Abstract
Awareness of pond conservation value is growing all over Europe. Ponds are recognized as important ecosystems supporting large numbers of species and several rare and threatened aquatic plants, macroinvertebrates and amphibians. Notwithstanding ponds, particularly temporary ones, are still neglected in Italy. There are some gaps in our understanding of the macrophyte ecology and the conservation value of Mediterranean small still waters. Therefore, this study investigated the macrophyte communities and physico-chemical characteristics of 8 permanent and 13 temporary ponds along the Tyrrhenian coast near Rome, with the aim to relate the distribution of aquatic plants to environmental variables, and to define the botanical conservation value of ponds. Throughout the study period (Spring 2002), Principal Component Analysis performed on abiotic variables clearly discriminated temporary ponds, smaller and more eutrophic, from permanent ponds, larger and with higher pH and oxygen concentration. A total of 73 macrophyte taxa were collected in the study ponds. Temporary waters hosted a smaller number of plant species than permanent ones. Besides hydroperiod length, the environmental factors related to plant richness were maximum depth, surface area, dissolved oxygen and nitrogen concentration in the water. Moreover, the Non-metric Multidimensional Scaling showed a high dissimilarity in the taxonomic composition of aquatic plants between temporary and permanent ponds. The former contained more annual fast-growing species (Callitriche sp. pl. and Ranunculus sp. pl.), while in the latter species with long life-cycles (i.e. Potamogeton sp. pl.) were more abundant. Our results highlighted that temporary and permanent ponds in central Italy have different macrophyte assemblages, with aquatic species (including some of conservation interest at regional scale) exclusively found in each pond type. This suggested that both type of ponds could give an irreplaceable contribution to the conservation of aquatic plant diversity of these freshwater ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As it has been demonstrated by the contributions of two recent European Ponds Workshops hosted in Geneva, Switzerland (2004) and in Toulouse, France (2006), the awareness of pond conservation value as biodiversity resource is growing all over Europe. Ponds are recognized as particularly important for amphibian (Beebee, 1997; Beja & Alcazar, 2003; Hazell et al., 2004), macroinvertebrate (Collinson et al., 1995; Oertli et al., 2000, 2002; Nicolet, 2001; Nicolet et al., 2004; Della Bella et al., 2005), and aquatic plant conservation (Grillas & Roché, 1997; Linton & Goulder, 2000, Oertli et al., 2000, 2002; Nicolet, 2001; Nicolet et al., 2004). They support large numbers of species and several rare and threatened species of all of these groups (Grillas et al., 2004a, b), and they strongly contribute to freshwater biodiversity at a regional level (Williams et al., 2004).
Ponds, particularly temporary ones, are aquatic habitats with multiple constraints due to their great abiotic variability, but this offers to species with particular adaptations many opportunities to succeed (Schwartz & Jenkins, 2000). For macrophytes inhabiting temporary waters, drought is the principal constraint. This constraint is even greater because of its unpredictability, and alternation of dry and wet-phase varies from year-to-year, especially in Mediterranean regions (Grillas & Roché, 1997). The survival strategies of macrophytes to fluctuations of environmental conditions involve resistant spores, seeds, dormant vegetative parts and flexibility of life cycles (Williams, 1985; Brock, 1988; Grillas & Roché, 1997; Grillas et al., 2004a; Nicolet et al., 2004; Cherry & Gough, 2006). The development of life cycles with different length, the dominance of one form of reproduction (sexual or vegetative), the major or minor investment in seed production and the germination patterns, might concur in structuring the macrophyte assemblages in wetlands with different hydroperiod length (Casanova & Brock, 1996; Grillas & Roché, 1997; Fernández-Aláez et al., 1999; Capon, 2003; Warwick & Brock, 2003; Grillas et al., 2004a). Seasonal wetland vegetation is mostly linked to semi-arid conditions and is widespread in regions with Mediterranean climate, such as the Mediterranean Basin, California, West Africa and Australia, where it has been thoroughly described (Grillas & Roché, 1997; Grillas et al., 2004a, b; Bagella et al., 2005; Barbour et al., 2005; Molina, 2005; Müller & Deil, 2005; Pignatti & Pignatti, 2005; Rudner, 2005). In Mediterranean temporary habitats, most plants are short-lived species with rapid life-cycles and with the ability to rapidly exploit periods of favourable conditions for germination and growth. Sexual reproduction is the dominant form of reproduction and therefore plants make a great investment in seed production to withstand alternate periods of flooding and desiccation (Grillas & Roché, 1997; Bissels et al., 2005; Pignatti & Pignatti, 2005; Rhazi et al., 2005). On the contrary, plants with long life-cycles have an advantage in permanent and more stable aquatic habitats and the vegetative reproduction is generally the commonest form of reproduction (Grillas & Roché, 1997). While the above studies have shown the influence of water regime on composition and distribution of wetland vegetation, comparative studies on the plant community characteristics in temporary and permanent ponds are still limited (Grillas, 1990; Williams et al., 1998; Bianco et al., 2001; Nicolet, 2001).
Macrophyte assemblages, species richness and botanical conservation value of ponds were investigated in some regions of Europe, such as UK (Jeffries, 1998; Williams et al., 1998; Linton & Goulder, 2000; Nicolet, 2001; Nicolet et al., 2004), Germany (Brose, 2001), Switzerland (Oertli et al., 2000, 2002) and France (Grillas, 1990; Grillas & Roché, 1997; Grillas et al., 2004a, b). In Italy the diversity of pond macrophytes has been little recognized. To date, the studies concerning pond vegetation in Italy are very limited (Bianco et al., 2001; Bagella et al., 2005) and there are some gaps in our understanding of macrophyte ecology in small still waters, and in their conservation value. The aims of this study were (i) to investigate the distribution of aquatic plants in some temporary and permanent ponds in central Italy, (ii) to determine the relationships between macrophyte richness and physico-chemical variables, and (iii) to define the botanical conservation value of the study ponds. Such results should allow us to provide useful advises on the management of these aquatic environments.
Methods
Study area
We conducted our study on 8 permanent and 13 temporary ponds located in four protected areas in central Italy, along the Tyrrhenian coast near Rome: WWF Oasis of Palo Laziale, Litorale Romano Natural State Reserve, Decima Malafede Natural Reserve and Presidential Estate of Castelporziano (Fig. 1). These protected areas include the last residues of the original Mediterranean plain forest formerly covering the Latium coast, now surrounded by an urban and agricultural landscape. All these four sites were proposed under the Birds Directive (CEC, 1979) and Habitats Directive (CEC, 1992) as part of the Natura 2000 network (IT6030022/5/7/8, IT6030053, IT6030084; Regione Lazio, 2004). Most of the permanent ponds are ground water fed. In spite of this, water level widely fluctuated during the study year (2002). The sampled temporary ponds may be considered as autumnal ponds (sensu Wiggins et al., 1980) and the length of their hydroperiod depends on rainfall, which usually peaks in autumn and spring. In the study year one temporary pond holded water for 60 days, three had a wet-phase duration between 100 and 200 days, and nine between 200 and 300 days.
Sampling and laboratory methods
Macrophytes
Macrophyte algae (or macroalgae) and vascular plants (submerged, floating and emergent) were collected by walking around and throughout ponds. In order to collect the highest number of species we repeated the surveys of ponds in March, May and June 2002, covering the flowering period of many plants to facilitate the identification. At the start of the study, the spring level of the studied ponds was similar to the winter level. We recorded all plants present in each sampling date within the perimeter of the pond, as defined by the water’s edge, and within 1 m of drawdown zone around it. Most taxa were identified to species level (see Electronic supplementary material), sometimes to genera, and rarely to family (Characeae and some species belonging to Gramineae and Umbelliferae). Species were assigned a distribution and conservation status according to the Regional Red List of Italian plants (Conti et al., 1997; Anzalone et al., in press).
Moreover, at the time of sampling, we also visually estimated macrophyte covers for each pond and the percentage of water surface covered by macrophytes was grouped in five class (0 = 0%, 1 = 1–25%, 2 = 26–50%, 3 = 51–75% and 4 = 76–100%), following the phytosociologic approach (Pignatti & Mengarda, 1962; Braun-Blanquet, 1976).
Physical and chemical data
The study ponds were characterised using variables describing morphology, water and sediment chemistry. At each visit, we measured the maximum depth and area of ponds as reported by Bazzanti et al. (1996). Conductivity, pH and dissolved oxygen were recorded by field metres. Water transparency was measured by visual judgement (1 = clear water, 2 = intermediate, 3 = turbid water). We determined organic matter, organic carbon, total phosphorus and total nitrogen contents and granulometric composition of the sediments, according to methods reported in Cummins (1962), Gaudette et al. (1974), Marengo & Baudo (1988), Bremner (1965), respectively. Nitrogen and phosphorus concentrations in waters were also measured following standard methods reported in IRSA (1994) and Wetzel & Likens (2000).
Data analysis
We employed Principal Component Analysis (PCA), based on morphology, water and sediment characteristics, to summarize variations among ponds and to highlight environmental gradients. Before the analyses, all variables were standardized following
Relationships between numbers of species [log10(x + 1) transformed] and environmental variables were explored using stepwise multiple regressions. Since physico-chemical data were inter-correlated, the first two axes extracted by PCA (PC factors) were used as independent variables in the subsequent regression analysis to determine how much variation in species richness could be accounted for by the environmental variables. Variables with factor loadings ≥|0.60| on an axis were considered important for that particular PC factor. Durbin–Watson test (Durbin & Watson, 1951; Olsen, 1995) was used to control autocorrelation of residuals due to temporal pseudoreplication (Hurlbert, 1984). Values of the test’s parameter (d) near 2 indicate absence of autocorrelation.
In order to measure similarity among pond macrophyte communities, we performed a 2-d Non metric Multidimensional Scaling (N-MDS) on the similarity matrix based on the Bray–Curtis similarity coefficient (Bray & Curtis, 1957; Clarke & Warwik, 1994) which was calculated on presence/absence data of all macrophyte taxa collected in the ponds (in the analysis three ponds were excluded because they hosted only one macrophyte species or were without aquatic vegetation). In order to identify which species were “typical” (found with consistent high frequencies in most of samples) of temporary or permanent pond, we used the Similarity Percentage analysis (SIMPER; Clarke & Warwick, 1994). This procedure decomposes the similarities of all within-pond type comparisons into their contributions from each species and lists species in decreasing order of their importance in typifying the two types of ponds.
Spearman rank coefficient of correlation (rs) was adopted to discover relationships between the environmental variables and PCA factor scores of ponds, and also their NMDS configuration. The Mann–Whitney U-test was used to highlight any significant differences between variables of temporary and permanent ponds.
We conducted our statistical analyses with Statistica (version 5) and PRIMER 5 (version 5.2.0) software.
Results
Physical and chemical characteristics of ponds
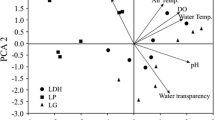
The first two components extracted in the PCA accounted for 57.6% of variance in the original data (Table 1 and Fig. 2). The strongest variations were in the sediment variables, with the contents of phosphorus, nitrogen, carbon and organic matter increasing on the first PC factor, along with silt and clay. The second factor defined a gradient from permanent ponds, with higher values of depth, surface area, pH and higher concentrations of dissolved oxygen in the water, to more temporary ponds, with higher concentrations of phosphorus and nitrogen in the water. Temporary ponds having shorter wet-phase duration (<200 days) are plotted on the most negative side of the second factor because of extreme values of these variables. There were significant differences in the values of these variables between temporary and permanent ponds at least for one sampling occasion, whereas granulometric analysis showed no significant differences in the fraction texture between the two types of ponds and the sediments resulted to be composed predominantly of silt and clay (Table 2).
Principal Component Analysis performed on physico-chemical and morphological variables (the percentage of variance explained by two first axes is reported in brackets and wet-phase duration of temporary ponds is indicated in the legend). Arrows indicate the correlation (significance at least P < 0.05) between axis pond scores and environmental variables
Macrophyte species richness and assemblages
A total of 73 taxa (88% identified to species level) were collected from 21 study ponds (Electronic supplementary material). Fifty-three (more than 70% of the total) were typical or exclusive species of wetland habitat and represent 13% of the aquatic species of Latium Region. During the entire study, 20 ponds hosted aquatic vegetation and the species richness of sites ranged between 1 and 26 (mean = 9 ± 1.6 S.E.; median = 6). The overall macrophyte richness was significantly higher (Mann–Whitney U-test; P < 0.01) in permanent ponds than in temporary ones (Fig. 3) although the maximum number of species was found in a pond with temporary character. On the contrary, the percentages of pond surface area covered by macrophytes did not result significantly different in two pond typologies. Multiple Regression Analysis (Durbin–Watson test for autocorrelation: d = 1.8) found significant relationships between PC factors and species richness of macrophytes. Both axes explained 44% of the variation in macrophyte richness (0.63 + 0.09 PC1 + 0.18 PC2; P < 0.001); but PC2 (P < 0.001) seemed to be more important than PC1 (P < 0.03). Besides hydroperiod length, the environmental factors related to plant richness were maximum depth, surface area, dissolved oxygen and nitrogen concentration in the water (Tables 1 and 3).
Non-metric Multidimensional scaling, performed on presence/absence of all macrophyte taxa collected within the ponds during the study year, showed a clear dissimilarity in the taxonomic composition of aquatic vegetation between temporary and permanent ponds (Fig. 4) along the first axis according to increasing values of nitrogen content in the water and decreasing values of wet-phase duration, surface area, depth, conductivity, pH, transparency, oxygen and phosphorus contents in the sediments. The Similarity Percentages Analysis (SIMPER) showed that temporary and permanent ponds had a Bray–Curtis dissimilarity of 90.6%. In Table 4 the species were listed in decreasing order of their importance in typifying the two groups of ponds. Permanent ponds were characterised by an exclusive presence of Veronica anagallis-aquatica, Mentha aquatica, Characeae, Myriophyllum spicatum and all species belonging to Potamogetonaceae (Potamogeton crispus, P. natans, P. nodosus, P. trichoides), and also by a high occurrence of Lythrum junceum, Sparganium erectum and Rumex conglomeratus. On the other hand, a lot of species belonging to Callitrichaceae (Callitriche truncata, C. stagnalis, C. hamulata) and Ranunculaceae family (Ranunculus ophioglossifolius, R. aquatilis, R. peltatus, R. trichophyllus) were exclusively collected in temporary ponds and Damasonium alisma and Lythrum portula were found with higher occurrence in this type of ponds than in permanent ones.
Finally, in order to assess the botanical conservation value of studied ponds, the number of species of conservation concern was determined at regional level. Out of 53 aquatic species evaluated, five were of conservation interest. One is Vulnerable and exclusively found in a temporary pond, and four were Lower Risk (IUCN, 1994), of which one was exclusively found in permanent ponds and two in temporary ones (Electronic supplementary material).
Discussion
Besides hydroperiod length, the size (maximum depth and surface area), pH, oxygen and nitrogen contents in the water seem to be the main physico-chemical variables which determine the separation between temporary and permanent ponds in central Italy. Principal Component Analysis discriminated temporary ponds, smaller and more eutrophic, from permanent ponds, larger and with higher pH and oxygen concentration along the second axis. However the analysis showed that most of variance among ponds seems to be explained by some sediment variables not related with wet-phase duration of ponds. Likely geology of the area and soil type are factors of primary importance in pond classification. To date there are very few studies on sediment characteristics of ponds, both temporary and permanent, to compare our findings that highlighted the role played by pond sediments. In freshwater ecosystems, sediments represent both nutrient accumulation level and release zone of nutrients (Häkanson, 1984; Salomons, 1985; Chapman, 1989) and, therefore, the sediments can provide an evaluation of the “history” of pond, in spite of the strong variability of their water characteristics.
This study also showed that ponds are valuable for wetland plant biodiversity. In the 21 studied ponds a high number of aquatic plants was found according to the most recent works in Europe. Similarly to our investigation, Williams et al. (1998) in a study of lowland ponds of Great Britain recorded a mean number of six plant species per pond with a range 0–25 species in temporary ponds and a mean of 11 with a range 0–35 in permanent ones. In another study on temporary ponds in Germany, Brose (2001) found a similar number of species per pond (9) with a range 1–24 species. In further investigations in Great Britain, some authors (Nicolet, 2001; Nicolet et al., 2004) recorded ponds, minimally impaired by anthropogenic activities, with an average of seventeen species per temporary pond (range 0–37) and 23 in permanent ponds (range 3–56). All these studies maintain that temporary ponds, although they generally have a few species when compared with permanent ones, could potentially host-plant communities rich in species. This assertion is confirmed by our investigation where the maximum number of species (26) was registered in a temporary pond. This can be attributable to many pioneer species from humid meadows colonising drawdown zone, free from hydrophyte competitors. Yet, median richness was higher in permanent ponds.
A part of wet-phase duration, we found that plant species richness seems mainly depend on pond surface area, depth and nitrogen in the water. In the ponds described here area, depth and nitrogen are highly correlated with hydroperiod because large and less eutrophic ponds are generally permanent. Therefore, it is difficult to discern the effect of hydroperiod, size, nitrogen enrichment on species richness variation as described in a previous work on macroinvertebrate communities hosted in these ponds (Della Bella et al., 2005). However, the relationship between area and macrophyte richness is well documented in aquatic systems (Rørslett, 1991; Vestergaard & Sand-Jensen, 2000; Oertli et al., 2000, 2002; Murphy, 2002; Jones et al., 2003; Rolon & Maltchik, 2006). For aquatic plants, the positive relationship between pond size and biodiversity can be considered a valid generalization for many cases (Gee et al., 1997; Jeffries, 1998; Oertli et al., 2000, 2002; Brose, 2001) although some authors found some controversial results (Friday, 1987; Linton & Goulder, 2000). The influence of water chemistry on aquatic plant richness was analysed in several studies. Generally, nutrient availability was described as major predictor of species distributions and the highest macrophyte diversity was observed in mesotrophic or slightly eutrophic ecosystems (Rørslett, 1991; Vestergaard & Sand-Jensen, 2000, Heegaard et al., 2001; Murphy, 2002). Consistently with our results, Oertli et al. (2000) found a negative relationship between nitrate concentration and the diversity of aquatic plants in ponds, while other authors failed to find this relationship in lakes (Jones et al., 2003) and wetlands (Rolon & Maltchik, 2006).
Moreover, our study highlighted a high dissimilarity in plant species composition between temporary and permanent ponds as shown in the ordination analysis where a clear separation is pointed out between the two types of ponds. The studied temporary waters were characterised by exclusive presence of many species of Callitriche and Ranunculus. These taxa are fast-growing species with an annual life cycle (Pignatti, 1982), and are capable to avoid the pond dry phase through a coincidence between the start of their life cycle and the filling of the basin with the autumn rainfall. Seed production occurs before summer then the plant dies when the pond dries up. Other species without this surviving strategy collected in our temporary ponds, like Damasonium alisma and Alisma lanceolatum, can survive if the moisture of soil is high or the wet-phase is long enough. These findings concur with other studies on plant community composition of temporary ponds and marshes (Grillas & Roché, 1997; Grillas et al., 2004a; Nicolet et al., 2004). On the contrary, the water presence all year round in permanent ponds allows the development of species with perennial life cycles that need more time for growth and flowering (Pignatti, 1982). In fact, in our permanent ponds we found hydrophytes, such as Potamogeton sp. pl., Myriophyllum spicatum and Veronica anagallis-aquatica, with long life-cycles that need to be continuously submerged and that do not tolerate dry soil, thus absent from temporary waters. Hydrological regime is recognized as one of the main factors determining the distribution and characteristics of aquatic vegetation in wetlands with different hydroperiod length (Capon, 2003; Warwick & Brock, 2003). In Mediterranean temporary waters most of plants are annuals, and the seed bank in the soil is of great importance for their survival. In these habitats, aquatic plants made major investment in seed production, thus sexual reproduction is the commonest form of reproduction (Grillas & Roché, 1997). Perennial species and vegetative reproduction dominate in more stable habitats, such as permanent waters, where this kind of plants are advantaged in competition for space, light and nutrients by their life form (Grillas & Roché, 1997). Comparative studies on the plant community composition between temporary and permanent ponds are still limited but they seem to confirm our findings. Bianco et al. (2001) in a previous study on some ponds in the Castelporziano Reserve (Italy) found plant communities dominated by Callitriche sp. pl. and Ranunculus aquatilis in temporary ponds, with turbid and eutrophic waters, and communities rich in Potamogeton species in permanent ones, with more transparent and oxygenated waters. Grillas (1990) investigated submerged macrophyte assemblages in the marshes of the Camargue (France) and found that Callitriche sp. pl., Ranunculus sp. pl. and other species (i.e. Tolypella sp.pl.) dominated communities in temporarily flooded oligohaline marshes whereas permanently flooded marshes are dominated by Potamogeton sp pl. and Myriophyllum spicatum. In the latter, they maintained an important cover and biomass in winter thus preventing the growth of annual species.
In conclusion, this study highlighted that temporary and permanent ponds in central Italy have different macrophyte species composition, with aquatic species exclusively found in each pond type. Permanent ponds are strictly aquatic habitats dominated by hydrophytes. Lowland temporary ponds, while capable of hosting some hydrophytes, are tightly linked to humid meadows. They have similar environmental conditions (temporariness of flooding period, low depth, hydromorphic soils) and they potentially can share similar species, such as Ranunculus ophioglossifolius, R. sardous and Lythrum portula. Our results also showed that the relationship between macrophyte species and pond wet-phase duration depends on pond size and some physico-chemical variables of water. For conservation purposes, the studied temporary and permanent ponds hosted some species of conservation interest at regional scale. Among these, Potamogeton trichoides and Callitriche truncata (Lower Risk Category) are exclusively found more than one time in only one type of pond (see Electronic supplementary material). Therefore, the two types of pond should be preserved or created because they both could give an irreplaceable contribution to the conservation of aquatic plant diversity of small still water bodies in Italy.
References
Anzalone, B., M. Iberite & E. Lattanzi. La flora del Lazio, in press.
Bagella, S., E. Farris, S. Pisanu & R. Filigheddu, 2005. Ricchezza floristica e diversità degli habitat umidi temporanei nella Sardegna Nord-Occidentale. Atti 100° Congresso della Società Botanica Italiana (Roma). Informatore Botanico Italiano 37: 112–113.
Barbour, M. G., A. I. Solomeshch, R. F. Holland, C. W. Witham, R. L. Macdonald, S. S. Cilliers, J. A. Molina, J. J. Buck & J. M. Hillman, 2005. Vernal pool vegetation of California: communities of long-inundated deep habitats. Phytocoenologia 35: 177–200.
Bazzanti, M., S. Baldoni & M. Seminara, 1996. Invertebrate macrofauna of a temporary ponds in Central Italy: composition, community parameters and temporal succession. Archiv für Hydrobiologie 137: 77–94.
Beebee, T. J. C., 1997. Changes in dewpond numbers and amphibian diversity over 20 years on Chalk Downland in Sussex, England. Biological Conservation 81: 215–219.
Beja, P. & R. Alcazar, 2003. Conservation of Mediterranean temporary ponds under agricultural intensification: an evaluation using amphibians. Biological Conservation 114: 317–326.
Bianco, P. M., M. De Lillis & A. Tinelli, 2001. Biodiversità in aree umide di recente realizzazione. In Accademia Nazionale delle Scienze detta dei XL (ed.), “Scritti e documenti” XXVI. 2. Il sistema ambientale della Tenuta Presidenziale di Castelporziano. Ricerche sulla complessità di un ecosistema forestale costiero mediterraneo. Segretariato Generale della Presidenza della Repubblica Italiana, 835–842.
Bissels, S., T. W. Donath, N. Hölzel & A. Otte, 2005. Ephemeral wetland vegetation in irregularly flooded arable fields along the northern Upper Rhine: the importance of persistent seedbanks. Phytocoenologia 35: 469–488.
Braun-Blanquet, J., 1976. Pflanzensoziologie. 3 Aufl. Springer, Vienna.
Bray, J. R. & J. T. Curtis, 1957. An ordination of the upland forest communities of Southern Wisconsin. Ecological Monograph 27: 325–349.
Bremner, J. M., 1965. Total nitrogen. In Black, C. A (ed.), Methods of soil analysis. 2. American Society of Agronomic, Inc., Vol. 9. Madison, 1149–1178.
Brock, M. A., 1988. Flexibility of life cycle pattern as a mechanism for tolerance of fluctuations of environmental conditions by aquatic plants. Verhandlungen der Internationale Vereinigung für theoretische und angewandte Limnologie 23: 1949–1953.
Brose, U., 2001. Relative importance of isolation, area and habitat heterogeneity for vascular plant species richness of temporary wetlands in east-German farmland. Ecography 24: 722–730.
Capon, S. J., 2003. Plant community responses to wetting and drying in a large arid floodplain. River Research and Applications 19: 509–520.
Casanova, M. T. & M. A. Brock, 1996. Can oospore germination patterns explain charophyte distribution in permanent and temporary wetland? Aquatic Botany 54: 297–312.
CEC, 1979. Council of European Communities Directive 79/409/EEC on the conservation of wild birds. Official Journal of European Communities, C103.
CEC, 1992. Council of European Communities Directive 92/43/EEC on the conservation of natural habitats and of wild fauna and flora. Official Journal of European Communities, L206.
Chapman, P. M., 1989. Current approaches to developing sediment quality criteria. Environment Toxicological Chemistry 8: 589–599.
Cherry, J. A. & L. Gough, 2006. Temporary floating island formation maintains wetland plant species richness: The role of the seed bank. Aquatic Botany 85: 29–36.
Clarke, K. R. & R. M. Warwick, 1994. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation. Natural Environment Research Council, UK.
Collinson, N. H., J. Biggs, A. Corfield, M. J. Hodson, D. Walker, M. Whitfield & P. J. Williams, 1995. Temporary and permanent ponds: an assessment of the effects of drying out on the conservation value of aquatic macroinvertebrate communities. Biological Conservation 74: 125–133.
Conti, F., A. Manzi & F. Pedrotti, 1997. Liste Rosse Regionali delle Piante d’Italia. WWF Italia, Società Botanica Italiana, Camerino.
Cummins, K. W., 1962. An evaluation of some technique for the collection and analysis of benthic sample with special emphasis on lotic water. American Midland Naturalist 67: 447–504.
Della Bella, V., M. Bazzanti & F. Chiarotti, 2005. Macroinvertebrate diversity and conservation status of Mediterranean ponds in Italy: water permancence and mesohabitat influence. Aquatic Conservation: Marine and Freshwater Ecosystems 15: 583–600.
Durbin, J. & G. S. Watson, 1951. Testing for serial correlation in least-squares regression. II Biometrika 38: 159–178.
Fernández-Aláez, C., M. Fernández-Aláez & E. Bécares, 1999. Influence of water level fluctuation on the structure and composition of the macrophyte vegetation in two small temporary lakes in the northwest of Spain. Hydrobiologia 415: 155–162.
Friday, L. E., 1987. The diversity of macroinvertebrate and macrophyte communities in ponds. Freshwater Biology 18: 87–104.
Gaudette, H. E., W. R. Flight, L. Toner & D. W. Folger, 1974. An inexpensive titration method for the determination of organic carbon in recent sediments. Journal of Sediment Petrology 44: 249–253.
Gee, J. H. R., B. D. Smith, K. M. Lee & S. W. Griffiths, 1997. The ecological basis of freshwater pond management for biodiversity. Aquatic Conservation: Marine and Freshwater Ecosystems 7: 91–104.
Grillas, P., 1990. Distribution of submerged macrophyte in the Camargue in relation to environmental factors. Journal of Vegetation Science 1: 393–402.
Grillas, P. & J. Roché, 1997. Vegetation of Temporary Marshes. Ecology and Management. Station Biologique de la Tour du Valat, Arles.
Grillas, P., P. Gauthier, N. Yavercovski & C. Perennou, 2004a. Mediterranean Temporary Pools, Vol. 1. Issues Relating to Conservation, Functioning and Management. Station Biologique de la Tour du Valat, Arles.
Grillas, P., P. Gauthier, N. Yavercovski & C. Perennou, 2004b. Mediterranean Temporary Pools, Vol. 2. Species Information Sheets. Station biologique de la Tour du Valat, Arles.
Häkanson, L., 1984. On the relationship between lake trophic level and lake sediments. Water Research 18: 303–314.
Hazell, D., J. M. Hero, D. Lindenmayer & R. Cunningham, 2004. A comparison of constructed and natural habitat for frog conservation in an Australian agricultural landscape. Biological Conservation 119: 61–71.
Heegaard, E., H. H. Birks, C. E. Gibson, S. J. Smith & S. Wolfe-Murphy, 2001. Species-environmental relationships of aquatic macrophtes in Northern Ireland. Aquatic Botany 70: 175–223.
Hurlbert, S. J., 1984. Pseudoreplication and the design of ecological field experiments. Ecological Monographs 54: 187–211.
IRSA, CNR, 1994. Metodi analitici per le acque. Istituto poligrafico e zecca dello stato, Roma.
IUCN, 1994. IUCN Red List Categories. Prepared by the IUCN Species Survival Commission. IUCN, Gland, Switzerland.
Jeffries, M. J., 1998. Pond macrophyte assemblages, biodisparity and spatial distribution of ponds in the Northumberland coastal plain, UK. Aquatic Conservation: Marine and Freshwater Ecosystems 8: 657–667.
Jones, J. I., W. Li & S. C. Maberly, 2003. Area, altitude aquatic plant diversity. Ecography 26: 411–420.
Linton, S. & R. Goulder, 2000. Botanical conservation value related to origin and management of ponds. Aquatic Conservation: Marine and Freshwater Ecosystems 10: 77–91.
Marengo, G. & R. Baudo, 1988. Forme del fosforo nei sedimenti lacustri. Acqua Aria 6: 717–721.
Molina, J. A., 2005. The vegetation of temporary ponds with Isoetes in the Iberian Peninsula. Phytocoenologia 35: 219–230.
Müller, J. V. & U. Deil, 2005. The ephemeral vegetation of seasonal and semipermanent ponds in tropical West Africa. Phytocoenologia 35: 327–388.
Murphy, K. J., 2002. Plant communities and plant diversity in softwater lakes of northern Europe. Aquatic Botany 73: 287–324.
Nicolet, P., 2001. Temporary ponds in the UK: a critical biodiversity resource for freshwater plants and animals. Freshwater Forum 17: 16–25.
Nicolet, P., J. Biggs, G. Fox, M. J. Hodson, C. Reynolds, M. Withfield & P. Williams, 2004. The wetland plant and macroinvertebrate assemblages of temporary ponds in England and Wales. Biological Conservation 120: 265–282.
Oertli, B., D. Auderset Joye, E. Castella, R. Juge & J. B. Lachavanne, 2000. Diversité biologique et typologie écologique des étangs et petits lacs de Suisse. Université de Genève, Office Federal de l’Environnement, des Forets et du Paysage (OFEFP), Genève.
Oertli, B., D. Auderset Joye, E. Castella, R. Juge, D. Cambin & J. B. Lachavanne, 2002. Does size matter? The relationship between pond area and biodiversity. Biological Conservation 104: 59–70.
Olsen, W., 1995. Regression Techniques. SAGE publishing inc., New York.
Pignatti, S., 1982. Flora d’Italia. 3 voll. Edagricole, Bologna.
Pignatti, S. & F. Mengarda, 1962. Un nuovo procedimento per l’elaborazione delle tabelle fitosociologiche. Accademia Nazionale dei Lincei. Rendiconti Lincei, Scienze Fisiche e Naturali, Serie 8, 32: 215–222.
Pignatti, E. & S. Pignatti, 2005. Ephemeral wetland vegetation of Western Australia. Phytocoenologia 35: 201–218.
Regione Lazio, 2004. La Rete Natura 2000 nel Lazio. Caratterizzazione dei Siti di Importanza Comunitaria e delle Zone di Protezione per l’attuazione della sottomisura I.1.2. Seconda edizione. Assessorato Ambiente, Dipartimento Territorio, Direzione Regionale Ambiente e Protezione Civile, Roma.
Rhazi, M., P. Grillas, F. Médail & L. Rhazi, 2005. Consequences of shrub clearing on the richness of aquatic vegetation in oligotrophic seasonal pools in Southern France. Phytocoenologia 35: 489–510.
Rolon, A. S. & L. Maltchik, 2006. Environmental factors as predictors of aquatic macrophyte and composition in wetlands of southern Brazil. Hydrobiologia 556: 221–231.
Rørslett, B., 1991. Principal determinants of aquatic macrophyte richness in northern European lakes. Aquatic Botany 39: 173–193.
Rudner, M., 2005. Seasonal and interannual dynamics in dwarf rush vegetation in the Southwestern Iberian Peninsula. Phytocoenologia 35: 403–420.
Salomons, W., 1985. Sediments and water quality. Environmental Technology Letters 6: 315–326.
Schwartz, S. S. & J. D. Genkins, 2000. Temporary aquatic habitats: constraints and opportunities. Aquatic Ecology 34: 3–8.
Vestergaard, O. & K. Sand-Jensen, 2000. Aquatic macrophyte richness in Danish lakes in relation to alkalinity, transparency, and lake area. Canadian Journal of Fisheries and Aquatic Science 57: 2022–2031.
Warwick, N. W. M. & M. A. Brock, 2003. Plant reproduction in temporary wetlands: the effects of seasonal timing, depth, and duration of flooding. Aquatic Botany 77: 153–167.
Wetzel, R. G. & G. E. Likens, 2000. Limnological Analyses. 3rd edn. Springer-Verlag, New York.
Wiggins, G. B., R. J. Mckay & I. M. Smith, 1980. Evolutionary and ecological strategies of animals in annual temporary pools. Archiv für Hydrobiologie Suppl. 58: 97–206.
Williams, W. D., 1985. Biotic adaptation in temporary lentic waters, with special reference to those in semi-arid and arid regions. Hydrobiologia 125: 85–110.
Williams, P., J. Biggs, C. J. Barr, C. P. Cummins, M. K. Gillespie, T. C. G. Rich, A. Baker, J. Baker, J. Beesley, A. Corfield, D. Dobson, A. S. Culling, G. Fox, D. C. Howard, K. Luursema, M. Rich, D. Samson, W. A. Scott, R. White & M. Whitfield, 1998. Lowland Pond Survey 1996. Department of the Environment, Trade and Regions, London.
Williams, P., M. Whitfield, J. Biggs, S. Bray, G. Fox, P. Nicolet & D. Sear, 2004. Comparative biodiversity of rivers, streams, ditches and ponds in an agricultural landscape in Southern England. Biological Conservation 115: 329–341.
Acknowledgements
The study was carried out as part of the PhD of V.D.B., and supported by a MURST 60% grant to M.B. We thank F. Grezzi for his valuable help in the field work. We wish to thank also the Presidential Estate of Castelporziano, WWF Lazio, Councils of Roma and Fiumicino, and Roma Natura for granting us permission to conduct our research within their natural reserves. We are also grateful to two anonymous referees for their suggestions and to B. Oertli for his editing care that improved the earlier version of the article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: R. Céréghino, J. Biggs, B. Oertli & S. Declerck
The ecology of European ponds: defining the characteristics of a neglected freshwater habitat
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Della Bella, V., Bazzanti, M., Dowgiallo, M.G. et al. Macrophyte diversity and physico-chemical characteristics of Tyrrhenian coast ponds in central Italy: implications for conservation. Hydrobiologia 597, 85–95 (2008). https://doi.org/10.1007/s10750-007-9216-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-9216-9