Abstract
Ephemeral ponds are vulnerable aquatic habitats which are difficult to protect given their dynamic nature and sensitivity to degradation during dry periods. Little information is available on these habitats in austral regions, with almost no information on food-web structure and complexity. The study aimed to assess trophic interactions among dominant organisms in an ephemeral pond food web, and investigate the importance of autochthonous and allochthonous carbon, using 13C and 15N isotopes. Results of the investigation suggest that the food web comprised four trophic levels, with the top predators being Notonectids (Notonecta sp.) and diving beetles (Cybister tripunctatus (Olivier)). Intermediary trophic levels comprised zooplankton (daphniids and copepodids), macroinvertebrates (e.g. micronectids and molluscs) and tadpoles. Generalist feeders dominated the higher trophic levels (>3) with specialists comprising the lower trophic levels (≤3). The consumers preferred autochthonous fine particulate organic matter, epiphyton and submerged macrophyte organic matter sources over allochthonous sources. Autochthonous organic matter was transferred to the food web via zooplankton and select macroinvertebrates including Micronecta sp. and Physa sp. The food-web structure within the pond appeared to reflect the secondary stage of trophic structural complexity in the evolution of ephemeral ponds over the course of their hydro-period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ephemeral ponds are an essential habitat for a variety of amphibians, invertebrates and fish and often represent important foraging ground for wading birds (Heyer et al., 1975; Ferreira et al., 2012; Polačik et al., 2014). Despite their ecological importance, the greater majority of these systems receive little or no protection and are often degraded or destroyed during the dry phase of their hydro-period (Palik et al., 2006). Ephemeral ponds are among the most difficult freshwater ecosystems to create or restore primarily because of their unique hydrological and ecological properties (Gebo & Brooks, 2012). Since ephemeral ponds are susceptible to degradation and loss, organisms dependent on these water bodies are particularly vulnerable (Gamble & Mitsch, 2009; Gebo & Brooks, 2012). One factor which makes it difficult to conduct ephemeral ponds surveys is that they are often not mapped due to their relatively small size and variable hydro-period. The development of strategies for the successful management of freshwater systems depends on our knowledge of biogeochemical dynamics and their dependence on hydrological regimes (Kopprio et al., 2014).
Ephemeral ponds provide convenient systems for testing ecological theories and represent important habitats for invertebrates and amphibians (Blaustein & Schwartz, 2001; De Meester et al., 2005). The organisms that inhabit these systems typically have characteristics associated with rapid growth and high-reproductive rates reflecting the temporal nature of these systems (Marcus & Weeks, 1997). Among the animal communities, crustaceans, branchiopods, hexapods and amphibians typically predominate, while fish are largely absent (Zacharias et al., 2007).
Many freshwater bodies receive high loadings of organic matter from adjacent environments and/or upland catchments, and consumers are often supported by these externally produced energy sources (Carpenter et al., 2005; Francis et al., 2011). Thus, freshwater ecosystems are exposed to subsidies from the terrestrial environments as a result of the aquatic ecosystems high perimeter to area ratios that characterize the amount of contact between aquatic and terrestrial systems (Francis et al., 2011). This is particularly pertinent for small ephemeral aquatic habitats, and stresses the importance of determining the relative contributions of allochthonous and autochthonous resources in food-web dynamics in these systems. One way to assess food-web dynamics within this context is to employ stable isotopes (Post, 2002; Carpenter et al., 2005; Kopprio et al., 2014; Yang et al., 2014). This method provides time-integrated information on the material assimilated by organisms thereby allowing the understanding of inorganic and organic carbon pools and changes therein (Arcagni et al., 2013). Carbon and nitrogen stable isotope compositions (δ13C and δ15N) are most commonly used in ecological studies (Abrantes et al., 2014). The δ13C changes little from food source to consumer (McCutchan et al., 2003) but can vary between different producers and is generally used as a source indicator (Mao et al., 2012). The trophic fractionation of δ15N, however, is mostly used as an indicator of trophic position (Post, 2002; McCutchan et al., 2003; Layman et al., 2007; Mao et al., 2012). The δ15N can also differ between sources, hence it can give information on diet, especially when combined with δ13C (Fry, 1991; Mao et al., 2012; Abrantes et al., 2014, Hill et al., 2015). As a result, δ13C and δ15N analyses can provide critical information about trophic relationships, food sources and the different energy pathways utilized (Huang et al., 2007; Mao et al., 2012; Arcagni et al., 2013). This approach has, however, been underutilized in African freshwater studies with the majority of published stable isotope food-web studies in the region having been conducted in estuaries or near shore marine environments (see for example, Froneman, 2002; Abrantes et al., 2014; Bergamino et al., 2014; Hill et al., 2015).
There are few studies on the food webs of freshwater environments in southern Africa, although there has been renewed interest in these systems (e.g. Harding & Hart, 2013), the focus has principally been on large freshwater river systems (e.g. Hecky & Hesslein, 1995; O’Reilly et al., 2002; Hill et al., 2015). The absence of food-web studies in small ponds within the temperate zone of South Africa is surprising, given the large number of such systems in the region (Ferreira et al., 2012). The current study, therefore, employed stable isotope analyses to assess the food-web structure and trophic interactions among the dominant faunal groups within a small-sized ephemeral pond located in the temperate Eastern Cape Province of South Africa. The main aim of this study was to assess the importance of autochthonous and allochthonous carbon sources in the food web and to assess trophic interactions among the major components of the zooplankton and macroinvertebrates using δ13C and δ15N isotope analysis in a small ephemeral pond. Given the small size of the pond, we hypothesized that allochthonous sources would be particularly important in the food web as previous research suggests that in oligotrophic freshwater systems, the detrital food web predominates. Moreover, we hypothesized that the detritus in the small system is comprised largely of subsidized material from adjacent terrestrial habitats.
Materials and methods
Study area
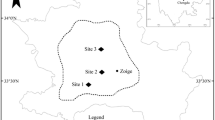
The small ephemeral pond (33°15′S, 26°26′E) lies approximately 10 km northwest of Grahamstown in the Eastern Cape Province of South Africa (Fig. 1). The pool is located within a temperate climatic zone, with warm atmospheric summer mean daily temperatures of 20.3°C (January) and mild winter mean daily temperatures of 12.3°C (June; Sinchembe & Ellery, 2010). Rainfall is distributed evenly over the entire catchment, with mean annual rainfall of ~680 mm, which is concentrated in the summer months from September to March (Sinchembe & Ellery, 2010). The pond, at the time of sampling had a maximum depth of 1.33 m, a length of 61.8 m a maximum width of 27.2 m and an estimated total surface area of 1680 m2 at full capacity (Suárez-Morales et al., 2015).
The vegetation in the immediate vicinity of the pond comprised grassland (Sporobolus africanus (Poir.) Robyns & Tournay), which is dominated by clumps of Acacia karroo Hyne shrubs (<1.5 m in total height). The littoral zone of the pond was characterized by extensive beds of Cyperus marginatus Thunb., Potamogeton schweinfurthii A. Benn., Lagarosiphon muscoides Harv. and Laurembergia repens P. J. Bergius subsp. brachypoda (Welw. ex Hiern) Oberm. The pond is situated on a privately owned farm and is utilized by cattle (Bos taurus Linnaeus) and sheep (Ovis aries aries var. merino Linnaeus) for drinking purposes. While the pond typically dries out during the dry periods, fine-scale hydro-period details are yet to be assessed.
Physical and chemical variables
Portable probes (CyberScan Series 600, Eutech Instruments, Singapore) were employed to measure conductivity, dissolved oxygen, pH, salinity, total dissolved solids and water temperature on site from four points in the pond (Fig. 1). Additionally, water samples (500 mL) were collected and placed on ice for the determination of dissolved ammonia, phosphate and nitrate concentrations in the laboratory using a HI 83203 multi-parameter bench photometer (Hanna Instruments Inc., Rhode Island). Concentrations were determined within 1 h of water sample collection.
Chlorophyll-a analysis
Chlorophyll-a (chl-a) measurements in the water column and macrophytes were analysed to give a proxy of the suspended and epiphyton algal concentrations from the four sampling points. Epiphyton brushed off 10 Cyperus marginatus 30 cm stalks using a toothbrush in 500 mL distilled water that were collected from four different sites of the pond together with 500 mL water samples for determination of suspended chl-a concentrations. Samples were stored under dark conditions until in the laboratory (within 1 h) whereby 250 mL of each sample was filtered and extracted as described in APHA (1995). Epiphyton chl-a concentration was presented as µg l−1 per 30 cm stalk and water column chl-a as µg l−1.
Isotope sample collection
Basal food sources and consumer samples were collected in July 2014, when the pond was at approximately half capacity. Four surface water samples, 30–40 cm depth, were collected using 20 l containers for the determination of fine particulate organic matter (FPOM, <500 μm size). Epiphyton samples were collected from submerged stalks of the dominant macrophyte species, C. marginatus, and were processed within an hour of collection. This was done in the laboratory, whereby the epiphyton brushed off the stalks, using a toothbrush, into distilled water with replicate samples (n = 4) attained from separate stalks. FPOM and epiphyton water were then pre-filtered through a 64 μm mesh to remove zooplankton and coarse particulate organic matter (CPOM). The water was then further filtered through pre-combusted (450°C, 5 h) Whatman GF/F filters. Visible zooplankton which passed through the pre-filtration was removed with forceps under a dissecting Olympus microscope operated at 100× magnification. Each GF/F filter was then placed in a separate labelled pre-combusted (450°C, 5 h) aluminium foil and stored at −20°C.
Using a van Veen grab, two independent sediment samples were collected (bite depth ≈ 1–2 cm) and placed into sterile plastic bags for laboratory analysis. Green leaves and stems of the macrophytes C. marginatus, L. muscoides, P. schweinfurthii and L. repens subsp. brachypoda were collected by hand, while CPOM was obtained from the surface sediment by hand picking. All samples were placed in separate labelled ziplock bags. Green leaves of the terrestrial shrub Acacia karroo Hayne and C4 grass S. africanus were hand collected and placed in labelled ziplock bags. Fresh cow and sheep dung were hand collected around the pond. In the laboratory, the sediment, macrophytes, terrestrial vegetation, cow and sheep dung were placed in pre-combusted foil envelopes and stored at −20°C.
During an exploratory survey to determine the size structuring of the zooplankton community, a 32 cm Ø mouth diameter 63 μm mesh zooplankton net was towed for 10 m through both open water and vegetated zones in the pond. For isotope collection, however, we employed a 200 μm mesh zooplankton net (50 cm Ø), in the same manner as this would collect the vast majority of zooplankton community, while ensuring a sample largely free of particulate organic matter. Zooplankton tows were conducted perpendicular to the littoral zone, while macroinvertebrate and tadpole samples were collected using a nylon hand net (500 μm mesh size, 30 × 30 cm dimension). Benthic invertebrate samples were collected by disturbing the sediment to suspend any organisms and collected using a hand net. All macroinvertebrate and frog species were identified to the lowest taxonomic level (genus or species level) using the field keys of du Preez & Carruthers (2009), Gerber & Gabriel (2002) and Suárez-Morales et al. (2015). The collected samples were sorted in the field and placed in separate containers, and the macroinvertebrate samples were left overnight to clear their guts. The macroinvertebrate samples were then placed in labelled pre-combusted aluminium foil envelopes and stored at −20°C. Tadpole tail muscle tissue samples were used in this study, since these are less variable in δ13C and δ15N than other tissue types (Pinnegar & Polunin, 1999; Mao et al., 2012), the whole body mass of invertebrates was used.
Stable isotope sample processing and analysis
All samples were freeze dried using a VirTis Benchtop 2 K freeze drier at −60°C for 36 h. The freeze-dried samples of sediment, macrophytes, macroinvertebrates, muscles, dung and terrestrial vegetation were further ground to a fine homogeneous powder using a mortar and pestle, and about 0.8–2 mg of the sample was placed in tin capsules. However, Physa sp., Cyzicus sp., Cypricercus sp. and Lynceus sp. had their shells removed prior to grinding, as these parts are typically carbon enriched. Before placement into tin capsules, dried samples of sediment were acidified by vortexing for 2 min in 2 M hydrochloric acid, centrifugation for 5 min at 3600 rpm, washed twice in deionised water followed again by centrifugation, dried at 50°C and homogenized in a Retsch Mixer Mill. Approximately, 1 mg each of dried epiphyton and FPOM material was collected from the Whatman GF/F filters and placed into tin capsules.
Stable isotope analyses were conducted at the IsoEnvironmental Laboratory at South African Institute for Aquatic Biodiversity (SAIAB) using a Europa Scientific 20–20 Isotope Ratio Mass Spectrometer linked to an ANCA SL Prep Unit. Carbon and nitrogen isotopic signatures were expressed as the relative differences between isotopic ratios in the sample and conventional standards (internal: beet sugar and ammonium sulphate, and certified Casein protein standard), using the standard equation:
where R is 13C/12C or 15N/14N.
The trophic positions (TP) of the various consumers in the pond were estimated using the formula of McCutchan et al. (2003) and Huang et al. (2007):
where δ15Nconsumer is the measured consumer δ15N for which TP needs to be estimated and δ15NDaphnia magna is the average δ15N of the primary consumer, in this case, Daphnia magna Straus, and 3.4 is the trophic fractionation for δ15N (Mao et al., 2012). The level 2 was consequently attributed, empirically, to D. magna (Mao et al., 2012).
Data analysis
The δ13C and δ15N values of the basal food sources and consumers were compared by means of a one-way ANOVA test using SPSS version16.0 for Windows software (SPSS Inc., 2007). The relative proportions of selected different basal food sources (epiphyton, FPOM, CPOM, L. repens subsp. brachypoda, C. marginatus, P. schweinfurthii, L. muscoides, sheep dung) and prey (Acari, Chironomus sp., D. magna, Lovenula raynerae Suárez-Morales, Wasserman & Dalu, Physa sp., Paradiaptomus lamellatus Sars, Micronecta sp., Xenopus laevis Loveridge tadpole small) based on the literature (e.g. Fernando & Leong, 1963; Vareschi & Jacobs, 1985; Giller, 1986; Pinder, 1986; Proctor & Pritchard, 1989; Allanson et al., 1990; Measey, 1998; Dillon, 2000; Ohba, 2009) to diets of the potential selected consumers (Acari, Cybister tripunctatus (Olivier), L. raynerae, Micronecta sp., Notonecta sp., Physa sp. P. lamellatus, X. laevis tadpoles) were assessed using a Bayesian mixing model, Stable Isotope Analyses in R (SIAR; Parnell et al., 2010). Following recommendations by Phillips et al. (2005, 2014) and Fry (2006), P. schweinfurthii and L. repens subsp. brachypoda were lumped together to form the group macrophytes (1) as they had very similar isotopic values. The SIAR model was run using data from the basal food sources and prey for the consumers in the pond. The Bayesian SIAR model incorporates uncertainty and variation in parameters (Parnell et al., 2010). Fractionation factors of δ15N 2.3 ± 0.18 and δ13C 0.5 ± 0.13 were used for all animals and δ15N 1.1 ± 0.29 and δ13C −0.21 ± 0.21 for all acidified samples (McCutchan et al., 2003; Huang et al., 2007; Mao et al., 2012).
The stable isotope Bayesian ellipses in R (SIBER) model in SIAR were employed to analyse the isotopic niche breadth and overlap among select dominant consumers: Acari, Notonecta sp., Micronecta sp., P. lamellatus, L. raynerae and X. laevis tadpoles using their δ13C and δ15N values (Layman et al., 2007; Jackson et al., 2011). Convex hull areas and ellipses represent the calculated isotopic niche breadths and widths for all individuals as described by Jackson et al. (2011). Standard ellipse area (SEAc), which provides a bivariate measure of mean isotopic niche, was calculated using SIBER (Jackson et al., 2011). The small letter ‘c’ indicates a small sample size correction for improving SEA values accuracy (Jackson et al., 2011). The SEAc measures the degree of niche overlap (%), with an absolute limit of 100% indicating complete overlap, which can then be used as a quantitative measure of dietary similarity between populations (see Layman et al., 2007 and Jackson et al., 2011 for detailed methodology). The SEA probability estimates of the relative contribution of dietary resources assimilated by the different consumers were obtained using bivariate, separate single-group mixing models in SIAR, with values >0.6 indicating ecologically significant dietary overlap and potentially direct resource competition (Parnell et al., 2010).
Results
Physical and chemical variables
Table 1 summarizes the physical and chemical characteristics of the ephemeral pond at the time of sample collection. Water column chl-a concentration was low with a mean of 7.33 (±1.44) µg l−1 compared to high concentrations observed for the epiphyton chl-a concentration, with a mean 22.35 (±7.79) µg l−1 per 30 cm. Nitrates and phosphates concentrations ranged from 1.2 to 5.2 mg l−1 and 0.3–1.4 mg l−1, respectively (Table 1).
Basal source composition
Macrophytes and terrestrial vegetation, fine- and coarse particulate organic matter (FPOM, CPOM), dung, epiphyton and sediment were considered as potential basal food sources (Table 2). Based on their δ13C values, the basal food sources were distinct from the consumers, ranging from −32.88 to −16.43‰ (Table 2, Fig. 2). All the submerged macrophytes fell within a similar range of 6 for the δ15N values. Allochthonous matter, A. karroo, was δ13C and δ15N depleted, while C4 grass S. africanus was δ13C enriched. The other basal food sources such as CPOM and epiphyton had similar δ13C and δ15N values (Table 2; Fig. 2). Sheep dung was δ13C depleted (−25.32 ± 1.35‰) and δ15N enriched (5.7 ± 0.69‰), whereas the inverse was observed for cow dung (Table 2; Fig. 2). In the present study, C:N ratios for all consumers ranged from 3.32 to 5.10, with autochthonous organic matter sources having C:N ratios of 9.31 ± 0.26 (epiphyton), 9.57 ± 0.80 (L. muscoides) and 7.88 ± 0.15 (FPOM), respectively. In comparison, allochthonous organic matter sources had C:N ratios of 20.52 ± 2.60 (A. karroo leaves), 24.69 ± 5.57 (cow dung), 25.05 ± 3.36 (CPOM), 29.69 ± 1.30 (C4 grass S. africanus) and 25.64 ± 4.33 (sheep dung), suggesting that the autochthonous organic matter was more readily utilized by most of the primary consumers than allochthonous organic matter.
Mean (±standard deviation) δ13C and δ15N isotope signatures (±standard deviation) for basal food sources (green diamonds), Anuran tadpoles (red triangles), macroinvertebrates (blue squares) and zooplankton (yellow circles) sampled from a small temperate pond. Abbreviations: Aca—Acacia karroo, NotB—Notonecta sp., Cypri—Cypricercus sp., Cop1—Lovenula raynerae, Cop2—Paradiaptomus lamellatus, Cyz—Cyzicus sp., Chir—Chironomus sp., CybL—Cybister tripunctatus larvae, Cyb—Cybister tripunctatus adult, Cyp—Cyperus marginatus, CowD—cow dung, CPOM—coarse particulate organic matter, Dap—Daphnia sp., Epi—epiphyton, FPOM—fine particulate organic matter, Lag—Lagarosiphon muscoides, Pot—Potamogeton schweinfurthii, Sed—sediment, ShpD—sheep dung, Shri—Lynceus sp., Snail—Physa sp., NotS—Micronecta sp., Spor—Sporobolus africanus (C4 grass), StroT—Strongylopus fasciatus tadpole, LauR—Laurembergia repens subsp. brachypoda, XenTL and XenTS—Xenopus laevis tadpole large and small
Consumer composition
During a preliminary survey using a 63 μm mesh zooplankton net, we observed that larger sized crustaceans contributed >95% of the zooplankton community and that small-sized zooplankton (e.g. nauplii and rotifers) were virtually absent (see Appendix Table S1). The δ13C and δ15N values of the consumers were clearly distinguishable from the primary producers (Table 2; Fig. 2). The clam shrimp, Cyzicus sp. had the most depleted δ13C values (−35.26 ± 0.79‰), while the water flea, D. magna, was the most δ15N (4.38 ± 0.42‰) depleted. Using one-way ANOVA analysis, significant differences were observed among consumers for both the δ13C (P < 0.01, F = 22.16, df = 64) and δ15N (P < 0.01, F = 34.97, df = 64) suggesting that they utilized different food sources.
Trophic positions
The trophic positions of various consumers in the pond were determined using δ15N values. We identified four trophic levels with Notonecta sp. at the top of the sampled food web (trophic position 4). The tadpoles and the selected macroinvertebrates occupied trophic position 3, while the majority of the zooplankton had intermediate values, and therefore occupied trophic position 2 (Table 2; Fig. 2). A notable exception in the zooplankton was the predacious copepod L. raynerae which occupied trophic level 3 (Table 2; Fig. 2).
Consumer diet proportions, niche breadth and overlaps
SIAR models outputs for the Notonecta sp. showed that it fed mostly on C. marginatus (mean proportion 19.1%) Physa sp. (17.7%), Acari (17.3%), Micronecta sp. (17.1%) and L. raynerae (16.4%), while C. tripunctatus adult showed a variable diet composition with D. magna (21.4%), macrophyte (1) (20.3%) and Micronecta sp. (17.4%) being the main food sources (Table 3). Chironomus fed mostly on FPOM (27.4%), CPOM (24.4%) and sediment (21.2%). Xenopus laevis tadpoles preferred to feed predominately on allochthonous organic matter; CPOM (27.8%) and sediment (30.5%) but autochthonous organic matter (epiphyton) contributed 41.6% of the individual food sources. Lagarosiphon muscoides (52.2%) and FPOM (13.2%) contributed 65.4% of the autochthonous basal food source diet of X. laevis tadpoles (Table 3). Of the consumers, Acari, L. raynerae, Micronecta sp. and P. lamellatus showed variable diets of macroinvertebrates, allochthonous and autochthonous, whereas L. raynerae and P. lamellatus had a slightly greater preference for P. lamellatus (23.9%) and D. magna (31.3%), respectively (Table 3).
SIBER analysis using convex hull areas and standard ellipses revealed relatively small isotopic niche width among the consumers (Table 4; Fig. 3), as highlighted by the trophic positions of the organisms (Table 2), and large isotopic niche overlap was observed between secondary consumers Notonecta sp. and C. tripunctatus (Table 4). L. raynerae showed the smallest isotopic feeding niche and substantial convex hull overlap with Acari and Notonecta sp., respectively (Table 3; Fig. 3). The niche breadth overlap, i.e. the overlap of standard ellipse areas (SEAc) between the Notonecta sp. and C. tripunctatus, was high (0.87). Niche breadth overlap for the majority of other consumers was low (<0.5), with the exception of Acari, which had moderate niche overlaps with Notonecta sp. (0.77), L. raynerae (0.68) and Micronecta sp. (0.52) (Table 4). The low dietary overlaps observed for X. laevis tadpoles and other consumers could be due to the different isotopic niche space positions and feeding at different trophic levels (Tables 2, 4).
SIBER output δ13C and δ15N biplot of the major predators (a) Notonecta sp., (b) C. tripunctatus, (c) Micronecta sp., (d) Acari, (e) Lovenula raynerae and (f) X. laevis tadpole (small). Convex hulls areas (dotted lines) and ellipses (solid lines) represent the calculated isotopic feeding niche widths of each species. The numbers in parenthesis represent the trophic positions
Contributions of autochthonous and allochthonous organic matter to consumers
Based on the trophic positions, the consumers were separated into five distinct groups, each serving a different role in transferring organic matter within the small ephemeral pond food web (Fig. 2). The copepods and other invertebrates in the pond gained organic matter directly from the basal food sources, i.e. autochthonous FPOM, epiphyton, macrophytes and benthic sediment or the allochthonous CPOM at various levels (Table 3). The filter feeders (e.g. D. magna and Cypricercus sp.), scrapers (e.g. Physa sp.), omnivores (e.g. X. laevis) and hyperbenthic predators (e.g. P. lamellatus) largely obtained their organic matter from autochthonous sources, although the allochthonous sources were represented (Table 3; Fig. 2).
Discussion
The present study highlights the contribution of both autochthonous and allochthonous organic carbon to food webs in ephemeral pond systems. However, autochthonous organic carbon (epiphyton, FPOM, macrophytes and sediment) appeared to be more readily incorporated into the food web than did the allochthonous material (CPOM and sheep dung). These findings are in agreement with similar studies in permanent aquatic environments (Huang et al., 2007; Mao et al., 2012; Harding & Hart, 2013) and suggest that in freshwater ecosystems, allochthonous processes are particularly important, irrespective of the type of freshwater environment. Numerous studies suggest that in low productive freshwater systems, the detrital food web predominates (Huang et al., 2007), but in our study, the food web was driven by autochthonous organic matter suggesting that it is relatively productive. The isotopic differences in CPOM with macrophytes and terrestrial vegetation suggest that the CPOM was composed of a mixture of all the vegetation fragments. The consumers generally preferred autochthonous FPOM, epiphyton and emerged macrophyte L. muscoides organic matter sources over allochthonous CPOM. Kopprio et al. (2014) and Huang et al. (2007) showed that consumers utilized organic matter with high nitrogen and low carbon content as it provided better quality food due to the presence of high proteins and energy levels plus low-fibre levels compared to allochthonous organic matter.
The animal community of the food web incorporated in the present study comprised a mixture of crustaceans (ostracods, water mites, branchiopods and copepods), hexapods, snails and anuran larvae (Table 2). The community composition within the pond was broadly similar to that recorded in other southern African ephemeral ponds (Ferreira et al., 2012; Riato et al., 2014) and indeed, in similar ponds in the northern hemisphere (O’Neil & Thorp, 2014). A notable exception was the poor representation of hexapods which have been shown to represent an important component of the biology within these systems. Insect diversity within temporarily pools is thought largely to be a function of pond size and age, as much of the insect community is a result of immigration from other environments (O’Neil & Thorp, 2014). While the study pond was small in size, we do not have sufficient information on the state of the hydro-period. Insects generally dominate these systems late in the ponds hydro-period, with the initial phase of the hydro-period expected to be depauperate of insects (O’Neil & Thorp, 2014). An additional explanation for the low hexapod diversity in the present study is one of seasonality. Insect activity within temperate regions of the world demonstrates strong seasonal patterns (Lencioni, 2004). It is therefore, possible that the hexapods would make a more important contribution to the animal community during the warmer summer months. Irrespective of season, however, the immigration of predators from other environments present potential confounds when assessing food-web dynamics within a system that is treated as closed, with regard to the analyses. This may explain the high variability in the isotopic signal observed for the top predators such as Notonecta sp. and Cybister tripunctatus during the current study. Alp et al. (2013) showed that adult feeding on terrestrial food sources could cause an isotopic shift during the terrestrial stage, which might explain the relatively high variability in isotopic signatures (δ13C and δ15N) observed for adult Notonectids (Fig. 2). This could also explain the difference between C. tripunctatus larvae and adult signatures. In addition to the potential isotope fractionation during metamorphosis from larval to adult life stage (Alp et al., 2013), larval C. tripunctatus signatures would be reflective of within-pond dynamics, while adults may represent isotopic values inclusive of signatures from other environments.
When assessing niche dynamics across the major groups, SIBER analysis revealed relatively small isotopic (trophic) niche width among the consumers (Fig. 3), with a large isotopic niche overlap being observed between secondary consumers. Acari, L. raynerae, Micronecta sp. and X. laevis tadpoles comprised the intermediate trophic group. Within this group, Acari and L. raynerae had the highest degree of overlap, with X. laevis tadpole and Micronecta sp. exhibiting more distinct niches. Micronecta sp. however had a particularly large feeding niche comprising a large C and N ranges. Of the secondary, Notonecta sp. and C. tripunctatus had a degree of overlap but with a fairly high degree of niche separation. SIBER analysis using convex hull areas and standard ellipses revealed relatively small isotopic niche width among the intermediate consumers of trophic levels 2 and 3 (Fig. 3), suggesting a small degree of resource partitioning between the various primary and secondary consumers. This is in contrast to previous studies, e.g. Pace et al. (1999), Layman et al. (2007) and Baiser et al. (2011) who suggest that freshwater ecosystems are characterized by high levels of “trophic redundancy” as evident from the high degree of overlap in dietary composition and predominance of generalists within these systems. The predominance of generalists is thought to support the so-called “insurance hypothesis” which predicts that high levels of trophic redundancy may minimize the effects of disturbance (Yachi & Loreau, 1999). In the present study, however, generalist feeders were scant. Even at trophic level 4, where the Notonecta sp. had the widest niche width, a degree of separation was observed with other groups.
The complexity of food webs within ephemeral ponds in the northern hemisphere has been strongly linked to diversity within systems, with high insect diversity ponds having more complex food webs than those systems dominated by crustacea (O’Neil & Thorp, 2014). While there was low hexapod diversity in the present study, insects comprised the top of the food web. This was consistent with findings of other studies on small freshwater systems that showed that Notonecta spp. were the top predators in these systems (Arnér et al., 1998; Blaustein, 1998; Klecka, 2014). Studies by Mazunder (1994) and Arnér et al. (1998) have highlighted the importance of Notonectids as a top predator within small ephemeral ponds. Notonectids have been demonstrated to be capable of co-existing with, as well as eliminating, zooplankton and other macroinvertebrates under natural conditions (Mazunder, 1994; Arnér et al., 1998). While Notonectids immigrate to ephemeral ponds early in their hydro-period, the zooplankton communities arise from the mass hatching of dormant eggs when ponds first fill up (Pinceel et al., 2013; O’Neil & Thorp, 2014). Within weeks of filling, these ponds are therefore dominated by mostly adult crustaceans that have matured, and in the case of the present pond, the predaceous paradiaptomid copepods (P. lamellatus and L. raynerae). The absence of small zooplanktonic organisms such as rotifers and crustacean nauplii in the present study (see Appendix Table S1), is likely due to a combination of life-history dynamics of the dominant zooplankton groups as mass hatching is often a once off event (Brendonck & De Meester, 2003), and predation given the large numbers of predators at the time of sampling (Vanni, 1986, 1988; Arnott & Vanni, 1993; Brönmark & Hansson, 2005).
In conclusion, the small ephemeral freshwater pond food web was largely sustained by autochthonous organic matter sources with allochthonous organic matter being of less importance. Autochthonous organic matter in the food web was transferred by zooplankton and other macroinvertebrates (e.g. Micronecta sp. and Physa sp.), with the latter also transferring some allochthonous organic matter. The ephemeral pond animal community comprised both generalist (trophic level ≥3) and specialist (trophic level <3) taxa during this hydro-period stage and was generally less complex than climax northern hemisphere studies (e.g. Klecka, 2014; O’Neil & Thorp, 2014; Riato et al., 2014). The current food-web structure therefore seems to reflect the primary to mid stage of trophic structural complexity in the evolution of ephemeral ponds over the course of their hydro-period (O’Neil & Thorp, 2014). It is likely that there will be more trophic redundancy in the summer months when hexapod diversity in this austral ephemeral pond increases. It is therefore crucial for future studies to assess these environments over the course of their hydro-period for the adequate understanding of their food-web structure and ecosystem functioning.
References
Abrantes, K. G., A. Barnett & S. Bouillon, 2014. Stable isotope-based community metrics as a tool to identify patterns in food web structure in east African estuaries. Functional Ecology 28: 270–282.
Allanson, B. R., R. C. Hart, J. H. O’Keeffe & R. D. Robarts, 1990. Planktonic and benthic invertebrates. In Allanson, B. R. (ed.), Inland Waters of Southern Africa: An Ecological Perspective. Springer, Dordrecht.
Alp, M., B. L. Peckarsky, S. M. Bernasconi & C. T. Robinson, 2013. Shifts in isotopic signatures of animals with complex life-cycles can complicate conclusions on cross-boundary trophic links. Aquatic Science 75: 595–606.
APHA, 1995. Standard Methods for the Examination of Water and Wastewater. Port City Press, Baltimore.
Arcagni, M., L. M. Campbell, M. A. Arribére, K. Kyser, K. Klassen, R. Casaux, M. L. Miserendino & S. G. Guevara, 2013. Food web structure in a double-basin ultra-oligotrophic lake in Northwest Patagonia, Argentina, using carbon and nitrogen stable isotopes. Limnologica 43: 131–142.
Arnér, M., S. Koivisto, J. Norberg & N. Kautsky, 1998. Trophic interactions in rockpool food webs: regualtion of zooplankton and phytoplankton by Notonecta and Daphnia. Freshwater Biology 39: 79–90.
Arnott, S. E. & M. J. Vanni, 1993. Zooplankton assemblages in fishless bog lakes: influence of biotic and abiotic factors. Ecology 74: 2361–2380.
Baiser, B., R. S. Ardeshiri & A. M. Ellison, 2011. Species richness and trophic diversity increase decomposition in a co-evolved food web. PLoS ONE 6: e20672.
Bergamino, L., T. Dalu, A. K. Whitfield, L. Carassou & N. B. Richoux, 2014. Stable isotope evidence of food web connectivity by a top predator (Argyrosomus japonicus: Sciaenidae: Teleostei) in the Kowie Estuary, South Africa. African Journal of Marine Science 36: 207–213.
Blaustein, L., 1998. Influence of the predatory backswimmer, Notonecta maculata, on invertebrate community structure. Ecological Entomology 23: 246–252.
Blaustein, L. & S. S. Schwartz, 2001. Why study ecology in temporary pools? Israel Journal of Zoology 47: 303–312.
Brendonck, L. & L. De Meester, 2003. Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. Hydrobiologia 491: 65–84.
Brönmark, C. & L.-A. Hansson, 2005. The Biology of Lakes and Ponds (Biology of Habitats), 2nd ed. Oxford University Press, Oxford.
Carpenter, S. R., J. J. Cole, M. L. Pace, M. V. De Bogert, D. L. Bade, D. Bastviken, C. M. Gille, J. R. Hodgson, J. F. Kitchell & E. S. Kritzberg, 2005. Ecosystem subsidies: terrestrial support of aquatic food webs from 13C addition to contrasting lakes. Ecology 86: 2737–2750.
De Meester, L., S. Declerck, R. Stoks, G. Louette, F. van de Meutter, T. de Brie, E. Michaels & L. Brendonck, 2005. Ponds and pools as ecological model systems in conservation biology, ecology and evolutionary biology. Aquatic Conservation and Freshwater Ecosystems 15: 715–725.
Dillon, R. T., 2000. The Ecology of Freshwater Mollusks. Cambridge University Press, New York.
du Preez, L. H. & V. C. Carruthers, 2009. A Complete Guide to the Frogs of Southern Africa. Struik Nature, Cape Town.
Fernando, C. H. & C. Y. Leong, 1963. Miscellaneous notes on the biology of Malayan Corixidae (Hemiptera: Heteroptera) and a study of the life histories of two species, Micronecta quadristrigata Bredd. and Agraptocorixa hyalinipennis (F.). Journal of Natural History 6: 545–558.
Ferreira, M., V. Wepener & J. H. J. van Vuren, 2012. Aquatic invertebrate communities of perennial pans in Mpumalanga, South Africa: a diversity and functional approach. African Invertebrates 53: 751–768.
Francis, T. B., D. E. Schindler, G. W. Holtgrieve, E. R. Larson, M. D. Scheuerell, B. X. Semmens & E. J. Ward, 2011. Habitat structure determines resource use by zooplankton in temperate lakes. Ecology Letters 14: 364–372.
Froneman, P. W., 2002. Food web structure in three contrasting estuaries determined using stable isotope (δ13C) analysis. African Journal of Aquatic Sciences 27: 107–115.
Fry, B., 1991. Stable isotope diagrams of freshwater food webs. Ecology 72: 2293–2297.
Fry, B., 2006. Stable isotope ecology. Springer, New York.
Gamble, D. & J. J. Mitsch, 2009. Hydro-periods of created and natural vernal pools in central Ohio: a comparison of depth and duration of inundation. Wetlands Ecology and Management 17: 385–395.
Gebo, N. A. & R. P. Brooks, 2012. Hygreomorphic (HGM) assessments of mitigation sites compared to natural reference wetlands in Pennsylvania. Wetlands 32: 321–331.
Gerber, A. & M. J. M. Gabriel, 2002. Aquatic Invertebrates of South African Rivers: Field Guide. Institute for Water Quality Studies, Pretoria.
Giller, P. S., 1986. The natural diet of the Notonectidae: field trials using electrophoresis. Ecological Entomology 11: 163–172.
Harding, W. R. & R. C. Hart, 2013. Food-web structure in the hypertrophic Rietvlei Dam based on stable isotope analysis: specific and general implications for reservoir biomanipulation. Water SA 39: 615–626.
Hecky, R. E. & R. H. Hesslein, 1995. Contributions of benthic algae to lake food webs as revealed by stable isotope analysis. Journal of the North American Benthological Society 14: 631–653.
Heyer, W. R., R. W. McDiarmid & D. L. Weigman, 1975. Tadpoles, predation and pond habitats in the tropics. Biotropica 7: 100–111.
Hill, J. M., R. W. Jones, M. P. Hill & O. L. F. Weyl, 2015. Comparisons of isotopic niche widths of some invasive and indigenous fauna in a South African river. Freshwater Biology 60: 893–902.
Huang, I.-Y., Y.-S. Lin, C.-P. Chen & H.-L. Hsieh, 2007. Food web structure of a subtropical headwater stream. Marine and Freshwater Research 58: 596–607.
Jackson, A. L., R. Inger, A. C. Parnell & S. Bearshop, 2011. Comparing isotopic niche widths among and within communities: SIBER − stable isotope Bayesian ellipses in R. Journal of Animal Ecology 80: 595–602.
Klecka, J., 2014. The role of a water bug, Sigara striata, in freshwater food webs. PeerJ 2: e389.
Kopprio, G. A., G. Kattner, R. H. Freije, S. J. de Paggi & R. J. Lara, 2014. Seasonal baseline of nutrients and stable isotopes in a saline lake of Argentina: biogeochemical processes and river runoff effects. Environmental Monitoring and Assessment 186: 3139–3148.
Layman, C. A., J. P. Quattrochi, C. M. Peyer & J. E. Allgeier, 2007. Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecology Letters 10: 937–944.
Lencioni, V., 2004. Survival strategies of freshwater insects in cold environments. Journal of Limnology 63: 45–55.
Mao, Z., X. Gu, Q. Zeng, L. Zhou & M. Sun, 2012. Food web structure of a shallow eutrophic lake (Lake Taihu, China) assessed by stable isotope analysis. Hydrobiologia 683: 173–183.
Marcus, V. & S. C. Weeks, 1997. The effects of pond duration on the life history traits of an ephemeral pond crustacean, Eulimnadia texana. Hydrobiologia 359: 213–221.
Mazunder, A., 1994. Patterns of algal biomass in dominant odd- versus even-link lake ecosystems. Ecology 75: 1141–1149.
McCutchan Jr, J. H., W. M. Lewis Jr, C. Kendall & C. C. McGrath, 2003. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102: 378–390.
Measey, G. J., 1998. Diet of feral Xenopus laevis (Daudin) in South Wales, U.K. Journal of Zoology 246: 287–298.
Ohba, S., 2009. Feeding habits of the diving beetle larvae, Cybister brevis Aubé (Coleoptera: Dytiscidae) in Japanese wetlands. Applied Entomology and Zoology 44: 447–453.
O’Neil, B. J. & J. H. Thorp, 2014. Untangling food-web structure in an ephemeral ecosystem. Freshwater Biology 59: 1462–1473.
O’Reilly, C. M., R. E. Hecky, A. S. Cohen & P.-D. Plisnier, 2002. Interpreting stable isotopes in food webs: recognizing the role of time averaging at different trophic levels. Limnology and Oceanography 47: 306–309.
Pace, M. L., J. J. Cole, S. R. Carpenter & J. F. Kitchell, 1999. Trophic cascades revealed in diverse ecosystems. Trends in Ecology and Evolution 14: 483–488.
Palik, B. D., P. Baxter & C. Kern, 2006. Upland forest linkages to seasonal wetlands: litter flux, processing and food quality. Ecosystems 9: 142–151.
Parnell, A. C., R. Inger, S. Bearhop & A. L. Jackson, 2010. Source partitioning using stable isotopes: coping with too much variation. PLOS one 5: e9672.
Phillips, D. L., S. D. Newsome & J. W. Gregg, 2005. Combining sources in stable isotope mixing models: alternative methods. Oecologia 144: 520–527.
Phillips, D. L., R. Inger, B. Stuart, A. L. Jackson, J. W. Moore, A. C. Parnell & B. X. Semmens, 2014. Best practices for use of stable isotope mixing models in food-web studies. Canadian Journal of Zoology 92: 823–835.
Pinceel, T., B. Vanschoenwinkel & L. Brendonck, 2013. Flexible dispersal dimorphism in zooplankton resting eggs: an example of repeated phenotypic coin flipping? Biological Journal of the Linnean Society 110: 749–756.
Pinder, L. C. V., 1986. Biology of freshwater chironomidae. Annual Review of Entomology 31: 1–23.
Pinnegar, J. K. & N. V. C. Polunin, 1999. Differential fractionation of δ13C and δ15N among fish tissues: implications for the study of trophic interactions. Functional Ecology 13: 225–231.
Polačik, M., R. Blažek, R. Řežucha, M. Vrtílek, E. Terzibasi Tozzini & M. Reichard, 2014. Alternative intrapopulation life-history strategies and their trade-offs in an African annual fish. Journal of Evolutionary Biology 27: 854–865.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703–718.
Proctor, H. & G. Pritchard, 1989. Neglected predators: water mites (Acari: Parasitengona: Hydrachnellae) in freshwater communities. Journal of the North American Benthological Society 8: 100–111.
Riato, L., C. van Ginkel & J. C. Taylor, 2014. Zooplankton and diatoms of temporary and permanent freshwater pans in the Mpumalanga Highveld region, South Africa. African Zoology 49: 113–127.
Sinchembe, M. & W. N. Ellery, 2010. Human impacts on hydrological health and the provision of ecosystem services: a case study of the eMthonjeni–Fairview Spring Wetland, Grahamstown, South Africa. African Journal of Aquatic Science 35: 227–239.
SPSS Inc. 2007. SPSS Release 16.0.0 for Windows. Polar Engineering and Consulting. Chicago, SPSS Inc.
Suárez-Morales, E., R. J. Wasserman & T. Dalu, 2015. A new species of Lovenula Schmeil (Copepoda, Diaptomidae) from the Eastern Cape province of South Africa. Crustaceana 88: 324–342.
Vanni, M. J., 1986. Competition in zooplankton communities: suppression of small species by Daphnia pulex. Limnology and Oceanography 31: 1039–1056.
Vanni, M. J., 1988. Freshwater zooplankton community structure: introduction of large invertebrate predators and large herbivores to a small species community. Canadian Journal of Fisheries and Aquatic Sciences 45: 1758–1770.
Vareschi, E. & J. Jacobs, 1985. The ecology of Lake Nakuru. Oecologia 65: 412–424.
Yachi, S. & M. Loreau, 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the inárance hypothesis. Proceeding of the National Academy of Science 96: 1463–1468.
Yang, C., G. M. Wilkinson, J. J. Cole, S. A. Macko & M. L. Pace, 2014. Assigning hydrogen, carbon, and nitrogen isotope values for phytoplankton and terrestrial detritus in aquatic food web studies. Inland Waters 4: 233–242.
Zacharias, I., E. Dimitriou, A. Dekker & E. Dorsman, 2007. Overview of temporary ponds in the Mediterranean region: Threats, management and conservation issues. Journal of Environmental Biology 28: 1–9.
Acknowledgments
This paper is dedicated to the memory of Sven Kaehler who carried out the stable isotope analysis. This study was made possible through financial and logistical support from Rhodes University and the National Research Foundation (NRF) of South Africa (UID: 77444). We thank PSR Weyl and T Bellingan (Department of Zoology and Entomology, Rhodes University) for assistance with macrophyte and macroinvertebrate identification, respectively, and Eduardo Suárez-Morales of El Colegio La Frontera Sur (ECOSUR), Mexico for copepod identification. In addition, we extend our gratitude to the White family for granting us access to their property. We thank the handling editor Mariana Meerhoff for her patience and two anonymous reviewers for their work and time through providing insightful and useful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Mariana Meerhoff
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dalu, T., Weyl, O.L.F., Froneman, P.W. et al. Trophic interactions in an austral temperate ephemeral pond inferred using stable isotope analysis. Hydrobiologia 768, 81–94 (2016). https://doi.org/10.1007/s10750-015-2533-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2533-5