnson & Omland, 2004). Finally, we used McFadden’s pseudo-r 2 to evaluate the goodness-of-fit of our best model. Accordingly, the log-likelihood of the intercept model was treated as a total sum of squares, and the log-likelihood of the selected model was treated as the sum of squared errors (Domenich & McFadden, 1975).
All analyses were carried out using R 2.15.2 (R Development Core Team, 2011). We used the glmer and anova functions from package ‘lme4’ (Bates et al., 2009) for GLMMs and likelihood ratio tests. We assessed the amount of variability in hatching time by means of Kaplan–Meyer survival analysis on the cumulative hatching curves of the different clones using the survift function from package ‘survival’ (Therneau, 2014). Resting eggs that died during the follow-up were conveniently treated as censored data. A non-parametric log-rank test was performed for testing the hypothesis of no difference in hatching profile among the clones studied. In addition, we used Cox’s proportional hazards regression model to examine the relationship of the cumulative hatching distributions to MA and LO as covariates. We fitted the Cox’s model using the coxph function also from package ‘survival’, with ‘Clon’ as stratified factor and LO treated as an ordinal covariate. The proportional hazards assumption was checked for each covariate, along with a global test for the model as a whole, with the cox.zph function available from the same R package.
Results
Nine out of the ten initial clones of B. plicatilis were finally analyzed in the experiments, as one of the clones did not grow during pre-experimental conditions. After fertilization bioassays, we achieved a total of 167 fertilized females to be monitored and their offspring subjected to hatching conditions. Fertilized females produced a total of 858 resting eggs, although only 410 of these eggs looked healthy at the moment of their production. The latest resting eggs produced were found to be more susceptible to deterioration, as showed by the significance of the binomial correlation found between the rate of dead resting eggs produced and mother age (β = 0.604, P value = 0.007; Fig. 1).
Probability of resting egg deterioration plotted against mother age. Symbols represent different clones: SAL2 (filled circle), SAL7 (open circle), SAL9 (filled inverted triangle), SAL14 (open triangle), SAL18 (filled square), SAL21 (open square), SAL22 (filled diamond), SAL23 (open diamond), and SAL29 (filled triangle)
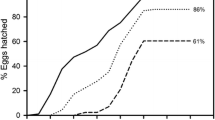
Life-history parameters of B. plicatilis fertilized females and their diapausing offspring are summarized by clone in Table 1. For simplicity, resting egg deterioration and hatching phenotype were expressed as probabilities in this table, although further analyses on these life-history traits were based on binary data (see “Materials and methods” section). Especially for the hatching phenotype, we observed that not all resting eggs produced by a given clone hatched after a first exposure to hatching-promoting conditions, therefore, we differentiated between ‘Early’ and ‘Late’ hatchers. Furthermore, we observed that single rotifer females within clones were able to produce both ‘Early’ and ‘Late’ hatchers. On average for clones, 75.5 ± 5.6% of females (n = 87) which produced two or more resting eggs had a variable offspring in terms of diapause duration. Hatching of ‘Early’ hatchers was not completely synchronous, but slightly extended in time (Fig. 2). The median hatching time for these ‘Early’ hatchers (i.e., time for 50% hatching) ranged 14–30 days among clones, which showed significantly different hatching patterns (Log-rank test, χ 2 = 144, df = 8, P value <0.001). Cox’s proportional hazards test showed that resting egg laying order significantly increased the probability of hatching in the clones studied (Table 2). Holding other factors and covariates constant, each additional resting egg produced by a rotifer female increased its probability of hatching by ca. 19%. We did not find evidence of non-proportional hazards either for each covariate (LO and MA) or the whole model (P value >0.05 in all cases).
Cumulative hatching curves of ‘Early’ hatcher resting eggs in the studied clones. Symbols for clones in legend are as in Fig. 1
The viability of ‘Late’ hatchers was tested by means of a second incubation round at the same hatching conditions. We observed that not all resting eggs initially classified as ‘Late’ hatchers hatched after the second incubation round, but some died during storage and others still remained healthy (Fig. 3). During the second exposure to hatching stimuli, we observed hatchlings in all clones except in two (SAL9 and SAL14). Numbers of hatchlings were low in general, and hatching fraction averaged 19.1 ± 6.1% for clones. Most of the resting eggs (70.7%) still looked healthy after this second exposure. We explored whether clones with large fractions of ‘Early’ hatchers did also hatch with a higher probability in the second exposure. We did not observe correlation between the proportion of eggs hatching in the first and second incubation round in the different clones studied (Pearson’s r 2 = 0.035, t = −0.506, df = 7, P value = 0.628).
As assessed by GLMMs, we observed statistically significant differences among clones for almost all the life-history traits studied, except fecundity (Table 3, C effect). On the other hand, REML ratio tests showed no significant differences among rotifer mothers within clones for any of the life-history traits related to the diapausing offspring (Table 3, M(C) effect). Thus, for the hatching phenotype, all mothers within a clone behaved in the same, but variable, manner.
Because resting eggs with LO above four were rare, we restricted our analyses of the counts of ‘Early’ and ‘Late’ hatchers up to the fourth resting egg produced. By doing this, we got a more balanced design among clones. Therefore, only 376 out of the initial 410 healthy-looking resting eggs were considered for the characterization of their hatching phenotype. Nonetheless, it is worthy to note here that we observed consistent results when the whole data set was considered (i.e., up to the sixth resting egg produced). Table 4 shows the set of nested models that were explored in order to identify the best fit of explanatory variables to observed data of ‘Early’ and ‘Late’ hatchers. Prior to final model selection, random effects due to ‘Mother’ variation within ‘Clone’ (M(C)) were discarded after performing REML ratio tests (P value >0.05 in all models). Model 6 was found to be the most parsimonious model without loss of information with respect to the saturated model (P value = 0.819 for the ML ratio test between models 1 and 6). This model revealed the existence of statistically significant differences due to ‘Clone’ (C) and ‘Laying order’ (LO) effects (see Table 4). No significant interaction effects were found in any of the GLMMs tested, what means that clones had different intercepts, but similar response to increasing levels in LO (Fig. 4). A possible ‘Mother age’ (MA) effect was discarded in all models where LO was involved, but models 5 and 7 showed that MA had a positive significant effect on the proportion of ‘Early’ to ‘Late’ hatchers in the absence of LO. A significant, positive correlation was found between MA and LO (Pearson’s r 2 = 0.533, t = 12.71, df = 408, P value <0.001).
Probability of being an ‘Early’ hatcher plotted against resting egg laying order. As in previous figures, symbols represent different clones: SAL2 (filled circle), SAL7 (open circle), SAL9 (filled inverted triangle), SAL14 (open triangle), SAL18 (filled square), SAL21 (open square), SAL22 (filled diamond), SAL23 (open diamond), and SAL29 (filled triangle)
Discussion
In this study, we have reported the existence of within-population phenotypic variation in diapause duration after defining two well-differentiated types of resting eggs from a single B. plicatilis population on the basis of their timing of hatching: (1) resting eggs which hatched after being subject to a first hatching stimulus, named ‘Early’ hatchers, and (2) resting eggs which did not hatch after the first stimulus but after being re-exposed to a second identical hatching stimulus—so with longer diapauses—and named ‘Late’ hatchers. Indeed, this variability was expressed within the offspring of single clones of the studied rotifer species, what suggests the existence of a risk-spreading strategy at the within-genotype level. It is worthy to note that clone offspring derived from intraclonal crosses, whose genotypes may differ genetically as a result of inbreeding recombination. Since males are haploids and double insemination is expected to be rare (Snell & Childress, 1987), most likely the offspring of a fertilized mother share the sire genes, and should be more related genetically among them than with the offspring of a different mother of the same clone. Nevertheless, heterozygosity levels for microsatellite loci have been observed to be low in the population studied (H e = 0.238; Campillo et al., 2009), and moreover, we have shown that among-mother variation within clones had no significant effects on the principal life-history traits that could affect our results (no significant M(C) effect after REML ratio tests). Interestingly, we observed that single mothers within clones were able to produce both ‘Early’ and ‘Late’ hatchers among their offspring.
Here, we found a mechanistic factor for diapause duration in B. plicatilis resting eggs is a mother condition related to her physiological age. Our results showed that the probability of being an ‘Early’ hatcher increased with resting egg laying order. Not only the incidence of ‘Early’ and ‘Late’ hatchers but also the timing for hatching (in days) observed during the first incubation period in our experiment was significantly reduced by resting egg laying order. We also explore the relationship of mother age to the incidence of ‘Early’ and ‘Late’ hatchers, but this covariate had no effect in most of the explanatory models explored (Table 3). Nonetheless, it is worth considering that mother age had an effect in those models where laying order was not taken into account. The correlation between both covariates was low but significant, what suggests that laying order has more predictive power and masks the effect of mother age. This may be due to the fact that laying order likely accounts for the effort invested by the mothers in producing previous eggs, and so represents a proxy of physiological age. Because diapause duration likely depends on (1) availability of resources during embryo development and (2) consumption of reserves during dormancy, we hypothesize that the first resting eggs produced would have longer diapauses as they might store more reserves than the last eggs produced, which in turn would hatch quickly as their reserves would not be enough to afford a long diapause period. In our study, we took care of providing an excess of food for resting egg production through the whole mother’s lifespan. Thus, we propose that aging may have an effect on the ability of females to allocate reserves to the offspring. The fact that deterioration rates of resting eggs increased with production age of the mother, as observed in this study and others (Kim & Hagiwara, 2011), supports this hypothesis.
We have proposed that variation in diapause duration in B. plicatilis resting eggs likely depends on individual egg availability of metabolic resources which, in turn, would be affected by food availability in the environment and the physiological condition of the mother. Such factors cannot reasonably predict whether the next growing season is going to be good or bad, as this quality likely depends on the length of the following season (e.g., an insufficient amount of rainfall refilling a temporary pond at the beginning of a new season may lead to a very short hydroperiod and a failure in the production of a new cohort of resting eggs, hence a bad season is defined; see Vanoberbeke & De Meester, 2009; García-Roger et al., 2014). Therefore, the condition for bet hedging that an ‘Early’ or ‘Late’ phenotype must be expressed in response to factors without predictive value for the next growing season seems to be fulfilled (see Menu & Desouhant, 2002; Crean & Marshall, 2009). Contrarily, Van Dooren & Brendonck (1998) have suggested that resting egg laying order (or age of the mother) could be a predictive cue under some circumstances: if the mother has managed to live long and produce more than one resting egg, conditions might be favorable for a while still, such that hatching sooner—even within the same season—could be advantageous. Interestingly, the shortest diapauses observed in our experiment lasted only 3–5 days (Fig. 3) and so can be compatible with this latter hypothesis of ‘anticipatory’ maternal effects (see Marshall & Uller, 2007). On the other hand, since sexual reproduction and resting egg formation typically occur close to the end of the growing season, hatching of diapausing eggs just after formation is not exempted of risk as the end of the season is inevitably closer. Given this controversy, at this moment, we cannot unequivocally conclude the existence of maternal-mediated bet hedging in this trait, but our main result is the demonstration of transgenerational plasticity related to resting egg laying order (or mother’s physiological age) in the probability of being a ‘Late’ hatcher.
In addition to the observed maternal control on resting egg-hatching phenotype, we also found a genetic (i.e., among-clone) component influencing the timing of resting egg hatching in this population, as observed in other rotifer life-history traits likely evolving bet-hedging strategies such as mixis induction (Carmona et al., 1994; Fussmann et al., 2007). Notwithstanding, these two factors explained only a low fraction of total variation in resting egg-hatching phenotype after implementing the calculation of the adjusted McFadden’s pseudo-r 2 for our binomial data (pseudo-r 2 = 0.13). Note, however, that McFadden’s pseudo-r 2 typically holds low values and simulations by Domenich & McFadden (1975) has established the equivalence of this value to ca. 30% of explained variance for a linear function. This value is still noticeably low because we controlled for food provisioning during resting egg formation, which was considered an important environmental source of variation in the timing of hatching. Even if we cannot conclude bet hedging from the above-described maternal effect, the finding of a still high residual component of variance in the hatching phenotype provides support for the claim that a risk-spreading strategy may exist. Thus, diversified bet hedging might be caused by factors other than those related to the physiological age of the mother, as for instance by ‘adaptive coin-flipping’ at the mother or embryo level (Oloffson et al., 2009; Childs et al., 2010), or by other unnoticed parental effects affecting resting egg sensitivity to hatching cues (e.g., shell thickness and permeability, concentration of light-absorbing pigments; see Pinceel et al., 2013) and resting egg reserves (e.g., mother size). We hypothesize that if different mothers (within a clone) each has offspring showing different hatching phenotypes probabilistically (all ‘Early’ or all ‘Late’, for the whole offspring of a single fertilized female), it could be evidence of coin-flipping at the maternal level. Despite we observed that single mothers were able to produce both ‘Early’ and ‘Late’ hatchers among their offspring, and that we did not find significant differences among mothers within clones in the counts of ‘Early’ to ‘Late’ hatchers, given the limited numbers of resting eggs produced per female, lack of statistical power impedes saying anything more conclusive on maternal coin-flipping. Alternatively, coin-flipping could also occur at the embryo level, as for instance due to random microenvironmental conditions during embryo development (Simons & Johnston, 1997, 2003), and evidence here would be found by demonstrating that the hatching phenotype changes probabilistically in successive eggs produced by a mother, and not as a consequence of laying order. Again, the few numbers of resting eggs produced by female prevented the implementation of appropriate randomness tests on the sequence of ‘Early’ and ‘Late’ hatchers in the offspring of single fertilized females (i.e., runs test). This is a critical point for further research because it seems difficult to enhance the production of resting eggs per fertilized female far beyond what has been achieved in this study. Under benign laboratory conditions as those described herein, resting egg-producing females in B. plicatilis typically lay 3–4 eggs on average (Snell & Garman, 1986), with maximum reported fecundities of six eggs (see Serra et al., 2008).
To summarize, we found some support that a maternal effect can provide the within-genotype variation required for bet hedging in the hatching phenotype of B. plicatilis resting eggs, and that this effect may be related to the amount of metabolic resources allocated to resting eggs as a function of laying order. While we cannot unequivocally claim whether this effect anticipates or not the environment the offspring of a fertilized female will experience in the next growing season, we call for the need to carry out further studies in order to support the hypothesis that the amount and quality of stored reserves (e.g., lipids) affect diapause duration (work now in progress). Furthermore, evidence of bet hedging in the timing of resting egg hatching would be strengthened by testing for the existence of correlation between the level of within-genotype variation in the trait (i.e., a more or less variable response based on the ratio of ‘Early’ to ‘Late’ hatchers; for details see García-Roger et al., 2014) and the degree of habitat unpredictability across different rotifer populations (see Simons, 2011; Gremer & Venable, 2014).
References
Bates, D., M. Maechler & B. Bolker, 2009. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-31. http://lme4.r-forge.r-project.org/.
Becks, L. & A. F. Agrawal, 2012. The evolution of sex is favoured during adaptation to new environments. PLoS Biology. doi:10.1371/journal.pbio.1001317.
Campillo, S., E. M. García-Roger, M. J. Carmona & M. Serra, 2009. Selection on life-history traits and genetic population divergence in rotifers. Journal of Evolutionary Biology 22: 2542–2553.
Carmona, M. J., M. Serra & M. R. Miracle, 1994. Effect of population density and genotype on life-histroy traits in the rotifer Brachionus plicatilis O.F. Müller. Journal of Experimental Marine Biology and Ecology 182: 223–235.
Childs, D. Z., C. J. E. Metcalf & M. Rees, 2010. Evolutionary bet-hedging in the real world: empirical evidence and challenges revealed by plants. Proceedings of the Royal Society B: Biological Sciences 277: 3055–3064.
Crean, A. J. & D. J. Marshall, 2009. Coping with environmental uncertainty: dynamic bet hedging as a maternal effect. Philosophical transactions of the Royal Society B: Biological Sciences 364: 1087–1096.
Domenich, T. & D. L. McFadden, 1975. Urban Travel Demand: A Behavioral Analysis. North-Holland Publishing Co, Amsterdam.
Evans, M. E. K. & J. J. Dennehy, 2005. Germ banking: bet hedging and variable release from egg and seed dormancy. The Quarterly Review of Biology 80: 431–451.
Fussmann, G. F., G. Kramer & M. Labib, 2007. Incomplete induction of mixis in Brachionus calyciflorus: patterns of reproduction at the individual level. Hydrobiologia 593: 111–119.
Gabaldón, C., J. Montero-Pau, M. Serra & M. J. Carmona, 2013. Morphological similarity and ecological overlap in two rotifer species. PLoS One. doi:10.1371/journal.pone.0057087.
García-Roger, E. M., M. J. Carmona & M. Serra, 2005. Deterioration patterns in diapausing egg banks of Brachionus (Müller, 1786) rotifer species. Journal of Experimental Marine Biology and Ecology 314(2): 149–161.
García-Roger, E. M., M. J. Carmona & M. Serra, 2006a. Patterns in rotifer diapausing egg Banks: density and viability. Journal of Experimental Marine Biology and Ecology 336(2): 198–210.
García-Roger, E. M., M. J. Carmona & M. Serra, 2006b. Hatching and viability of rotifer diapausing eggs collected from pond sediments. Freshwater Biology 51: 1351–1358.
García-Roger, E. M., M. Serra & M. J. Carmona, 2014. Bet-hedging in diapausing egg hatching of temporary rotifer populations—a review of models and new insights. International Review of Hydrobiology 98: 1–11.
Gremer, J. R. & D. L. Venable, 2014. Bet hedging in desert winter annual plants: optimal germination strategies in a variable environment. Ecology Letters 17: 380–387.
Guillard, R. R. L., 1975. Culture of phytoplankton for feeding marine invertebrates. In Smith, W. L. & M. H. Chanley (eds), Culture of Marine Invertebrate Animals. Plenum Press, New York: 26–60.
Johnson, J. B. & K. S. Omland, 2004. Model selection in ecology and evolution. Trends in Ecology and Evolution 19: 101–108.
Kim, H. J. & A. Hagiwara, 2011. Effect of female aging on the morphology and hatchability of resting eggs in the rotifer Brachionus plicatilis Müller. Hydrobiologia 662: 107–111.
King, C. E., 1969. Experimental studies of aging in rotifers. Experimental Gerontology 4: 63–79.
King, C. E. & M. R. Miracle, 1980. A perspective on aging in rotifers. Hydrobiologia 73: 13–19.
Kotani, T., M. Ozaki, K. Matsuoka, T. W. Snell & A. Hagiwara, 2001. Reproductive isolation among geographically and temporally isolated marine Brachionus strains. Hydrobiologia 446(447): 283–290.
Logan, M., 2010. Biostatistical Design and Analysis Using R: A Practical Guide. Blackwell Publishing, Oxford.
Marcus, N. H., R. Lutz, W. Burnett & P. Cable, 1994. Age, viability and vertical distribution of zooplankton resting eggs from an anoxic basin: evidence of an egg bank. Limnology & Oceanography 39: 154–158.
Marshall, D. J. & T. Uller, 2007. When is a maternal effect adaptive? Oikos 116: 1957–1963.
Menu, F. & E. Desouhant, 2002. Bet-hedging for variability in life cycle duration: bigger and later-emerging chestnut weevils have increased probability of a prolonged diapause. Oecologia 132: 167–174.
Oloffson, H., J. Ripa & N. Jonzen, 2009. Bet-hedging as an evolutionary game: the trade-off between egg size and number. Proceedings of the Royal Society B: Biological Sciences 276: 2963–2969.
Philippi, T. & J. Seger, 1989. Hedging one’s evolutionary bets, revisited. Trends in Ecology and Evolution 4: 41–44.
Pinceel, T., B. Vanschoenwinkel, J. Uten & L. Brendonck, 2013. Mechanistic and evolutionary aspects of light-induced dormancy termination in a temporary pond crustacean. Freshwater Science 32: 517–524.
Pinheiro, J. C. & D. M. Bates, 2000. Mixed-Effects Models in S and S-PLUS. Springer, New York.
R Development Core Team, 2011. The R Project for Statistical Computing. R Foundation for statistical computing, Vienna.
Scheuerl, T. & C.-P. Stelzer, 2013. Patterns and dynamics of rapid local adaptation and sex in varying habitat types in rotifers. Ecology and Evolution. doi:10.1002/ece3.781.
Schröder, T., 2005. Diapause in monogonont rotifers. Hydrobiologia 181: 291–306.
Schröder, T. & J. J. Gilbert, 2004. Transgenerational plasticity for sexual reproduction and diapause in the life cycle of monogonont rotifers: intraclonal, intraspecific and interspecific variation in the response to crowding. Functional Ecology 18: 458–466.
Serra, M., E. Aparici & M. J. Carmona, 2008. When to be sexual: sexual allocation theory and population density-dependent induction of sex in cyclical parthenogens. Journal of Plankton Research 30: 1207–1214.
Simons, A. M., 2009. Fluctuating natural selection accounts for the evolution of diversification bet-hedging. Proceedings of the Royal Society B: Biological Sciences 276: 1987–1992.
Simons, A. M., 2011. Modes of response to environmental change and the elusive empirical evidence of bet-hedging. Proceedings of the Royal Society B: Biological Sciences 278: 1601–1609.
Simons, A. M. & M. O. Johnston, 1997. Developmental instablility as a bet hedging strategy. Oikos 80: 401–406.
Simons, A. M. & M. O. Johnston, 2003. Suboptimal timing of reproduction in Lobelia inflate may be a conservative bet-hedging strategy. Journal of Evolutionary Biology 16: 233–243.
Simons, A. M. & M. O. Johnston, 2006. Environmental and genetic sources of diversification in the timing of seed germination: implications for the evolution of bet hedging. Evolution 60: 2280–2292.
Snell, T. W. & M. Childress, 1987. The effect of age on male and female fertility in the rotifer Brachionus plicatilis. Hydrobiologia 147: 329–334.
Snell, T. & B. L. Garman, 1986. Encounter probabilities between male and female rotifers. Journal of Experimental Marine Biology and Ecology 97: 221–230.
Snell, T. W., J. Kubanek, W. Carter, A. B. Payne, J. Kim, M. K. Hicks & C.-P. Stelzer, 2006. A protein signal triggers sexual reproduction in Brachionus plicatilis (Rotifera). Marine Biology 149: 763–773.
Stelzer, C. P. & T. W. Snell, 2003. Induction of Sexual reproduction in Brachionus plicatilis (Monogononta, Rotifera) by a density-dependent chemical cue. Limnology & Oceanography 48: 939–943.
Therneau, T. M., 2014. Package ‘survival’. R package version 2.37-7. http://r-forge.r-project.org/.
Tortajada, A. M., M. J. Carmona & M. Serra, 2009. Does haplodiploidy purge inbreeding depression in rotifer populations? PLoS One 4(12): e8195.
Van Dooren, T. J. M. & L. Brendonck, 1998. The hatching pattern of Branchipodopsis wolfi (Crustacea: Anostraca): phenotypic plasticity, additive genetic and maternal effects. Archiv für Hydrobiologie 52: 219–227. (Special Issues on Advanced Limnology).
Vanoberbeke, J. & L. De Meester, 2009. Within season short-term hatching delays suggest risk-spreading behavior in populations of the freshwater cladoceran Daphnia. Écoscience 16: 441–451.
Acknowledgments
We thank R. Ortells, J. Montero-Pau, M.J. Carmona and M. Serra for helpful comments on the manuscript. Two anonymous reviewers contributed to improve the final version of the manuscript. C. Gabaldón kindly provided the studied rotifer clones from her own collection. This research was supported by funds from the projects UV-INV-PRECOMP12-80525 (University of València) and CGL2012-30779 (Spanish Ministry of Economy and Competitivity, co-financed by FEDER).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Diego Fontaneto
Rights and permissions
About this article
Cite this article
Martínez-Ruiz, C., García-Roger, E.M. Being first increases the probability of long diapause in rotifer resting eggs. Hydrobiologia 745, 111–121 (2015). https://doi.org/10.1007/s10750-014-2098-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-014-2098-8