Abstract
In the rotifer Brachionus calyciflorus mictic-female production is density-dependent and appears to be induced by a chemical—a quorum sensing molecule—produced by the females themselves. Even at the highest densities, however, populations never become entirely mictic: i.e., some amictic females continue to be produced. Surprisingly, the phenomenon also occurs in clonal laboratory populations with genetically identical individuals. Here, we study how this ecologically adaptive phenomenon is generated at the level of individual reproducing females. In a life-history experiment we subjected 123 amictic females of a clone of B. calyciflorus separately to a daily renewed stimulus of culture medium conditioned at a density of 30 females ml−1. For each of these mothers we isolated the lifetime offspring individually and recorded whether these females were amictic or mictic. Mothers produced on average 16 offspring but none of the mothers produced 100% mictic offspring; the average proportion of mictic females was 30%, despite the extremely strong stimulus. The distribution of amictic vs. mictic offspring was not uniform over the mothers’ lifetime. Early and late offspring had a low probability of being mictic whereas mid-aged mothers produced the highest proportion of mictic daughters (up to 56%). We conclude that not all oocytes of B. calyciflorus can be turned into mictic females, even when the mictic-female-inducing stimulus is extremely high. Propensity to become a mictic female also depends on the rank of an egg within a female’s offspring production. Despite these regularities, we observed considerable stochastic variability with respect to individual mothers’ life histories.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a monogonont rotifer, Brachionus calyciflorus has a heterogonic life cycle in which mictic (sexual) reproduction is separated by several generations of amictic (asexual) reproduction. Although amictic reproduction via female parthenogenesis allows rotifer populations to grow rapidly, most monogononts intersperse bisexual reproduction. Mixis is often adaptive because it results in production of resting stages that enable rotifers to survive unfavourable conditions. In many monogononts environmental stimuli are known to trigger mictic reproduction. Stimuli such as photoperiod (Pourriot, 1963) or cold shock (Ruttner-Kolisko, 1964) probably serve as direct indicators of unfavourable conditions, which will soon follow. However, rotifer populations may also switch to mictic reproduction under favourable conditions, a strategy that allows rotifers to deposit large numbers of high-quality diapausing eggs into the sediment egg-bank (Gilbert & Schröder, 2004).
In the genus Brachionus, mixis can be induced by crowding, i.e., by a high concentration of conspecific females which release an unknown chemical (but see: Snell et al., 2006) into the surrounding water (Gilbert, 1963; Carmona et al., 1993; Stelzer & Snell, 2003). This phenomenon is termed quorum sensing (Wallace et al., 2006). For B. calyciflorus, chemical induction of mixis (in the physical absence of conspecifics) has not been shown yet. However, we were able to induce mixis in low-density cultures of B. calyciflorus by adding conditioned medium, that is, medium which we filtered rotifer-free but which had contained high numbers of B. calyciflorus previously (unpubl. obs.). In the laboratory, Snell & Boyer (1988) and Fussmann et al. (2003) observed a linear relationship between Brachionus concentration and the percentage of mictic females. On the other hand, mixis induction appears to be almost never complete since we know only one observation of field or laboratory rotifer populations that entirely consisted of mictic females (Pourriot, 1965).
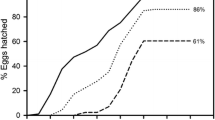
Two additional observations make incomplete mixis an especially interesting and puzzling phenomenon. First, complete mixis cannot be induced in laboratory cultures even when the stimulus (the density of Brachionus) is orders of magnitudes more intense than normally encountered in the field (Snell & Boyer, 1988; Gilbert, 2003b; Stelzer & Snell, 2003). Second, mixis is also incomplete in clonal cultures of B. calyciflorus (Gilbert, 1963, 2003b) indicating that the phenomenon is not simply a consequence of different clonal sensitivities towards the stimulus in natural, multi-clonal populations. Because all mothers and their oocytes are genetically identical in a clonal population, one would expect that the production of mictic offspring (and hence the relative frequency of mictic females in the population) would increase sharply from 0 to 100% around a critical stimulus concentration (Fig. 1).
Mictic ratio (mictic females/total females) as a function of rotifer population density. Qualitative plots of the expected (dotted line) and typically observed (solid line) relationship for a clonal rotifer population with density-dependent mixis induction (e.g., Brachionus). Because all mothers are genetically identical mixis ratio is expected to increase sharply to 100% at a threshold stimulus concentration. Empirical studies show that mixis ratio tends to increase gradually and to level off below 100% (incomplete mixis induction)
Here, we investigate the phenomenon of incomplete mixis at the level of individual mothers in a clonal population of B. calyciflorus. Gilbert (2003a) suggested that “some oocytes of amictic mothers are programmed to develop into amictic females, no matter how strong the mictic-female-inducing stimulus”. We sought support for this hypothesis in the laboratory by exposing individual B. calyciflorus amictic females to a strong mictic-female-inducing stimulus. More specifically, we conducted a life-table experiment to answer the following three questions. (1) Is the mixis response really incomplete at very high B. calyciflorus densities? (2) Does the mixis response vary predictably with the age of the mother? (3) Do individual differences exist in the mixis response among mothers? To do this we reared each mother’s lifetime offspring production to adulthood, recorded the sexual status (amictic or mictic) of these daughters and kept track of the order in which the daughters were produced. We confirmed that mixis induction in B. calyciflorus is incomplete, revealed high variability for overall lifetime mictic offspring production and found mictic offspring production to be non-uniformly distributed over the mothers’ lifetime.
Methods
Rotifer and algal cultures
We isolated a clone of Brachionus calyciflorus from Beaver Lake, on top of Mount-Royal in Montreal, Canada in August 2005. We cultured the clonal population in chemostat vessels using CHU-10 medium (modified according to the University of Toronto Culture Collection) and Monoraphidium minutum (Chlorophyta; strain 243-1, Culture Collection of Algae, University of Göttingen, Germany) as food. We were able to permanently keep the B. calyciflorus population at high densities of >10 ml−1 at 12°C; at the same time, the clone maintained a high propensity to produce mictic offspring throughout its cultivation in the laboratory.
Experimental procedure
The goal of our experiment was to study patterns of mictic vs. amictic offspring reproduction of individual females exposed to a strong mixis-inducing stimulus. Consequently, we transferred amictic females (future mothers) into individual cultures where they were exposed to medium conditioned by high densities of conspecifics. The whole experiment was conducted at 12°C (including microscopic inspection of cultures). At this temperature, reproduction of B. calyciflorus was sufficiently slow to allow us to isolate each mother’s offspring in the order it hatched. We raised each of these daughters to maturity and scored them as amictic or mictic offspring. This is only possible after females have attached their first egg, which is either a small haploid, male (mictic) egg or a bigger diploid, female (amictic) egg; non-ovigerous amictic and mictic females are morphologically indistinguishable.
At the beginning of the experiment we removed juvenile female B. calyciflorus from a clonal culture with a density of ≈40 ml−1. To clean the juveniles we placed them for 2 h in 6-well cell culture plates (Corning costar®) containing CHU-10 medium. A total of 288 juveniles were then transferred each to a single well of a 48-well cell culture plate (Corning costar®) containing 0.5 ml of conditioned medium and 4 × 106 cells ml−1 of M. minutum. We prepared conditioned medium from a separate high-density population of the same clone of B. calyciflorus that we used in the experiment. We measured the density of this stock culture (which was > 30 ml−1 throughout the duration of the experiment) and filtered the medium through a 0.7 μm GF/F filter (Whatman). We then added a concentrated Monoraphidium solution (in CHU-10 medium) to the filtrate to obtain a solution with a stimulus concentration representative of 30 B. calyciflorus ml−1 and a density of 4 × 106 M. minutum cells ml−1. We concentrated M. minutum through gentle centrifugation for 45 s (International Equipment Company CL A8661H-2) before adding the algae to the filtrate.
The 288 juveniles were allowed to mature in the 48-well plates. We discarded mictic individuals and 123 amictic mothers remained for the experiment. We transferred these into three new 48-well plates with fresh conditioned water, which we changed every 24 h until the end of the experiment. We ran the entire experiment in a walk-in growth chamber at 12°C and a 16:8 light/dark cycle using cool white fluorescent bulbs. We kept the 48-well plates on a shaking table (Eberbach) to keep M. minutum suspended. For the major part of the experiment, we checked plates every 6 h at 6 am, 12 noon, 6 pm and 12 midnight; at the beginning (first–third offspring) and end (19th–25th offspring) of the experiment we checked cultures less frequently but at least every 12 h. On these occasions we removed newly hatched offspring and transferred these daughters into individual wells containing 0.4 ml of a Rhodomonas minuta algal culture (we preferred Rhodomonas over Monoraphidium because these flagellated algae remained in suspension without shaking which reduced B. calyciflorus mortality; we did not use R. minuta during the life-table experiment because phototactic movements of the cells could have led to inhomogeneous food concentrations across replicates). This procedure allowed us to keep track of the rank of each offspring as we rarely found two offspring in a single well after 6 h. When we found two offspring, we considered the larger of the two to be the oldest. We then allowed the offspring to mature at 24°C and scored them as mictic or amictic. These changes in temperature and algal food were permissible because the sexual fate (amictic vs. mictic female) is already determined when the offspring hatches (Ruttner-Kolisko, 1964). The experiment ended when the last mother died.
Statistical analysis
Our experimental results showed that the probability of producing mictic daughters varied with age of the mother. More specifically, we collected data on the proportion of mictic offspring produced with respect to the rank of the offspring (with rank 1 being the first offspring produced, rank 2 the second, and so forth). For each rank we tested whether the proportion of mictic offspring observed differed significantly from the proportion expected under the assumption that mothers randomly assign mictic and amictic offspring. The assignment mictic/amictic can be modelled as a binomial process if we assume that mothers have a certain proportion of oocytes in their ovary that are bound to be mictic/amictic. Under the null hypothesis mothers produce mictic/amictic offspring with probabilities p and 1 − p, respectively, where p is the proportion of oocytes with mictic destiny. Here, we tested for rank-related divergence from expected mictic frequencies and estimated p by the overall mictic proportion p = 0.304 during the experiment (see “Results”). The probability of observing n mothers producing more than x mictic (and less than n − x amictic) daughters as their ith offspring is then given by the cumulative binomial distribution:
Based on this distribution and the number of mothers (n) surviving to produce offspring of rank i we constructed 95%-confidence intervals (CI) for the observed number of mictic offspring x. Because x is a positive integer we constructed CIs conservatively, assuring that values outside the CI represent a significant deviation from the expected distribution at the α ≤ 0.05 level.
We tested whether the patterns of mixis induction at different observation times (0, 6, 12 and 18 h after the change of medium and transfer of rotifers) differed from one another. We standardized the number of mictic offspring at each rank inspected at exact 6-h intervals (4–18) and each treatment level (0, 6, 12, 18 h) for the total number of offspring in each treatment and performed a Monte–Carlo randomisation of the data (Gotelli & Ellison, 2004). We conducted pairwise comparisons between all six treatment combinations by randomly drawing two groups of 15 from the combined 30 standardized mictic offspring numbers of two treatments. We used the difference between the sums of the two groups as a test statistic (this test statistic is equal to the difference of the average mictic proportions because we used standardized data). We computed the randomised test statistic 25,000 times and compared it with the observed difference in mictic proportions (straight lines in Fig. 5). We then calculated the P-value as the relative frequency of randomisations that produced a test statistic as great as or greater than the differences of the mictic proportions observed in the experiment. Finally, we adjusted the level of statistical significance for performing multiple statistical tests (Bonferroni correction).
Results
The experiment lasted 16 days (calculated from the birth of the mothers). After 15.7 days the last offspring had hatched. The maximum number of offspring observed among the 123 amictic mothers was 25 daughters; the average number of offspring was 15.6. For the presentation and interpretation of our results we found it convenient to scale time by the rank of the offspring hatched (from 1 to 25). This scale can be converted to the average lifetime elapsed until the daughter of rank i hatched. In our figures we show absolute time on the upper x-axis and rank of offspring on the lower x-axis; the non-equidistant tick marks reflect time between hatching events. Whether the offspring was amictic or mictic did not influence the time interval between consecutive hatching events (t-test, P = 0.88; Fig. 2). However, the time interval between offspring hatchings itself ranged from 0.35 to 0.91 days, with low intervals between offspring ranks 4–10 and the longest intervals towards the end of the experiment (Fig. 2).
Survivorship of the initial 123 amictic mothers decreased almost linearly over the duration of the experiment (Fig. 3). About 60% of the mothers were still alive after the first half of the experiment. Because some amictic B. calyciflorus females gave birth to more than 20 daughters we conclude that part of the experimental population died before releasing their entire potential offspring.
Survivorship of experimental amictic mothers (n = 123 = 100% for rank 1). “Rank” refers to ith offspring of a mother hatched, i.e., about 50% of the mothers lived to give birth to 18 or more daughters. The upper x-axis is labelled with the average lifetime elapsed until the ith offspring hatched. For the time from the mother’s birth to the birth of her first daughter we measured an average time of 3.1 days for this clone of B. calyciflorus at 12°C (unpublished data)
The combined effects of applying conditioned medium and confining individuals in wells with 0.5 ml of medium resulted in a strong and persistent yet incomplete mixis response (<100%) in the experimental amictic mothers (Fig. 4). Overall, 30.4% of the observed offspring grew up to be mictic females. The proportion of mictic offspring varied considerably with the rank of the offspring (Fig. 4). We found mixis frequency to be unimodally distributed among the ranks of offspring, with the maximum mictic proportion of 56.5% occurring at the ninth offspring. Confidence intervals in Fig. 4 reveal that early ranks (1, 2, 3) and late ranks (16, 18, 21) tended to have significantly lower mictic offspring production than expected by chance (based on the assumption that mictic and amictic offspring are produced by chance with a mictic probability of 0.304; see “Methods”) while intermediate ranks (7–11) had significantly higher mictic offspring production. Note that due to low sample size (surviving mothers) significant deviations from the average mictic proportion were difficult to detect towards the end of the experiment.
Incomplete mixis induction at a stimulus intensity equivalent to 30 Brachionus ml−1. Percentage of mictic offspring among total offspring of all experimental mothers producing their ith offspring (i = “Rank”). Straight line: average mictic offspring percentage of total offspring produced. Dotted lines: upper and lower limits of 95% confidence interval, assuming random mictic/amictic offspring production (see “Methods”). Upper x-axis as in Fig. 3
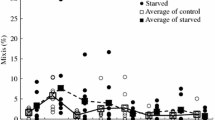
We inspected our experimental cultures every 6 h for newly hatched offspring but mothers were transferred to fresh medium with fresh stimulus only every 24 h. We therefore tested whether the patterns of mixis induction found at different observation times (0, 6, 12 and 18 h after change of medium) differed from one another. A statistical randomisation procedure showed significant differences among the six possible pairwise comparisons (Table 1). In essence, the two treatments with high mixis induction (6 and 12 h; Fig 5b, c) are not different from one another, neither are those with low mixis induction (0 and 18 h; Fig. 5 a, d). All other differences are significant, except that the difference between the 0-h and 6-h group is only marginally statistically significant (P = 0.03, but Bonferroni correction applies). Note that this analysis only includes mothers inspected exactly on a 6-h schedule (ranks 4–16).
Mixis induction observed at the time or several hours after the daily transfer of amictic mothers and renewal of the inducing stimulus. (a) 0 h (=at the time of the renewal); n = 252 (total number of daughters). (b) 6 h after renewal; n = 325. (c) 12 h after renewal; n = 228. (d) 18 h after renewal; n = 280. Straight line: average mictic offspring percentage (ranks 4–16) for respective plot
We also found highly variable patterns of mictic offspring production among individual mothers (Table 2). No single mother produced 100% mictic offspring; mictic offspring production ranged between 0 and 75%. Roughly three quarters of the mothers produced between 15 and 45% mictic offspring.
Discussion
The partial response of rotifers to mixis-inducing stimuli is a widely observed phenomenon (Gilbert, 1963; Snell & Boyer, 1988; Gilbert, 2003b; Stelzer & Snell, 2003) and can be interpreted as a diversified bet-hedging strategy (Philippi & Seger, 1989; Gilbert, 2003a). Although high stimulus concentrations may indicate environmental conditions under which sexually reproducing individuals have higher fitness, the long-term fitness of the rotifer population in an unpredictably changing environment will be higher if not all individuals switch to sexual reproduction (Serra & King, 1999).
Most laboratory studies assessed the relative frequency of sexual reproduction by measuring the mixis ratio (the proportion of mictic females among all females) in an experimental culture. Although claims of incomplete mixis derived from such studies are likely to be true, the method is inaccurate because (1) it does not account for the delay between mixis stimulus and reproductive response, (2) results may be derived from a population with unstable age structure and (3) the reproductive mode of non-ovigerous females remains unknown. In our experimental study we took a life-table approach that allowed us to individually monitor the life-time offspring of a large synchronized cohort of females subject to an intense chemical stimulus supplied by conspecifics. Therefore, our study provides new insights into how reproductive patterns at the individual level are orchestrated to generate the ecologically adaptive phenomenon of incomplete mixis at the population level.
Is the mixis response really incomplete at very high B. calyciflorus densities?
We confirmed incomplete mixis for our strain of B. calyciflorus, which produced an average of 30.4% mictic offspring despite the strong chemical stimulus (equivalent of 30 females ml−1). The maximum values were 75% for an individual mother and 56.5% for age-specific reproduction (ninth offspring). Several studies have reported higher mixis rates (but <100%) of Brachionus at lower crowding stimuli than ours (Pourriot & Rougier, 1986; Gilbert 2003b; Stelzer & Snell, 2003).
Our study is one of the few to use conditioned medium as a crowding stimulus, which has been shown to elicit a mixis response that is somewhat lower than that observed in a mass culture of comparable density (Stelzer & Snell, 2003). Very high-density stimuli can obviously only be administered via conditioned medium because, otherwise, the volume available for individual culture becomes prohibitively small (e.g., 33 μl at our stimulus intensity). Although we have some preliminary evidence that mixis can directly be induced by conditioned medium in B. calyciflorus populations (unpubl. obs.) the quantitative effectiveness of the chemical stimulus is yet to be established. In this study, we attempted to maximize the potential effect of the mixis stimulus by using conditioned medium and culturing individual rotifers in low-volume wells, which increases the probability of auto-induction. Our results show that we may have underestimated the true potential of mictic reproduction. In Fig. 5 and Table 1 we provide evidence that the probability of observing mictic offspring is higher 6 or 12 h after renewing the stimulus than it is at the time of renewal or 18 h after renewal. Keeping in mind that observations at the time of stimulus renewal truly reflect responses 24 h after renewal, these results are consistent with a rapidly decaying activity of the stimulus after administration. Further experimentation is required to show that the observed pattern of cyclically decreasing mixis rates cannot just be attributed to the fact that rotifers are transferred to new culture wells with fresh food and that eggs are susceptible to the stimulus 6–12 h before hatching (or 6–12 h plus n times 24 h before hatching).
In conclusion, the exact rate of mixis at a given concentration of conditioned medium may be difficult to determine but in our study it never reached 100%, even at densities that are much higher than observed in natural environments. This result begs the question of how a clonal population of rotifers may consistently produce non-zero mixis rates below 100%.
Does the mixis response vary predictably with the age of the mother?
An appealing solution to the phenomenon of partial mixis in clonal rotifer populations is the possibility that some oocytes of amictic mothers are pre-programmed to develop into amictic females (Gilbert, 2003a). The mictic ratio among the oocytes would then pre-determine the mictic ratio of a future rotifer population with overlapping generations and stable age structure. A rigorous test of this hypothesis would require the experimenter to follow the development of individual rotifer oocytes to maturity, which—if possible at all—lay beyond the scope of the present study. Our data, however, show that incomplete mixis can be traced to patterns of reproduction that occur at the individual level. Hence, incomplete mixis in B. calyciflorus is not a phenomenon solely arising from the coupling of population dynamics and density-dependent mixis initiation. This scenario is conceivable if rotifers adopt a “bang-bang” strategy of reproduction where mixis is turned on and off around a threshold density (Serra & King, 1999).
If the ovary or the pseudocoelom (where the oocytes develop) contain amictic and mictic oocytes in a certain proportion, the life-time offspring of a mother can either hatch in a random or non-random order. Our results show clear deviations from the uniform distribution of mictic (and amictic) offspring production expected under the random scenario (Fig. 4). This probably means that the chemical induction of the oocytes does not occur directly in the mother’s body cavity but is mediated by the physiology of the mother (Gilbert, 2003a); in our study we surmise a physiological component that changes systematically over the mothers’ life-time. On the other hand, our data also suggest that a probabilistic component is involved in oocyte induction since mictic offspring production was never 0 or 100% for a particular egg rank and not all egg ranks deviated significantly from the production expected with random amictic vs. mictic daughter assignment.
Several studies report mictic offspring production that varies with maternal age although none of these studies provided the single-offspring resolution over the mothers’ life-time that we achieved in our study. The most prevalent pattern is one of decreasing propensity of Brachionus to produce mictic daughters with maternal age (Pourriot & Rougier, 1976, 1986; Rougier & Pourriot, 1977; Gilbert & Schröder, 2007). Two studies report a similar unimodal pattern of mictic offspring production as we observed it, one study for Brachionus plicatilis (Lubzens & Minkoff, 1988), the other for B. calyciflorus (Pourriot & Rougier, 1986). Pourriot & Rougier (1986) observed the unimodal pattern only at cold experimental temperatures of 10 and 14°C (similar to the 12°C in our study) while a decreasing pattern occurred at 20°C. It is possible that only the elongated life-time of the rotifers at colder temperatures allows the unimodal pattern of mictic offspring production to develop.
Do individual differences exist in the mixis response among mothers?
The previous section emphasized commonalities among the reproductive behaviour of 123 rotifer females but our results also revealed striking differences among the life histories of individual mothers. Although all individuals were members of a genetically identical clone and were raised under the exact same environmental conditions we observed great phenotypic differences with regard to how many mictic offspring these genotypes produced (Table 2) and at what maternal age they produced them. Such variability may arise due to the random nature of chemical reactions within the individual or its cells, especially with respect to gene expression (Raser & O’Shea, 2005). This variability may again be interpreted as ecologically adaptive bet-hedging (Philippi & Seger, 1989): i.e., the individual phenotypic variance prevents a synchronous switch from an all-amictic to all-mictic population. A recent theoretical study showed that, in unpredictable dynamic environments, stochastic production of this variability may impart higher fitness to the population than probing of and adaptive response to the environment (Kussell & Leibler, 2005). It appears that rotifers of the genus Brachionus have settled on a mixed strategy: the phenotype (propensity to produce mictic offspring) is largely determined by an environmental stimulus (Brachionus density) perceived by the individuals but stochastic variability makes this relationship less rigid.
References
Carmona, M. J., M. Serra & M. R. Miracle, 1993. Relationships between mixis in Brachionus plicatilis and preconditioning of culture medium by crowding. Hydrobiologia 255: 145–152.
Fussmann, G. F., S. P. Ellner & N. G. Hairston, 2003. Evolution as a critical component of plankton dynamics. Proceedings of the Royal Society of London Series B-Biological Sciences 270: 1015–1022.
Gilbert, J. J., 1963. Mictic female production in the rotifer Brachionus calyciflorus. Journal of Experimental Zoology 153: 113–124.
Gilbert, J. J., 2003a. Environmental and endogenous control of sexuality in a rotifer life cycle: developmental and population biology. Evolution & Development 5: 19–24.
Gilbert, J. J., 2003b. Specificity of crowding response that induces sexuality in the rotifer Brachionus. Limnology and Oceanography 48: 1297–1303.
Gilbert, J. J. & T. Schröder, 2004. Rotifers from diapausing, fertilized eggs: unique features and emergence. Limnology and Oceanography 49: 1341–1354.
Gilbert, J. J. & T. Schröder, 2007. Intraclonal variation in propensity for mixis in several rotifers: variation among females and with maternal age. Hydrobiologia doi:10.1007/s10750-007-9040-2.
Gotelli, N. J. & A. M. Ellison, 2004. A Primer of Ecological Statistics. Sinauer, Sunderland, MA.
Kussell, E. & S. Leibler, 2005. Phenotypic diversity, population growth, and information in fluctuating environments. Science 309: 2075–2078.
Lubzens, E. & G. Minkoff, 1988. Influence of the age of algae fed to rotifers (Brachionus plicatilis O. F. Müller) on the expression of mixis in their progenies. Oecologia 75: 430–435.
Philippi, T. & J. Seger, 1989. Hedging ones evolutionary bets, revisited. Trends in Ecology & Evolution 4: 41–44.
Pourriot, R., 1963. Influence du rythme nycthemeral sur le cycle sexuel de quelques rotifères. Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences 256: 5216–5219.
Pourriot, R., 1965. Sur le déterminisme du mode de reproduction chez les rotifères. Schweizerische Zeitschrift für Hydrologie 27: 76–87.
Pourriot, R. & C. Rougier, 1976. Influence de l’âge des parents sur la production de femelles mictiques chez Brachionus calyciflorus (Pallas) et B. rubens Ehr. (Rotifères). Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences Série D 283: 1497–1500.
Pourriot, R. & C. Rougier, 1986. Rhythmes de production de femelles sexuées chez le rotifère Brachionus calyciflorus, en élevage à température constante. Bulletin de la Societé Zoologique de France 111: 203–209.
Raser, J. M. & E. K. O’Shea, 2005. Noise in gene expression: origins, consequences, and control. Science 309: 2010–2013.
Rougier, C. & R. Pourriot, 1977. Aging and control of reproduction in Brachionus calyciflorus (Pallas) (Rotatoria). Experimental Gerontology 12: 137–151.
Ruttner-Kolisko, A., 1964. Űber die labile Periode im Fortpflanzungszyklus der Rädertiere. Internationale Revue der gesamten Hydrobiologie 49: 473–482.
Serra, M. & C. E. King, 1999. Optimal rates of bisexual reproduction in cyclical parthenogens with density-dependent growth. Journal of Evolutionary Biology 12: 263–271.
Snell, T. W. & E. M. Boyer, 1988. Thresholds for mictic female production in the rotifer Brachionus plicatilis (Muller). Journal of Experimental Marine Biology and Ecology 124: 77–85.
Snell, T. W., J. Kubanek, W. Carter, A. B. Payne, J. Kim, M. K. Hicks & C. P. Stelzer, 2006. A protein signal triggers sexual reproduction in Brachionus plicatilis (Rotifera). Marine Biology 149: 763–773.
Stelzer, C. P. & T. W. Snell, 2003. Induction of sexual reproduction in Brachionus plicatilis (Monogononta, Rotifera) by a density-dependent chemical cue. Limnology and Oceanography 48: 939–943.
Wallace, R. L., T.W. Snell, C. Ricci & T. Nogrady, 2006. In Segers, H. (ed.) Rotifera: Volume 1 Biology, Ecology and Systematics, 2nd edn. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World 23. Kenobi Productions, Ghent, and Backhuys Publishers, Leiden.
Acknowledgements
We acknowledge financial support through an NSERC discovery grant to GFF. We thank Mark Romer for growth chamber set-up.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: S. S. S. Sarma, R. D. Gulati, R. L. Wallace, S. Nandini, H. J. Dumont & R. Rico-Martínez.
Advances in Rotifer Research.
Rights and permissions
About this article
Cite this article
Fussmann, G.F., Kramer, G. & Labib, M. Incomplete induction of mixis in Brachionus calyciflorus: patterns of reproduction at the individual level. Hydrobiologia 593, 111–119 (2007). https://doi.org/10.1007/s10750-007-9041-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-9041-1