Abstract
Mytilopsis sallei is a small marine bivalve and is considered as a serious pest. We assume that the invasive bivalve M. sallei changed the community structure of fouling macrofauna and reduced the species diversity index in Yundang Lagoon, Xiamen, China. In order to verify the above hypothesis, test panels were submerged seasonally at five stations during four seasons in Yundang Lagoon, and some chemical parameters were determined. The results showed there were significant differences in density and biomass of M. sallei and other fouling macrofauna with season and with station. The species diversity of the macrofaunal fouling community at stations B and F was low in summer, because high density of M. sallei was found at two stations. There were significantly positive correlations between density and biomass of M. sallei and water temperature and COD, and significantly negative correlations with pH. The results confirmed that this invasive species changed the density and biomass compositions of fouling macrofauna, reduced the species diversity index during the summer period, and somewhat worsened the aquatic environmental quality in Yundang Lagoon, because the pH and the DO were the lowest, and the BOD and the COD were the second lowest in summer among four seasons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The introduction or immigration of foreign species is a major cause of biodiversity loss (Crooks, 2002). The impact of alien species might affect three major biodiversity aspects: species, their genetic structure, and landscape (Wiegleb et al., 2013). Among bivalves, some dreissenids have become invasive pests when being introduced to or invading new environments (Kennedy, 2011a). As sessile suspension feeders, dreissenid and mytilid bivalve molluscs have become some of the most important invasive organisms in marine, brackish, and freshwater ecosystems (Aldridge et al., 2008). Mytilopsis sallei has successfully invaded several major ports in East Asia (Wong et al., 2011). M. sallei was reported in an oyster bed at Tapeng Bay, near Tungkang Port, in Taiwan, in 1977 (Chang, 1985) and in Kiyomizu harbour, Japan, in 1979 (Ishibashi & Kasaka, 1980). M. sallei was collected alive from Hong Kong waters (Tolo Harbour) attached to a floating wreckage (Morton, 1980). M. sallei was introduced to Maluan Bay in Xiamen as feed for cultured fishes and shrimps at the beginning of the 1990s, and soon it became a dominant species within its fouling community (Wang et al., 1999). A high mortality of Balanus reticulatus was noted when M. sallei occurred at high density in Maluan Bay, Xiamen, China (Cai et al., 2005, 2006). The Caribbean false mussel M. sallei (Récluz, 1849) occurs in tropical monsoon drains of Singapore (Tan & Morton, 2006). M. sallei colonized Yundang Lagoon in the year 2000 (Cai et al., 2010). The bivalve M. sallei was reported as an invasive species in Indian waters (Gaonkar et al., 2010a, b). In the south-western region of Taiwan, M. sallei causes not only abundance declines of native hard clam, but also undesirable changes in aquaculture systems and economic losses (Liao et al., 2010).
Other species of the genus Mytilopsis were found abundantly in several countries. Conrad’s false mussel, M. leucophaeata, is a highly euryhaline species, originating from the Atlantic coast of the United States and the Gulf of Mexico (Marelli & Gray, 1983, 1985), and now settles in industrial cooling water systems in Europe (Jenner et al., 1998), with settlement densities as high as 5.5 million m−2 (Rajagopal et al., 2003). Rajagopal et al. (1997) reported M. leucophaeata to be the dominant macrofouling organism of electricity-generating stations in the Noordzeekanaal of the Netherlands. Laine et al. (2006) reported that M. leucophaeata was near a power plant’s cooling water discharge in the Baltic Sea. Originating from the Pacific coast of Panama, M. adamsi appeared later in a shrimp farm along the Pacific coast of Mexico (Salgado-Barragán & Toledano-Grandos, 2006). Since the discovery of M. trautwineana within a shrimp farm at the Caribbean coast of Colombia, the mussel has reached high abundances in the beds of some ponds, where it builds up layers of up to 10 cm (Aldridge et al., 2008).
From mussels to barnacles to algae, studies suggest that such “hull-fouling” organisms could pose an invasion threat that is “equally strong if not stronger” than that from organisms transported by ballast water (Dahms et al., 2004a; Strain, 2012). M. leucophaeata usually occurs in very low numbers and has rarely been mentioned in field survey reports; However, occasionally in its native habitat and often in habitats where it has been introduced (as in Europe and Brazil), it may undergo population break-downs for no clear reason (Kennedy, 2011a).
To assess the invasive ability of M. sallei, both spatial and temporal variables should be considered. Local biotic effects such as on top-down control by predatory fish and any relationship to the benthic community need to be surveyed and then be extrapolated to the entire ecosystem (Karatayev et al., 2007). This particularly holds for the suspension-feeding ability of M. sallei (Borthagaray & Carranza, 2007). The system-wide effects depend not only on the characteristics of the water bodies (invasibility), but also on the invasiveness of M. sallei. Its initial impact is expected to be modified over time (Hicks, 2004).
Predicting the ecosystem consequences of simultaneous gains (invasion) and losses (extinction) requires that we first understand which biological traits predispose life forms to higher probabilities of extirpation or establishment (response traits), and detail how response traits covary with traits that drive ecosystem functioning (effect traits) (Cardinale 2012; Wiegleb et al., 2013). We assume that the invasive bivalve M. sallei changed the community structure of fouling macrofauna (the proportions among fouling macrofauna groups), reduced the species diversity index, and increased some chemical materials in Yundang Lagoon, Xiamen, China. Following this hypothesis, the present study aims to analyze the temporal and spatial distribution of M. sallei and the relationship between density and biomass of M. sallei and environmental factors, as well as between M. sallei and other fouling macrofauna in Yundang Lagoon, Xiamen, China.
Materials and methods
Study site
Yundang Lagoon (24°29′N, 118°04′E), which used to be a natural bay called Yundang Harbour, is located in the western bay of Xiamen, China. Since the early 1970s, a great number of land reclamation projects were carried out by the Xiamen government, and the Yundang Harbour gradually became an almost-closed salt lake, with its area reduced from the original 10 to 2.2 km2. Floodwater draining channels in the urban district were built along the site of Yundang Lagoon. A sea wall was constructed to keep Yundang Lagoon from the Western Sea of Xiamen. However, in order to circulate the water, the incoming waters of natural tides were utilized by regulating the water gate diurnally. This way of circulating seawater was transported by tidal force. The circumference of Yundang Lagoon is now a main shopping center, residential area, and recreation park of Xiamen.
Field methods
Five stations, identified as B–F, were investigated in terms of fouling macrofauna that settled on pine test panels (Fig. 1). Station B was at the drainage channel; more residential sewage outlets were there. Station C was at an inner lake. Station D was at the channel between the inner and outer lake. Station E was at the outer lake, and Station F was close to the water outlet. Two seasonal test panels were placed submerged in the water for 90 days and replaced by another set of two panels after retrieval at a panel station. Each panel measured 20 cm × 20 cm × 2 cm.
All samples and retrieved panels were immediately fixed in 5% formalin until examination in the laboratory. In the laboratory, the samples were sieved through a 0.5 mm mesh. Fouling macrofauna retained on the sieve was stored in 70% ethanol. Fouling macrofauna was identified to species level or to the lowest taxonomic level possible, e.g., genus, which was then treated as a distinct taxon in the analysis. Fouling macrofauna was counted under a dissection microscope and weighed using an electronic balance (sensitivity as 0.1 mg) (see Dahms et al., 2004b).
Eight environmental factors, i.e., water temperature, salinity, pH, DO (dissolved oxygen), BOD (biochemical oxygen demand), COD (chemical oxygen demand), NH3-N (nitrate nitrogen), and DIP (dissolved inorganic phosphate = reactive phosphate) were calculated for every sampling (see Dahms & Qian, 2005).
Statistical analysis
The species diversity index of each station was calculated using Shannon–Weaver index (H′).
Multi-factorial Analysis of Variance (ANOVA) from SPSS software was used to investigate differences between seasons (summer, winter, spring, and autumn) and stations (B–F) for density and biomass of fouling macrofauna. To identify homogeneous subsets of means, ANOVA was followed by pairwise multiple comparison using Tukey’s posthoc test. Cluster analysis and Multidimensional scaling (MDS) ordinations were used to visually assess spatial and seasonal differences in macrofaunal fouling communities in Yundang Lagoon (Clarke & Warwick, 1994; Clarke & Gorley, 2006). MDS ordination was constructed from Bray–Curtis similarity matrices, after fourth root transformation and row standardization of density data. Non-parametric correlations (Spearman-rank) were performed between density and biomass of dominant fouling organisms and environmental factors. The BIOENV function was used to highlight the key factors, or a combination of factors, that accounted for the macrofaunal fouling community.
Results
Density and biomass of M. sallei and fouling macrofauna in Yundang Lagoon
A total of 28 species of sessile macrofauna were identified from the test panels in Yundang Lagoon taken throughout all four seasons. There were 11 polychaetes, 6 bivalves, 6 crustaceans, 1 platyhelminthes, 1 nemertean, 1 bryozoan, and 1 urochordate.
The density and biomass of M. sallei were both highest in summer, second highest in autumn, third highest in spring, and lowest in winter. The density of fouling macrofauna was highest in summer, second highest in autumn, third highest in winter, and lowest in spring, while the biomass of fouling macrofauna was highest in summer, second highest in autumn, third highest in spring and lowest in winter (Table 1).
The density of M. sallei was highest at station F, second highest at station B, third highest at station D, fourth highest at station C, and lowest at station E, while the biomass of M. sallei was highest at station F, second highest at station D, third highest at station C, fourth highest at station E, and lowest at station B (Table 1). The spatial distribution of other fouling macrofauna was the same as that of M. sallei.
Univariate tests on the distribution of M. sallei and fouling macrofauna revealed that M. sallei and fouling macrofaunal density and biomass were significantly influenced by season, station, and season × station (Table 2).
Percentage of each group within the macrofaunal fouling community in Yundang Lagoon
The density percentage of each group within the macrofaunal fouling community was different for each season. Bivalves occupied 49.50% of the area in summer when M. sallei was abundant. But bivalves occupied only 0.25% in summer supposed there was no M. sallei (Fig. 2). Polychaetes and crustaceans occupied 3.83 and 46.57%, respectively, in summer when there was M. sallei. But polychaetes and crustaceans occupied 17.90 and 81.71% in summer, respectively, supposed there was no M. sallei. The density percentage of polychaetes and crustaceans in the fouling macrofaunal community changed little seasonally in autumn, winter, and spring, irrespective whether there was M. sallei or not.
The biomass percentage of each group in the macrofaunal fouling community was the same as the density percentage of each group (Fig. 3).
Cluster and MDS analysis
There was significant seasonal variation for the macrofaunal fouling community in Yundang Lagoon (Fig. 4). A MDS plot showed similar results as the cluster analysis (Fig. 5). The polychaetes Capitella capitata and Polydora ciliata, pollution resistant species, were found during all seasons at station B.
Species diversity of macrofaunal fouling community in Yundang Lagoon
The species diversity of the macrofaunal fouling community at stations B and F was no more than 0.6 in summer. The species diversity indices at stations B, F in summer were all lower than those at stations B, F in autumn and winter, respectively (Fig. 6). The density and biomass of M. sallei at stations B and F were all higher than at other stations (Table 1).
Relationship between M. sallei and environmental factors in Yundang Lagoon
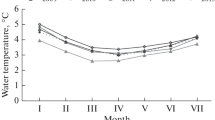
The mean water temperature was highest in summer, second highest in autumn, third highest in spring, and lowest in winter. The seasonal variation of water temperature was the same as that of density and biomass of M. sallei. The seasonal variations of salinity, pH, DO, BOD, COD, and NH3-N did not show congruence with that of density and biomass of M. sallei, and were different from each other. The mean pH and DO values in summer were lower than during the other three seasons (Fig. 7).
There was a significantly positive correlation between density and biomass of M. sallei with water temperature and COD. There was a significantly negative correlation with pH but no significant correlation with salinity, DO, BOD, NH3-N, and DIP (Table 3). BIOENV analysis showed that water temperature, pH, COD, and DIP were related to the macrofaunal fouling community in Yundang Lagoon (Table 4).
Discussion
As an exotic macrofauna species, M. sallei invaded the fouling community and became a dominant species in Yundang Lagoon in summer (Cai et al., 2010). Our results showed that M. sallei changed the species composition of macrofaunal fouling community. For example, if there was no M. sallei, the dominant groups in summer would be crustaceans, the sessile Balanus reticulatus, and the soft bottom inhabiting amphipod Corophium uenoi (both of them are crustaceans) that are known to be the dominant macrofaunal species before the invasion of M. sallei (Wang et al., 1996; Cai et al., 2010). This could be shown for another species, M. adamsi. By attaching to each other, M. adamsi could even colonize several substrata and cause rapid changes in local communities, because the mussel commonly builds up dense layers that excluded other sessile organisms (Wangkulangkul & Lheknim, 2008). However, mussels of the genus Mytilopsis subsequently also provide a new habitat for associated fauna, such as amphipods and polychaetes to colonize with the potential of changing the community structure even further as has been described before for dreissenids and mytilids (Crooks & Khim, 1999; People, 2006; Borthagaray & Carranza, 2007). Positive correlations could be demonstrated between M. sallei and the polychaetes Capitella capitata and Polydora ciliata in Xiamen Maluan Bay (Cai et al., 2006). Both Capitella capitata and Polydora ciliata are opportunists and pollution resistance species; if they were abundant, the ecosystem would be changed (Cai et al., 2006, 2010). Our results confirmed that appropriate density of M. sallei is propitious to increase the biodiversity within the macrofaunal fouling community. For example, the higher density of M. sallei and the lower diversity index at stations in Yundang Lagoon in summer (Fig. 6). The densities of M. sallei at stations B, C, E, and F in autumn were lower than those at stations B, C, E, and F in summer, respectively, but the diversity indices of macrofaunal fouling community at stations B, C, E, and F in autumn were higher than those at stations B, C, E, and F in summer, respectively. Biodiversity in turn commonly improves water quality (Cardinale, 2011).
Temperature is not the main restricting environmental variable for adult M. leucophaeata (Verween et al., 2007). This species was recorded in fluctuating water temperatures ranging from 5°C in Finland (Laine et al., 2006) up to 30°C in Miami (Siddall, 1980). A seasonal effect was clearly reflected in dissolved oxygen levels; when temperatures dropped from autumn to winter, DO levels increased (Morton, 1989). However, temperature remained an important factor for the initiation of spawning (de Vooys, 1999). According to our results, there was a significantly positive correlation between density and biomass of M. sallei and water temperature because many juveniles of M. sallei were found attached to the panels during summer and autumn, but no juveniles were found in winter. A few large individuals of M. sallei were found on panels in winter could have moved there from other places. M. sallei is capable of detaching itself and reattaching to new surfaces (Morton, 1989). M. leucophaeata moves and reattaches to substrata which are protected from predators (Kennedy, 2011b). The seasonal variation of M. sallei density in Yundang Lagoon confirmed that water temperature was a limiting factor for its hatching larvae since this species originated from tropical waters.
Mytilopsis sallei is often found in polluted inner bays and lagoons (Huang & Chen, 2002; Cai et al., 2005, 2006, 2010; Wangkulangkul & Lheknim, 2008; Gaonkar et al., 2010a). M. sallei is not reported from Xiamen western waters and other open sea waters (Wang et al., 1996; Cai et al., 2010). The habitat of M. sallei is similar to M. leucophaeata which was only found on tidal mud flats along river banks and none was found at seven other locations in the Wadden Sea area of the North Sea (Buschbaum et al., 2012).
Zebra mussel populations are capable of removing over 90% of organic matter from the water (MacIsaac, 1996). About 50% of precipitated matter was utilized within zebra mussel populations, while the rest was deposited as feces and pseudofeces, and served as sources for organic pollution in the eastern Gulf of Finland (Orlova et al., 2004). The biodeposition of zebra mussels increased with temperature and water Chl a content, but these effects were less effective than those of salinity (Lauringson et al., 2007). Fouling organisms that settled on cages or buoys contributed much to phytoplankton depletion, oxygen consumption and the increase of ammonia and phosphate concentrations in waters during the peak fouling period (Su et al., 2007). The content of DO in summer in Yundang Lagoon was lower than during other seasons. One of the reasons was certainly related to oxygen consumption by M. sallei because of its high density.
The effects of the globally invasive species M. sallei on macrofaunal fouling communities are attributed to its competitive advantages in colonizing polluted inner bays, its contribution to changes of species composition and diversity of the macrofaunal fouling communities during the summer period (Cai et al., 2006, 2010). M. sallei consumed a large amount of nutrient substance from the water in Maluan Bay, Xiamen (Ji, 1998).
As other dreissenids, Dreissena polymorpha provides a number of physical, chemical, and biological characteristics that influence the structure of co-occurring macroinvertebrates (Laine et al., 2006; Lauringson et al., 2007). Mollusk shells are abundant, persistent, and ubiquitous physical structures in aquatic habitats (Kennedy, 2011b). Sardiña et al. (2008) carried out an experimental study with the golden mussel Limnopernea fortunei in order to examine the influence of this newly created habitat on benthic invertebrate communities. They showed that the lowest abundance and diversity of benthic invertebrates are recorded in substrates lacking golden mussels. Oligochaetes were an exception to this rule, as they were significantly more abundant on substrata with live mussels (Sardiña et al., 2008). Here, oxygen levels are probably low within the interstitial spaces of the mussel shells due to bacterial decomposition of the accumulated organic deposits, the low oxygen levels will favor the presence of oligochaete animals. This indicates that physical attributes of the environment, as much as biological attributes of the golden mussel, influence the structure, diversity, and abundance of the benthic community. Clumped mussels have abundant interstitial spaces that serve as refugia from disturbance and predation for small organisms. It should be mentioned that dense coverage of hard surfaces by mussels may reduce or displace native species as shown by the golden mussel L. fortunei (Darrigran & Damborenea, 2011).
High density of mussels consumes a large of phytoplankton from the water which results in the lack of primary producers (Baudinet, et al., 1990). The Zebra mussel, D. polymorpha, a major consumer of phytoplankton, exerts a significant top-down control on riverine phytoplankton (Caraco et al., 1997). Before M. sallei invaded Xiamen Maluan Bay, the phytoplankton abundance was high, after the establishment of M. sallei, the phytoplankton abundance was low and stabilized at a low abundance (Lin & Yang, 2006).
Conclusions
To understand the impact of the invasive bivalve M. sallei on a native macrofaunal fouling community, it is essential to determine the spatial and temporal distribution of M. sallei and other fouling macrofauna as well as the relationship between M. sallei and environmental factors. Our results demonstrated significant positive correlations between density and biomass of M. sallei and water temperature and COD, and a significantly negative correlation with pH. BIOENV analysis showed that water temperature, pH, COD, and DIP were key environmental factors affecting the community structure of fouling macrofauna in Yundang Lagoon. These results confirmed that the invasive species changed the density and biomass composition of fouling macrofauna and reduced the species diversity index during the summer period, and somewhat worsened the aquatic environmental quality in Yundang Lagoon because the pH and the DO were the lowest, and the BOD and the COD were the second lowest in summer among four seasons.
References
Aldridge, D. C., M. Salazar, J. Serna & J. Cock, 2008. Density-dependent effects of a new invasive false mussel, Mytilopsis trautwineana (Tryon 1866), on shrimp, Litopenaeus vannamei (Boone 1931), aquaculture in Colombia. Aquaculture 281: 34–42.
Baudinet, D., E. Alliot, B. Berlang, C. Grenz, M.-R. Plante-Cuny, R. Plante & C. Salen-Picard, 1990. Incidence of mussel culture on biogeochemical fluxes at the sediment-water interface. Hydrobiologia 207: 187–196.
Borthagaray, A. I. & A. Carranza, 2007. Mussels as ecosystem engineers: their contribution to species richness in a rocky littoral community. Acta Oecologica 31: 243–250.
Buschbaum, C., D. Lackschewitz & K. Reise, 2012. Non-native macrobenthos in the Wadden Sea ecosystem. Ocean & Coastal Management 68: 89–101.
Cai, L. Z., Y. Gao, G. S. Zeng, L. Yang, W. M. Liu & X. C. Lin, 2005. Spatial and temporal distribution of exotic species, Mytilopsis sallei, in sea water outside of shrimp ponds in Maluan Bay, Xiamen. Journal of Xiamen University (Natural Science) 44: 54–57.
Cai, L. Z., Y. Gao, W. M. Liu, X. C. Lin, X. P. Zhou, L. Jin, L. Yang & G. S. Zeng, 2006. Effect of exotic species, Mytilopsis sallei, on macrozoobenthos in Maluan Bay. Xiamen in China. Acta Oceanologica Sinica 28(5): 83–89.
Cai, L. Z., H. S. Lin, K. Huang, W. Wang, S. J. Fu & X. P. Zhou, 2010. The effect of an exotic species, Mytilopsis sallei (Bivalvia), on the ecosystem of Yundang Lagoon, Xiamen, China. In Symposium of 13th World Lake Conference. Chinese Society for Environmental Science, China Agricultural University Press, Beijing: 1906–1912.
Caraco, N. F., J. J. Cole, P. A. Raymond, D. L. Strayer, M. L. Pace, S. E. G. Findlay & D. T. Fischer, 1997. Zebra mussel invasion in a large, turbid river: phytoplankton response to increased grazing. Ecology 18: 588–602.
Cardinale, B. J., 2011. Biodiversity improves water quality through niche partitioning. Nature 472: 86–89.
Cardinale, B. J., 2012. Biodiversity loss and its impact on humanity. Nature 486: 59–67.
Chang, K. M., 1985. Newly introduced false mussel to Taiwan (Bivalvia: Dreissenidae). Bulletin of Malacology, Republic of China 11: 61–67.
Clarke, K. R. & R. N. Gorley, 2006. PRIMER v 6: User Manual/Tutorial. PRIMER-E, Plymouth, UK.
Clarke, K. R. & R. M. Warwick, 1994. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation. Natural Environment Research Council, Plymouth, UK: 144 pp.
Crooks, J. A., 2002. Characterizing ecosystem-level consequences of biological invasions: the role of ecosystem engineers. Oikos 97: 153–166.
Crooks, J. A. & H. S. Khim, 1999. Architectural vs. biological effects of a habitat altering, exotic mussel, Musculista senhousia. Journal of Experimental Marine Biology and Ecology 240: 53–75.
Dahms, H.-U. & P.-Y. Qian, 2005. Exposure of biofilms to meiofaunal copepods affects the larval settlement of Hydroides elegans (Polychaeta). Marine Ecology Progress Series 297: 203–214.
Dahms, H.-U., S. Dobretsov & P.-Y. Qian, 2004a. The effect of bacterial and diatom biofilms on the settlement of the bryozoan Bugula neritina. Journal of Experimental Marine Biology and Ecology 313: 191–209.
Dahms, H.-U., T. Harder & P.-Y. Qian, 2004b. Effect of meiofauna on macrofauna recruitment: settlement inhibition of the polychaete Hydroides elegans by the harpacticoid copepod Tisbe japonica. Journal of Experimental Marine Biology and Ecology 311: 47–61.
Darrigran, G. & C. Damborenea, 2011. Ecosystem engineering impacts of Limnoperna fortunei in South America. Zoological Science. 28: 1–7.
De Vooys, C. G. N., 1999. Numbers of larvae and primary plantigrades of the mussel Mytilus edulis in the western Dutch Wadden Sea. Journal of Sea Research 41: 189–201.
Gaonkar, C. A., S. S. Sawant, A. C. Anil, V. Krishnamurthy & S. N. Harkantra, 2010a. Changes in the occurrence of hard stratum fauna: a case study from Mumbai harbor, India. Indian Journal of Marine Sciences 39(1): 74–84.
Gaonkar, C. A., S. S. Sawant, A. C. Anil, V. Krishnamurthy & S. N. Harkantra, 2010b. Mumbai harbor, India: gateway for introduction of marine organisms. Environmental Monitoring and Assessment 163: 583–589.
Hicks, G., 2004. Turning the Tide: is aquatic bioinvader research heading in the right direction? Aquatic Invaders 15: 9–20.
Huang, Z. G. & L. S. Chen, 2002. Preliminary study on the biofouling in two harbours of Taiwan Province of China. Acta Oceanologica Sinica 24(6): 92–98.
Ishibashi, I. & M. Kasaka, 1980. First record of an introduced mussel, Mytilopsis sallei (Recluz, 1849) in Shimizu Harbour. Marine Fouling 2: 60.
Jenner, H. A., J. W. Whitehouse, C. J. L. Taylor & M. Khalanski, 1998. Cooling water management in European power stations: biology and control. Hydroecology Applications 1–2: 1–225.
Ji, W. D., 1998. Relationship of biogeochemistry from organic pollution and eutrophication in the Maluan Bay of Xiamen. Acta Oceanologica Sinica 20: 134–143.
Karatayev, A. Y., D. Boltovskoy, D. K. Padilla & L. E. Burlakova, 2007. The invasive bivalves Dreissena polymorpha and Limnoperna fortunei: parallels, contrasts, potential spread and invasion impacts. Journal of Shellfish Research 26: 205–213.
Kennedy, V. S., 2011a. The invasive dark falsemussel Mytilopsis leucophaeata (Bivalvia: Dreissenidae): a literature review. Aquatic Ecology 45: 163–183.
Kennedy, V. S., 2011b. Biology of the uncommon Dreissenid Bivalve Mytilopsis leucophaeata (Conrad, 1831) in central Chesapeake Bay. Journal of Molluscan Studies 77: 154–164.
Laine, A. O., J. Matitila & A. Lehoinen, 2006. First record of the brackish water dreissenid bivalve Mytilopsis leucophaeata in the northern Baltic Sea. Aquatic Invasions 1: 38–41.
Lauringson, V., E. Mälton, J. Kotta, K. Kangur, H. Orav-Kotta & I. Kotta, 2007. Environmental factors influencing the biodeposition of the suspension feeding bivalve Dreissena polymorpha (Pallas): comparison of brackish and freshwater populations. Estuarine Coastal and Shelf Science 75: 459–467.
Liao, C. M., Y. R. Ju, C. P. Chio & W. Y. Chen, 2010. Risk-based probabilistic approach to assess the impact of false mussel invasions of farmed hard clams. Risk Analysis 30(2): 310–323.
Lin, G. M. & Q. L. Yang, 2006. Impacts of alien species Mytilopsis sallei on phytoplankton at Maluan Bay in Xiamen, Fujian, China. Journal of Tropical Oceanography 25(5): 63–67.
MacIsaac, H. J., 1996. Potential abiotic and biotic impacts of zebra mussels on the inland waters of North America. American Zoology 36: 287–299.
Marelli, D. C. & S. Gray, 1983. Conchological redescriptions of Mytilopsis sallei and Mytilopsis leucophaeta of the brackish Western Alantic. Veliger 25: 185–193.
Marelli, D. C. & S. Gray, 1985. Comments on the status of recent members of the genus Mytilopsis (Bivalvia: Dreissenidae). Malacological Review 18: 117–122.
Morton, B., 1980. Mytilopsis sallei recorded from Hong Kong: an introduction by Vietnamese refugees? Malacological Review 13: 90–92.
Morton, B., 1989. Life-history characteristics and sexual strategy of Mytilopsis sallei (Bivalvia: Dreissenacea), introduced into Hong Kong. Journal Zoology of London 219: 469–485.
Orlova, M., S. Golubkov, L. Kalinina & N. Ignatieva, 2004. Dreissena polymorpha (Bivalvia: Dreissenidae) in the Neva Estuary (eastern Gulf of Finland, Baltic Sea): is it a biofilter or source for pollution? Marine Pollution Bulletin 49: 196–205.
People, J., 2006. Mussel beds on different types of structures support different macroinvertebrate assemblages. Austral Ecology 31: 271–281.
Rajagopal, S., G. Van Der Velde & H. A. Jenner, 1997. Shell valve movement response of dark false mussel, Mytilopsis leucophaeta, to chlorination. Water Research 31: 3187–3190.
Rajagopal, S., M. Van der Gaag, G. Van der Velde & H. A. Jenner, 2003. How effective is intermittent chlorination to control adult mussel fouling in cooling water systems? Water Research 37: 329–338.
Salgado-Barragán, J. & A. Toledano-Grandos, 2006. The false mussel Mytilopsis adamsi Morrison, 1946 (Mollusca: Bivalvia: Dreissenidae) in the Pacific waters of Mexico: a case of biological invasion. Hydrobiologia 563: 1–7.
Sardiña, P., D. H. Cataldo & D. Boltovskoy, 2008. The effects of the invasive mussel, Limnoperna fortunei, on associated fauna in South American freshwaters: importance of physical structure and food supply. Fundamental applications in limnology. Archiv für Hydrobiologie 173: 135–144.
Siddall, S. E., 1980. Early development of Mytilopsis leucophaeata. Veliger 22(4): 378–379.
Strain, D., 2012. Invasive species. Researchers set course to blockade ballast invaders. Science 336: 664–665.
Su, Z. X., Y. Yan & L. M. Huang, 2007. Effect of fouling on feeding, oxygen consumption and waste excretion of pearl oyster Pinctada martensii in Daya Bay cultivation. Marine Science Bulletin 9(2): 34–42.
Tan, K. S. & B. Morton, 2006. The invasive Caribbean bivalve Mytilopsis sallei (Dreissenidae) introduced to Singapore and Johor Bahru, Malaysia. The Raffles Bulletin of Zoology 54: 429–434.
Verween, A., M. Vincx & S. Degraer, 2007. The effect of temperature and salinity on the survival of Mytilopsis leucophaeata (Mollusca, Bivalvia): the search for environmental limits. Journal of Experimental Marine Biology and Ecology 348: 111–120.
Wang, J. J., Z. G. Huang, C. Y. Li, C. X. Zheng, N. Lin & S. K. Yan, 1996. Fouling organisms in cage cultural areas in Xiamen Harbour. Acta Oceanologica Sinica 18: 93–102.
Wang, J. J., Z. G. Huang, C. X. Zheng & N. Lin, 1999. Population dynamics and structure of alien species Mytilopsis sallei in Fujian, China. Journal of Oceanography in Taiwan Strait 18(4): 372–377.
Wangkulangkul, K. & V. Lheknim, 2008. The occurrence of an invasive alien mussel Mytilopsis adamsi Morrison, 1946 (Bivalvia: Dreissenidae) in estuaries and lagoons of the lower south of the Gulf of Thailand with comments on their establishment. Aquatic Invasions 3(1): 325–330.
Wiegleb, G., U. Bröring, G. Choi, H.-U. Dahms, K. Kanongdate, C.-W. Byeon & L. G. Ler, 2013. Ecological restoration as precaution and not as restitutional compensation. Biodiversity and Conservation 22: 1931–1948.
Wong, Y. T., R. Meier & K. S. Tan, 2011. High haplotype variability in established Asia populations of the invasive Caribbean bivalve Mytilopsis sallei (Dreissenidae). Biological Invasions 13: 341–348.
Acknowledgements
This work was supported by the Chinese National Foundation (project number 40776066). Our panel test was supported by the Xiamen Yundang Lagoon Management Office. This research was a part of the project “Long-term change of structure and function in marine ecosystems of Korea,” funded by the Ministry of Oceans and Fisheries. This work was supported by a grant of NRF (2012R1A2A2A02012617).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Jiang-Shiou Hwang & Koen Martens / Challenges in Aquatic Sciences
Rights and permissions
About this article
Cite this article
Cai, LZ., Hwang, JS., Dahms, HU. et al. Effect of the invasive bivalve Mytilopsis sallei on the macrofaunal fouling community and the environment of Yundang Lagoon, Xiamen, China. Hydrobiologia 741, 101–111 (2014). https://doi.org/10.1007/s10750-014-2012-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-014-2012-4