Abstract
Macrophyte complexity has been associated with high abundance and richness of macroinvertebrates. While the effect on richness has been attributed to an increase in the number of niches, the effect on abundance has been explained by a higher availability of space for small individuals, refuge, and/or food. For studying effects of complexity on macroinvertebrates, we used complementary approaches of laboratory choice and field colonization experiments, with macrophytes (Egeria densa and Elodea ernstae) and plastic imitations of contrasting fractal dimension. We investigated whether macroinvertebrates may actively select complex habitats by Hyalella sp. choice experiments. Then, we tested effects of complexity on macroinvertebrate density, biomass, richness, diversity, and body size using colonization experiments. Finally, a caging experiment was performed to study interacting effects of complexity and predation. The active choice of complex substrates by Hyalella sp., and the significant positive relationship between macrophyte fractal dimension and macroinvertebrate density support the existence of a positive effect of complexity on abundance. As macroinvertebrate length was not associated with fractal dimension, such differences could not be attributed to a higher space available for smaller invertebrates in complex plants. Finally, neither macroinvertebrate density nor size was reduced by fish predation in the Las Flores stream.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The quantification of the structural complexity is an issue of paramount importance because it affects population dynamics, community structure, and ecosystem functioning (Cooper et al., 1997). The structurally complex habitats have been found to support higher richness and number of organisms than the simple ones (Taniguchi et al., 2003; Hauser et al., 2006; Thomaz et al., 2008), and influence biotic interactions and body size distributions (Stewart et al., 2003; MacAbendroth et al., 2005).
In aquatic ecosystems, complexity has been usually studied by assessing invertebrate abundance and richness on macrophytes of contrasting architecture (Taniguchi et al., 2003; Hauser et al., 2006; Thomaz et al., 2008; Lucena-Moya & Duggan, 2011). The positive effect of macrophyte complexity on richness has been attributed to an increase in the number of niches (MacArthur & MacArthur, 1961; Stewart et al., 2003), whereas the effect on abundance has received several explanations. The microhabitats hypothesis suggests that plants with complex architecture present more space available for the establishment of small individuals (Morse et al., 1985), so that the total number of organisms is increased. The refugia hypothesis postulates that complex architecture has a negative effect on fish predation (Coull & Wells, 1983; Russo, 1987; Beukers & Jones, 1998; Warfe & Barmuta, 2004), and/or reduces the impact of physical stress factors such as current (Gregg & Rose, 1982; Dodds & Biggs, 2002). Finally, the food availability hypothesis suggests that complex architecture favors the presence of epiphytic algae and detritus (Cattaneo & Kalff, 1980; Taniguchi et al., 2003; Gosselain et al., 2005; Warfe & Barmuta, 2006), which attract herbivorous and detritivorous invertebrates.
In addition, macrophyte complexity has been found to influence body size distributions (Stewart et al., 2003; MacAbendroth et al., 2005; Ferreiro et al., 2011). The microhabitats hypothesis indicates that complexity per se has a negative effect on invertebrate size (Williamson & Lawton, 1991). In addition, predation per se has a negative effect on macroinvertebrate size (Crowder & Cooper, 1982; Diehl, 1992) and complexity reduces predation of invertebrates by fishes (Coull & Wells, 1983; Russo, 1987). Thus, it is expected that in complex macrophyte beds the importance of predation is reduced, and so invertebrates reach greater size (Tolonen et al., 2003).
The most common approximation in studying macrophyte complexity has been qualitatively differentiating two levels of complexity (broad versus dissected leaves–simple versus complex macrophytes) (Taniguchi et al., 2003; Gosselain et al., 2005; Warfe & Barmuta, 2006; Lucena-Moya & Duggan, 2011). Alternatively, some authors have applied quantitative index based on structural characteristic of plants (Lillie & Budd, 1992; Dibble et al., 1996; Bartholomew & Shine, 2008; St. Pierre & Kovalenko, 2014). During the last years, the fractal dimension has also been used for studying complexity in aquatic ecosystems (MacAbendroth et al., 2005; Thomaz et al., 2008; Ferreiro et al., 2013). While indexes constructed by counting and measuring macrophyte interstitial spaces account for size and abundance of structural elements, the fractal dimension may only fully encompasses the abundance/density of structural elements (Tokeshi & Arakaki, 2012).
From the point of view of population ecology, the absence of a species in a place at any time is first attributed to the limitation of physicochemical factors, and inability of individuals to reach such place. Only when there is evidence that a species may move and live in an area but it does not, it is appropriate to test the hypothesis of habitat selection (Krebs, 1986). This appears to be a case for macrophyte-associated macroinvertebrates, as several authors have found differences in invertebrate communities among plant species from the same macrophyte patch (Krecker, 1939; Ferreiro et al., 2011). When studying habitat selection, we may consider whether the species selection is active or passive. Passive selection is related to the existence of a limitation in survivorship (e.g., high predation and low food availability), while active selection is related to the existence of a choice behavior. Some authors have reported that invertebrates do not actively select the substrate but are predated differently depending on complexity (Russo, 1987). This, plus the protection from predation being the only demonstrated effect of complexity (Coull & Wells, 1983; Warfe & Barmuta, 2004), led us to consider the importance of survivorship factors in habitat selection.
The aim of this study was to investigate experimentally the influence of macrophyte complexity on several attributes (density, biomass, body size distribution, taxon richness, and diversity) of the macroinvertebrate community associated with aquatic plants in a Pampean stream. First, we report three active selection experiments where macrophytes and plastic imitations of different fractal dimensions were selected by Hyalella sp. Our hypothesis is that amphipods select structures with high fractal dimension. Then, we present the results of three colonization field experiments of macrophytes and plastic imitations with different fractal dimensions, in the presence of different predation risks. Our hypothesis is that macrophyte complexity increases the density, biomass, richness, and diversity of macroinvertebrates. In addition, we hypothesize that higher fractal dimension is linked to smaller invertebrates and reduced effect of predation.
Materials and methods
Study site
The study was conducted in the Las Flores stream (34°27′25″S, 59°03′56″W), a second-order stream that is a tributary of the Luján River. The stream is situated in the Pampean region, a vast grassy plain that covers central Argentina, where climate is temperate humid with a mean annual temperature of 16°C, and a mean annual precipitation between 600 and 1,200 mm. The physicochemical characteristics of the Las Flores stream are described elsewhere (Giorgi et al., 2005). A lack of riparian forest, low current velocities, and high nutrient levels in Pampean streams allow the development of dense and diverse macrophyte communities (Feijoó & Lombardo, 2007).
Field sampling
Egeria densa Planch. and Elodea ernstae St. John, referred to hereafter by their genus names, were selected to perform experiments because both are among the most common submerged macrophyte species in this ecosystem and have quite different complexity levels. Active selection experiments were performed with Hyalella sp. because amphipods are the dominant group on submerged macrophytes in the Las Flores stream (Ferreiro et al., 2011).
Macrophytes and associated macroinvertebrates were collected with a cylindrical mesh bag sampler with 460-μm mesh (Cheruvelil et al., 2000). The sampler was 40-cm long and 30-cm diameter and had a drawstring at the bottom to close the sampler and prevent the escape of actively swimming organisms. The sampler was gently moved over the plant until the plant was inside and then it was closed. Once the sample was collected, the sampler was inverted and rinsed with filtered stream water over a plastic tray, and contents were stored in 1,000-ml plastic containers. In the laboratory, macroinvertebrates were separated from macrophytes and preserved in 70% ethanol within 2 hours since the sampling time.
Samples processing
Estimation of macrophyte fractal dimension, surface area, and biomass
Macrophytes were put into a white plastic tray filled with tap water. Samples were photographed with a digital camera at 7× magnification. All photographs had the same format, size, and resolution (JPEG, 15.7 × 10.5 cm2, and 560 dpi, respectively). The images were modified, eliminating shade and reflections to improve resolution using the GIMP software (Kimball & Mattis, 1996–2008). Then, they were converted into black and white, and boundary line images of the plants were obtained. Fractal dimension was estimated by the box-counting method (Sugihara & May, 1990) using the ImageJ software (Rasband, 1997–2008). Twenty grids with box side-length (S) from 10 to 110 pixels were placed on each image and the number of occupied boxes (N) was counted. Fractal dimension was estimated as the slope of log N and log 1/S. The number of grids and side-length range of boxes was selected following recommendations by Halley et al. (2004). Then, leaves and branches from each sample were separated, put into a plastic bag, and scanned. Surface area (A) was estimated as the double of scanned area and calculated using the ImageJ software. Macrophyte samples were dried at 60°C until constant weight to determine dried weight (DW).
Estimation of periphyton biomass
Macrophyte fragments were introduced in glass beakers and sonicated three times for 3 min, separated by intervals of 1 min. We have previously observed sonicated leaves under microscope and determined that 90% of algae are removed by this process, and sonication does not break plant cells. A 200-m subsample was taken from the final suspension and filtered through a pre-weighed Whatman GF/F glass fiber filter to determine particulate organic matter content (POM). Filters were dried at 60°C until constant weight and combusted at 500°C for 4 h. POM was determined as the difference between dry weight and ash-free dry weight. Another 100-ml subsample was filtered through a Whatman GF/F filter and photosynthetic pigments were extracted in 90% acetone at 4°C for 24 h. The extract was then measured using a spectrophotometer, and chlorophyll-a content (Chl-a) was estimated following APHA (1995).
Estimation of macroinvertebrate density, biomass, richness, rarefied richness, diversity, maximal length, and size spectra
In the laboratory, macroinvertebrates were fixed in 70% ethanol and kept until they were counted and identified to the lowest possible taxonomic level (usually genus). Macroinvertebrate biomass was obtained by drying samples in pre-weighed aluminum envelopes at 60°C until constant weight. An analytical digital balance was used to weigh envelopes.
We refer to the number of taxa and not the number of species because in our system the identification of many aquatic macroinvertebrates is difficult, due to the high diversity and the fact that invertebrates associated to macrophytes are usually immature stages (available taxonomic keys refer to adults). To account for the influence of the number of individuals on the estimation of taxon richness, rarefaction curves were constructed from our macroinvertebrate sample data (Gotelli & Colwell, 2001) using the EstimateS Win 8.0 program (Colwell, 2006). Curves were adjusted according to the Clench equation (Clench, 1979), and rarefied taxon richness was estimated for each macroinvertebrate sample. The taxon diversity was estimated by the Shannon–Wiener index.
The macroinvertebrate maximal length (L) was obtained at 100× (±0.05 mm) under stereomicroscope as the linear measurement from the proximal end of the head to the distal end of the individual. Gastropoda L was estimated by the height of the shell. The size spectra were defined following Morse et al. (1985) and Williamson & Lawton (1991), as the regression of log (N) vs. log (L). Amphipoda size spectra were approximately linear for lengths higher than the mode, as shown for other invertebrates (Williamson & Lawton, 1991; Gunnarsson, 1992). Then, experiment treatments where size was measured were compared with confidence intervals at 95% for slope and intercepts were calculated based on ordinary least squares regression between log (N) and log (L).
Laboratory experiments
In order to test the active habitat choice between macrophytes, we examined differences in invertebrate distribution in paired sets of macrophytes and in paired sets of plastic macrophyte imitations. Each experiment (Laboratory Experiments 1–3 below) was performed in three plastic trays with 10 l of tap water, where bunches of macrophytes or plastic imitations of contrasting architecture were set in opposing sides. Groups of 60 individuals of Hyalella sp. obtained from the Las Flores stream were released in the center of each tray and allowed to choose between treatments (following the protocol described in Hansen et al., 2011). After 24 h, macroinvertebrates and macrophytes were sampled with plastic containers of 1,000 ml. Amphipods were separated and counted. All experiments were performed in the absence of observers and in a normal light-darkness cycle, with macrophytes and plastic imitations free of periphyton and invertebrates. The number of amphipods and biomass of macrophytes used in experiments was chosen so each treatment had final densities similar to those found in the Las Flores stream.
Laboratory experiment 1: selection between plastic imitations of low and high D
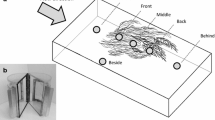
Commercial plastic imitations of macrophytes of contrasting architecture (Fig. 1) were cut to obtain fragments with identical surface area. Plastic imitations’ D was obtained from photographs 7× as described above. The “leaf” area of each type of imitation was obtained from scanning then the total surface area was estimated by multiplying “leaf” area by the number of leaves per fragment. Four fragments of each type were set on opposite sides for each tray.
Laboratory experiment 2: selection between Elodea of low D and Elodea of high D
Fragments of 30 cm of Elodea were sampled and cleaned from invertebrates and periphyton. Half of fragments were randomly assigned to the control group, and the other half to a treatment which consisted in cutting the leaves at two consecutive nodes, thus simplifying architecture and reducing the D (Online Resource 1). Elodea was chosen for this experiment because this procedure was not able to change significantly the D of the other macrophyte species. Macrophytes were preserved at 4°C for 24 h before performing experiment. Two fragments of each Elodea treatment were set on the opposite sides of each tray.
Laboratory experiment 3: selection between Egeria and Elodea
Fragments of 30 cm of Egeria and Elodea were sampled from the Las Flores stream and cleaned from invertebrates and periphyton. In each tray, two fragments of Egeria and four of Elodea were set on opposite sides. After active choice experiment, macrophyte D, A, and DW were estimated as previously described.
The data were analyzed by ANOVAs (factor = complexity level, 3 replicates) for D as a confirmatory predictor variable and Amphipoda density as a response variable. In the laboratory experiment 2, Amphipoda density could not be normalized, so a non-parametric test (Mann–Whitney test) was applied.
Field experiments
In order to study field macroinvertebrate community on different macrophytes, we performed three colonization experiments of macrophytes and plastic imitations with different D, in the presence of different levels of predation.
As we know, samples from the same stream cannot be truly independent of each other. The degree to which invertebrate community is influenced by the upstream colonization would generally depend on the importance of drift that depends on current velocity, and invertebrate size and habits (Wilzbach et al., 1988). The invertebrates observed in this study (Gastropoda, Amphipoda, Ephemeroptera, Odonata, Oligochaeta) are closely associated with macrophytes (mainly clingers and crawlers), and the current velocity in the Las Flores stream is very slow (usually <20 cm s−1; Giorgi et al., 2005). Therefore, we considered that a separation among replicates of 5–30 m and 15–30 days of colonization was enough to approximate to independence of experimental units.
Field experiment 1: colonization of plastic imitations of different fractal dimensions
In November 2010 (spring), plastic imitations described above were separated in bunches of different complexities and set along the Las Flores stream (4 imitations per bunch, 6 replicates). Pairs of bunches of different complexities were set along a 25 m reach, following a block design (5-m separation among blocks), and after 2 weeks of colonization, the imitations were recovered with 1,000-ml plastic containers. In the laboratory, macroinvertebrates were counted and identified. A subsample of plant imitations from each group was used to estimate Chl-a and POM. The block factor was found non-significant by ANOVA, so that data were analyzed by one-way ANOVA (factor = complexity level, 6 replicates) for variables D, density, taxon richness, rarefied taxon richness and diversity of invertebrates, and density of the most abundant taxon (Oligochaeta), Chl-a and POM.
Field experiment 2: colonization of Elodea with low and high D
In January 2011 (summer), 150 fragments of Elodea of 25 cm were sampled in the Las Flores stream. They were separated from invertebrates and randomly assigned to 8 groups of 18 fragments each, 4 controls and 4 treatments. While controls remained untouched, Elodea in the low D treatment was systematically cut in order to simplify its architecture as described in the previous laboratory experiment (Online Resource 1). Once treatment was applied, 5 fragments were selected at random in each group to take photographs and determine mean D per group at the beginning of experiment. Then, a randomly selected fragment of Elodea in each group was processed to determine initial Chl-a and POM. The fragments left in each group were tied together in bunches, which were attached to eight identical black plastic basket (1.3 × 1.3 cm2 mesh size) with polystyrene floats and identification marks (Fig. 2). The plastic baskets were set along a 30 m reach of the Las Flores stream, in a block design, where pairs of baskets (control and treatment) were about 7 m from each other.
After 25 days in stream, baskets were recovered. At the laboratory, macroinvertebrates were counted, identified and measured under stereomicroscope, and dried to estimate biomass. Five Elodea fragments from each group were photographed to estimate final mean D. A randomly selected fragment from each sample was processed to estimate final POM and Chl-a. Finally, all Elodea A and DW were estimated. Data were analyzed by ANOVAs (factor = complexity level, 4 replicates) for variables D, density, biomass, taxon richness, rarefied taxon richness, diversity, mean L of macroinvertebrates, and initial and final POM and Chl-a. When the block factor was significant, block ANOVAs were performed (this was the case for macroinvertebrate density, taxon richness, rarefied taxon richness, and diversity). Product–moment correlations were performed between D and all macroinvertebrate variables
Field experiment 3: colonization of Egeria (low D) and Elodea (high D) in the presence of different levels of predation
In March 2011 (late summer), 100 25-cm fragments of Elodea and 50 fragments of Egeria were collected from the Las Flores stream. Plants were cleaned from invertebrates and algae, and randomly assigned to 8 groups of Egeria (5 fragments per group), and 8 groups of Elodea (9 fragments per group). One fragment was randomly selected from each group to corroborate that initial Chl-a and POM were very low. The other fragments in each group were tied together in bunches and preserved at 4°C till the next day.
Once in the Las Flores stream, half of Egeria and Elodea groups were assigned to two treatments with different mesh sizes (small mesh size = 1.2 × 1 cm2 and big mesh size = 2.5 × 2.5 cm2) which determined the levels of predation. To control the effect of mesh size in colonization, both mesh sizes were present in baskets during the colonization process. After 24 days, the predation level was assigned to experimental units keeping only one mesh size according to the treatment (1.2 × 1 cm2 for low predation and 2.5 × 2.5 cm2 for high predation). Baskets were set in a 30 m reach of the Las Flores following a block design (Egeria high predation, Elodea high predation, Egeria low predation, and Elodea low predation), similarly to field experiment described above (7 m of separation between blocks).
After a week of removing outside mesh, a month after the beginning of experiment, baskets were sampled. In the laboratory, plants were separated from invertebrates, which were fixed for later counting, identification, measurement, and estimation of biomass. Three macrophytes from each basket were photographed to estimate D, and a randomly selected fragment was used to estimate final POM and Chl-a. Then, macrophytes were processed to estimate A and DW. Data were analyzed by the two-way ANOVA (factor 1 = species, factor 2 = level of predation, 4 replicates) for variables D, density, biomass, taxon richness, rarefied taxon richness, diversity, and initial and final POM and Chl-a. In the case of macroinvertebrate density, the block was found significant so that block two-way ANOVA was applied. Product–moment correlations were performed between D and all macroinvertebrate variables and macroinvertebrate density was log transformed to meet the normality assumption.
In addition, fish sampling was performed in the studied reach of the Las Flores stream at the beginning of April. This sample was aimed to register fish predator species and size, in order to determine the ability of fish to enter baskets with different mesh sizes. First, we performed a diurnal sample with a Danish seine of low selectivity (wing mesh size = 15 mm, bag mesh size = 5 mm) along 5 m of macrophyte beds. Then, a trammel net of low selectivity (big mesh size = 150 mm, small mesh size = 15 mm) was set surrounding a big Egeria bed and left for 24 h to capture nocturnal fishes. All sampled fishes were counted and identified, and maximal length and body depth were measure to quantify the size of individuals. A total of 10 fish taxa were sampled among macrophytes, of which Astyanax eigenmanniorum, Pseudocorynopoma doriae, Australoheros facetus, Gymnogeophagus meridionalis, Oligosarcus sp., and Cheirodon interruptus would regularly feed on macroinvertebrates. Astyanax eigenmanniorum and C. interruptus showed body depths between 1 and 2 cm, which would not allow them to enter small mesh size baskets. As expected, Elodea D was higher than Egeria D (D: Egeria = 1.35 ± 0.04 and Elodea = 1.47 ± 0.02; mean ± SD, F 1,3 = 30.50, P = 0.008). Therefore, macrophyte species and mesh size baskets selected would represent different levels of complexity and predation for invertebrates, respectively.
Data analysis
All macroinvertebrate and periphyton variables were analyzed against macrophyte A and DW. Most of the results were similar when analyzing variables referred to A or DW, so we only report data per square centimeter of macrophyte. When differences between both kinds of variables were observed, results for both variables are reported.
All variables were checked for normality (Shapiro–Wilk, P > 0.05) and homogeneity of variances (Levene, P > 0.05) before performing ANOVAs and product–moment correlations. Statistical analyses were performed using SPSS 11.5.1.
Results
Laboratory experiments
After 24 h, all amphipods were found over experimental substrates. Hyalella sp. actively selected complex instead of simple substrates in experiments where they had to choose between simple–complex plastic imitations and Egeria–Elodea. In the experiment performed with simple–complex Elodea, a higher amphipod density was found on the simple (cut) Elodea (Table 1; Fig. 3).
Field experiment 1: colonization of plastic imitations of different fractal dimensions
A total of 1,665 individuals belonging to 14 taxa of macroinvertebrates were sampled. The community was dominated by Oligochaeta, which represented 56% of the total of individuals (Fig. 4). There were no differences between plastic imitations in density and biomass of macroinvertebrates (Table 1). However, the density of the dominant taxon was significantly higher on complex imitations (F 1,10 = 8.13, P = 0.017; Table 2).
No differences in taxon richness between plastic imitations were found (F 1,10 = 3.35, P = 0.080), but rarefied taxon richness (F 1,10 = 5.49, P = 0.041) and diversity were higher on simple imitations (F 1,10 = 13.13, P = 0.005; Table 3). The Chl-a (F 1,10 = 1.02, P = 0.337) and POM (F 1,10 = 0.25, P = 0.629) were similar in both treatments.
Field experiment 2: colonization of Elodea with low and high D
A total of 3,350 macroinvertebrates belonging to 26 taxa were sampled. The community was dominated by Amphipoda (52% of total individuals), Gastropoda (20%), and Oligochaeta (13%). Macrophyte leaflet removal was able to reduce the D of Elodea at the beginning of the experiment (F 1,6 = 30.50, P = 0.001). By the end of the experiment D of both type of plants were reduced and although the difference in complexity between treatments tended to persist, it was reduced and became non-significant (F 1,6 = 5.57, P = 0.077; Fig. 5). There were no differences in Chl-a between treatments at the beginning or at the end of experiment. The POM only showed significant differences between treatments at the beginning (POM: simple Elodea = 0.19 ± 0.04 mg cm−2 and complex Elodea = 0.13 ± 0.01 mg cm−2; Mean ± SD; F 1,6 = 7.07, P = 0.038).
The macroinvertebrate density was not significantly different between the high and low D treatments (Table 1), but it correlated positively with final D (R = 0.845, P = 0.008; Fig. 6). Macroinvertebrate biomass was neither affected by the treatment (simple Elodea = 0.6 ± 0.8 mg cm−2; complex Elodea = 0.5 ± 0.5 mg cm−2; mean ± SD) nor correlated with D. No significant differences were found between simple and complex Elodea taxon richness (F 1,6 = 1.36, P = 0.287), rarefied taxon richness (F 1,6 = 1.06, P = 0.344), or diversity (F 1,6 = 0.46, P = 0.524; Table 3). In addition, there was no correlation of D with taxon richness, rarefied taxon richness, or diversity.
Macroinvertebrate length did not differ between treatments (simple Elodea = 3.8 ± 0.7 mm and complex Elodea = 5 ± 1 mm; mean ± SD), and did not correlate with D. The size spectra (log N vs. log L) were polymodal as a result of the superposition of different taxa spectra. Among dominant groups, Hyalella sp. (Amphipoda) and Ampullariidae (Gastropoda) showed bimodal distributions, whereas Heleobia sp. (Gastropoda) had a unimodal distribution (Fig. 7). Regarding the linear part of Hyalella sp. size spectra (L > 4 mm), non-significant differences were found between the slopes and intercepts of treatments (Table 4).
Field experiment 3: colonization of Egeria (low D) and Elodea (high D) in the presence of different levels of predation
A total of 10,595 macroinvertebrates were sampled. They belonged to 26 taxa, mainly Gastropoda (43% of total individuals), Amphipoda (30%), and Oligochaeta (11%). At the beginning of the experiment, there were no differences in Chl-a and POM between macrophyte species. However, a higher biomass of periphyton was detected on Elodea than Egeria after a month in the stream (Chl-a: F 1,3 = 17.24, P = 0.025 and POM: F 1,3 = 22.76, P = 0.017).
Two-way ANOVA for macrophyte species and predation level factors with density of macroinvertebrates as a dependent variable only found significant differences for level of predation (Table 1). One-way ANOVA for Egeria showed that high predation level baskets had higher macroinvertebrate density than low predation level baskets (F 1,3 = 44.52, P = 0.007; Fig. 8). When macroinvertebrate abundance was referred to macrophyte dry weight, non-significant differences in density of macroinvertebrates were found for macrophyte species and predation level factors (Online Resource 2).
Neither macrophyte species nor predation level affected macroinvertebrate biomass. The D correlated positively with macroinvertebrate density (R = 0.546, P = 0.029) but not with biomass. Finally, taxon richness, rarefied taxon richness, and diversity were similar for the four treatments (Table 3).
The macroinvertebrate L did not differ between treatments and did not correlate with D. The size spectra (log N vs. log L) were polymodal and similar in the four treatments (Online Resource 3). Besides, no differences were found in slopes and intercepts between treatments for the linear part of Hyalella sp. size spectra (Table 4).
Discussion
The preference of Hyalella sp. for structures with high D in two out of three laboratory experiments (simple vs. complex plastic imitations, and Egeria vs. Elodea), and the positive correlation between macroinvertebrate density and D in two of our field experiments (simple vs. complex Elodea, and Egeria vs. Elodea) support the existence of a positive effect of complexity on invertebrate abundance. As L was not correlated with D in any of the experiments, differences in density could not be explained by the presence of a higher area for small invertebrates establishment in macrophytes of high D. Fish predation had no effect on the size of macroinvertebrates. As for macroinvertebrate density, in the presence of high fish predation risk, it was increased in Egeria but unaffected in Elodea in the Las Flores stream.
Laboratory experiments
Hyalella sp. actively selected complex imitations and Elodea, high D structures, contradicting the findings of Russo (1987) who tested amphipod preference for simple and complex artificial substrates and did not report any active selection for a type of structure but a passive selection mediated by fish predation. Nevertheless, our results are supported by Hansen et al. (2011), whose experiments found an active selection of complex macrophytes and plastic imitations by Gammarus oceanicus (Amphipoda).
Hyalella selection of simple Elodea instead of complex Elodea was surprising as the opposite result was expected. However, this preference for simple (cut) Elodea could be explained by the liberation of soluble substances that may attract amphipods even 24 h after macrophyte tissue damage. This may have ecological meaning as it is expected that old senescent plants (e.g., at the end of summer) have a higher release of intracellular substances.
Field experiments
In the plastic imitations experiment, the dominance of Oligochaeta indicates that the macroinvertebrate community was not representative of that found on the Las Flores stream macrophytes, usually dominated by Amphipoda and Gastropoda (Ferreiro et al., 2011). Non-significant differences in the density of macroinvertebrates were found between simple and complex imitations. However, when the density of Oligochaeta was analyzed separately, data dispersion was reduced and the difference between complexity treatments was significant. This result may be explained by the ability of Oligochaeta to roll up around cylindrical structures such as complex imitation “leaves.”
The negative effect of plastic imitation complexity on rarefied richness and diversity was surprising, as it was opposite to the expected. Analyses of the density of main taxa on each type of imitation showed that this effect may be attributed to the presence of several species of Gastropoda on simple imitations, which were absent on the complex substrate. Some authors have reported that gastropods are excluded from complex inflexible artificial substrates with small inter-structural space sizes (Kelaher, 2003; Bartholomew & Shine, 2008), so these habitats may have reduced species richness relative to simpler habitats.
As for macrophyte field experiments, the dominance of Amphipoda and Gastropoda on macrophytes agrees with the community composition reported in a previous field study in the Las Flores stream (Ferreiro et al., 2011). The lack of effect of D on taxon richness and diversity does not agree with the results of other studies (Taniguchi et al., 2003; Thomaz et al., 2008), but has been previously reported in the Las Flores stream (Ferreiro et al., 2011). The strong positive correlation between D and macroinvertebrate density does not agree with the lack of significant difference in macroinvertebrate density between treatments detected by ANOVA. This may be explained by D detecting, at the end of the experiment, differences in the structure of plants assigned to the same complexity level treatment.
In the experiment with simple and complex Elodea, cutting Elodea reduced D at the beginning of experiment. However, after 25 days in the stream, the difference in complexity between treatments was considerably reduced. This was explained by the natural growth of plants, as the addition of new branches causes an increase in D. Then, at the end of experiment, one of the replicates of the cut Elodea had a D similar to that of the uncut treatment (Fig. 6). In addition, no differences in periphyton biomass were detected between treatments at the beginning and end of the colonization period, so this experiment tested the effect of complexity in the absence of differences in food availability. However, in the Egeria and Elodea experiment, periphyton biomass was significantly higher on the complex treatment after a month in the stream. Then, in this experiment, food availability would have been higher in complex macrophyte (Elodea), especially to the end of the colonization period. Finally, as macrophytes had been cleaned at the beginning of the experiment, this evidence supports some previous field sampling and experimental results from the Las Flores stream (Ferreiro et al., 2013) who had indicated that D may have a positive effect on the growth of epiphytic algae.
Macrophyte complexity and macroinvertebrate size spectra
The size–frequency distributions of structural elements of a habitat affect the body size distributions, so an increase in the number of small habitat elements would lead to size spectra with lower number of big body size individuals (Tokeshi & Arakaki, 2012). We considered that an increase in the number/density of habitat elements is followed by a simultaneous reduction in the number of large habitat elements. So, we expected that treatments with high D had a higher slope of the abundance–length relation. However, size spectra we found were not linear as proposed by Morse et al. (1985) and Schmid (2000), but normal (Mittelbach, 1981; Armstrong & Nudds, 1985; Gunnarsson, 1992) or polymodal (Gaston & Lawton, 1988; Stead et al., 2005).
Some authors have found that complexity leads to an increase in the abundance of small individuals, and a simultaneous decrease in the number of large individuals (MacAbendroth et al., 2005; Ferreiro et al., 2011). However, a peak in the abundance of large invertebrates for intermediate levels of complexity has also been reported, and attributed to the existence of an ideal interstice size, where invertebrates may fit and avoid predation by larger predators (Bartholomew & Ebeid, 2011). In the presence of predation, normal size spectra may be expected if the survivorship of intermediate size invertebrates is maximized, as they are able to fit through the spaces that their larger predators cannot and displace smaller individuals. In addition, Schmid et al. (2000) stated that the fact that abundance–body size relations are non-linear and may be caused by sampling procedures that lead to a bias against small and rare species. Finally, it may be argued that larger, more mobile invertebrates are more difficult to capture. However, our data showed a bimodal distribution for Amphipoda and Ampullariidae, where two peaks in abundance–size relation were observed for different size ranges, so alternative explanations are necessary.
A higher slope was observed for the regression of log (N) and log (L) of amphipods in complex than simple Elodea, however, this difference was non-significant (Table 4). This agrees with the lack of correlation between D and mean L and indicates that under these experimental conditions, D had no effect on macroinvertebrate size.
Fish predation risk and macroinvertebrate density
The lack of effect of fish predation risk on macroinvertebrate density in Elodea treatments was surprising, but may be expected if macroinvertebrates are very well adapted to avoid predation in these macrophyte beds. The positive effect of high predation risk on macroinvertebrate density in Egeria treatments was even more surprising.
There is a general consensus that predation is reduced in complex macrophyte stands (Heck & Crowder, 1991). However, while some authors have reported lower predation rates in aquatic plants with complex structure (Dionne & Folt, 1991; Warfe & Barmuta, 2004), the opposite was observed by Warfe & Barmuta (2006). Warfe et al. (2008) further addressed this question pointing out that the role of vegetation as refuge is related to the absolute value of interstitial space available to prey but unavailable to predators. Bartholomew et al. (2000) have proposed a dimensionless index of structural habitat complexity that measures the extent to which the structure interferes with a predator’s ability to move through the habitat in search of, or while pursuing, prey. When values of average inter-structural space size (S p) divided by the size of the predator (P r) are below 1, the predator cannot move through the habitat and prey survivorship should be uniformly high. As the average S p/P r increases beyond 1, the number of spaces that the predator can fit through accumulates the predator’s maneuverability within these spaces also increases, and prey survivorship should decrease rapidly.
We have previously performed estimations of the average inter-structural space size from the Las Flores macrophyte photographs, assessing the length of interstices with a ruler positioned at the center of macrophyte fragments (Dibble et al., 1996). In April 2008, we found an average interstice length of 0.9 ± 0.3 cm for Egeria and 1.7 ± 0.9 cm for Elodea (mean ± SD, N = 6) (Ferreiro et al., 2010). As body depths of fishes only entering in high predation risk baskets were between 1 and 2 cm, we may expect Sp/Pr values between 0.9 and 0.5 (±0.3) for Egeria and between 1.7 and 0.9 (±0.9) for Elodea. This suggests that the lack of effect of fish predation risk in Elodea treatments macroinvertebrate density could not be attributed to a lack of predator’s maneuverability.
Fish predation risk and macroinvertebrate body size
As predation per se has a negative effect on macroinvertebrate size (Crowder & Cooper, 1982; Diehl, 1992), an increase in predation would lead to size spectra with lower number of big body size individuals. As macrophyte complexity would have a negative effect on predation (Coull & Wells, 1983; Russo, 1987; Warfe & Barmuta, 2004), we may expect that the increase in the slope log N–log L was higher in Egeria (low D) than Elodea (high D).
The regression between log (N) and log (L) to the right of the mode in amphipods showed higher slopes for high predation level treatments in both macrophyte species. However, non-significant differences could be inferred from data (Table 4). This, plus the lack of difference in mean L detected by two-way ANOVA, indicates that in our experiments there were no differences in macroinvertebrate size that could be attributed to predation-complexity effects.
Final considerations
The preference for complex structures of Hyalella sp. indicates that active selection may be important for macroinvertebrate distribution on substrates of contrasting complexity. The positive relationship between fractal dimension and macroinvertebrate density corroborates the existence of a positive effect of macrophyte complexity on invertebrate abundance. As body length was not associated with fractal dimension, such differences could not be attributed to the existence of a higher space available for smaller invertebrates in complex plants. In addition, neither macroinvertebrate density nor size was reduced by predation in the Las Flores stream.
References
APHA, 1995. Standard Methods for the Examination of Water and Wastewater. American Public Health Association Inc., Washington, DC.
Armstrong, D. P. & T. D. Nudds, 1985. Factors influencing invertebrate size distributions in prairie potholes and implications for coexisting duck species. Freshwater Invertebrate Biology 4: 41–47.
Bartholomew, A. & K. Ebeid, 2011. Habitat complexity influences the microhabitat choices of desert beetles. Israel Journal of Ecology and Evolution 57: 213–221.
Bartholomew, A. & R. L. Shine, 2008. Space size relative to prey width (Sp/Py) influences macrofaunal colonization of artificial structures. Marine Ecology Progress Series 358: 95–102.
Bartholomew, A., R. J. Diaz & G. Cicchetti, 2000. New dimensionless indices of structural habitat complexity: predicted and actual effects on a predator’s foraging success. Marine Ecology Progress Series 206: 45–58.
Beukers, J. S. & G. P. Jones, 1998. Habitat complexity modifies the impact of piscivores on a coral reef fish population. Oecologia 114: 50–59.
Cattaneo, A. & J. Kalff, 1980. The relative contribution of aquatic macrophytes and their epiphytes to the production of macrophytes beds. Limnology & Oceanography 25: 280–289.
Cheruvelil, K. S., P. A. Soranno & R. D. Serbin, 2000. Macroinvertebrates associated with submerged macrophytes: sample size and power to detect effects. Hydrobiologia 441: 133–139.
Clench, H., 1979. How to make regional lists of butterflies: some thoughts. Journal Lepidopterist’s Society 33: 216–321.
Colwell, R. K., 2006. EstimateS: Statistical estimation of species richness and shared species from samples, Version 8. Persistent. purl.oclc.org/estimates.
Cooper, S., L. Barmuta, O. Sarnelle, K. Kratz & S. Diehl, 1997. Quantifying spatial heterogeneity in streams. Journal of the North American Benthological Society 16: 174–188.
Coull, B. C. & J. B. J. Wells, 1983. Refuges from fish predation: experiments with phytal meiofauna from the New Zealand rocky intertidal. Ecology 64: 1599–1609.
Crowder, L. B. & W. E. Cooper, 1982. Habitat structural complexity and the interaction between bluegills and their prey. Ecology 63: 1802–1813.
Dibble, E. D., K. J. Killgore & G. O. Dick, 1996. Measurement of plant architecture in seven aquatic plants. Journal of Freshwater Ecology 11: 311–318.
Diehl, S., 1992. Fish predation and benthic community structure-the role of omnivory and habitat complexity. Ecology 73: 1646–1661.
Dionne, M. & C. L. Folt, 1991. An experimental analysis of macrophyte growth forms as fish foraging habitat. Canadian Journal of Fisheries and Aquatic Sciences 48: 123–131.
Dodds, W. K. & B. J. F. Biggs, 2002. Water velocity attenuation by stream periphyton and macrophytes in relation to growth form and architecture. Journal of the North American Benthological Society 21: 2–15.
Feijoó, C. & R. Lombardo, 2007. Baseline water quality and macrophytes assemblages in Pampean streams: a regional approach. Water Research 41: 1399–1410.
Ferreiro, N., C. Feijoó & L. Leggieri. 2010. Efecto de la complejidad de las macrófitas sobre la densidad de los macroinvertebrados acompañantes en un arroyo pampeano. Libro de resúmenes de la IV Reunión Binacional de Ecología. Asociación Argentina de Ecología (ASAE) – Sociedad de Ecología de Chile (SOCECOL). http://www.conicet.gov.ar/new_scp/detalle.php?keywords=&id=23411&congresos=yes&detalles=yes&congr_id=906863.
Ferreiro, N., C. Feijoó, A. Giorgi & L. Leggieri, 2011. Effects of macrophyte heterogeneity and food availability on structural parameters of the macroinvertebrate community in a Pampean stream. Hydrobiologia 664: 199–211.
Ferreiro, N., A. Giorgi & C. Feijoó, 2013. Effects of macrophyte architecture and leaf shape complexity on structural parameters of the epiphytic algal community in a Pampean stream. Aquatic Ecology 47: 389–401.
Gaston, K. J. & J. H. Lawton, 1988. Patterns in the distribution and abundance of insect populations. Nature 331: 709–712.
Giorgi, A., C. Feijoó & G. Tell, 2005. Primary producers in a Pampean stream: temporal variation and structuring role. Biodiversity and Conservation 14: 1699–1718.
Gosselain, V., C. Hudon, A. Cattaneo, P. Gagnon, D. Planas & D. Rochefort, 2005. Physical variables driving epiphytic algal biomass in a dense macrophyte bed of the St. Lawrence River (Quebec, Canada). Hydrobiologia 534: 11–22.
Gotelli, N. J. & R. K. Colwell, 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4: 379–391.
Gregg, W. W. & F. L. Rose, 1982. The effects of aquatic macrophytes on the stream microenvironment. Aquatic Botany 14: 309–324.
Gunnarsson, B., 1992. Fractal dimension of plants and body size distribution in spiders. Functional Ecology 6: 636–641.
Halley, J., S. Hartley, A. Kallimanis, W. Kunin, J. Lennon & S. Sgardelis, 2004. Uses and abuses of fractal methodology in ecology. Ecology Letters 7: 254–271.
Hansen, J. P., S. A. Wikström, H. Axemar & L. Kautsky, 2011. Distribution differences and active habitat choices of invertebrates between macrophytes of different morphological complexity. Aquatic Ecology 45: 11–22.
Hauser, A., M. Attrill & P. Cotton, 2006. Effects of habitat complexity on the diversity and abundance of macrofauna colonizing artificial kelp holdfasts. Marine Ecology Progress Series 325: 93–100.
Heck, K. & L. Crowder, 1991. Habitat structure and predator prey interactions in vegetated aquatic systems. In Bell, S., E. McCoy & H. Mushinsky (eds), Habitat Structure: The Physical Arrangement of the Objects in Space. Chapman and Hall, London: 281–299.
Kelaher, B. P., 2003. Changes in habitat complexity negatively affect diverse gastropod assemblages in coralline algal turf. Oecologia 135: 431–441.
Kimball, S. & P. Mattis, 1996–2008. GNU Image Manipulation Program (GIMP). http://www.gimp.org.
Krebs, C. J., 1986. Capítulo 4: 19–20. In Krebs, C. J. (ed.), Ecología: análisis experimental de la distribución y abundancia, 2ª ed. Pirámide, Madrid.
Krecker, F. H., 1939. A comparative study of the animal populations of certain submerged plants. Ecology 20: 553–562.
Lillie, R. A. & J. Budd, 1992. Habitat architecture of Myriophyllum spicatum L. as an index to habitat quality for fish and macroinvertebrates. Journal of Freshwater Ecology 7: 113–125.
Lucena-Moya, P. & I. C. Duggan, 2011. Macrophyte architecture affects the abundance and diversity of littoral microfauna. Aquatic Ecology 45: 279–287.
MacAbendroth, L., P. Ramsay, A. Foggo, S. Rundle & D. Bilton, 2005. Does macrophyte fractal complexity drive invertebrate diversity, biomass and body size distributions? Oikos 111: 279–290.
MacArthur, R. H. & J. W. MacArthur, 1961. On bird species diversity. Ecology 42: 594–598.
Mittelbach, G. G., 1981. Patterns of invertebrate size and abundance in aquatic habitats. Canadian Journal of Fisheries and Aquatic Sciences 38: 896–904.
Morse, D., J. Lawton, M. Dodson & M. Williamson, 1985. Fractal dimension of vegetation and the distribution of arthropod body length. Nature 314: 731–733.
Rasband, W., 1997–2008. ImageJ. U.S. National Institutes of Health, Bethesda, MD. http://rsb.info.nih.gov/ij/.
Russo, A. R., 1987. Role of habitat complexity in mediating predation by the gray damselfish Abudefduf sordidus on epiphytal amphipods. Marine Ecology Progress Series 36: 101–105.
Schmid, P. E., 2000. Fractal properties of habitat and patch structure in benthic ecosystems. Advances in Ecological Research 30: 339–401.
Schmid, P. E., M. Tokeshi & J. M. Schmid-Araya, 2000. Relation between population density and body size in stream communities. Science 289: 1557–1560.
St. Pierre, J.I., & K.E. Kovalenko, 2014. Effect of habitat complexity attributes on species richness. Ecosphere 5: 1–10.
Stead, T. K., J. M. Schmid-Araya, P. E. Schmid & A. G. Hildrew, 2005. The distribution of body size in a stream community: one system, many patterns. Journal of Animal Ecology 74: 475–487.
Stewart, T., T. Shumaker & T. Radzio, 2003. Linear and nonlinear effects of habitat structure on composition and abundance in the macroinvertebrate community of a large river. American Midland Naturalist 149: 293–305.
Sugihara, G. & R. May, 1990. Application of fractals in ecology. Trends in Ecology & Evolution 5: 79–86.
Taniguchi, H., S. Nakato & M. Tokeshi, 2003. Influences of habitat complexity on the diversity and abundance of epiphytic invertebrates on plants. Freshwater Biology 48: 718–728.
Thomaz, S., E. Dibble, L. Evangelista, J. Higuti & L. Bini, 2008. Influence of aquatic macrophyte habitat complexity on invertebrate abundance and richness in tropical lagoons. Freshwater Biology 53: 358–367.
Tokeshi, M. & S. Arakaki, 2012. Habitat complexity in aquatic systems: fractals and beyond. Hydrobiologia 685: 27–47.
Tolonen, K. T., H. Hämäläinen, I. J. Holopainen, K. Mikkonen & J. Karjalainen, 2003. Body size and substrate association of littoral insects in relation to vegetation structure. Hydrobiologia 499: 179–190.
Warfe, D. M. & L. A. Barmuta, 2004. Habitat structural complexity mediates the foraging success of multiple predator species. Oecologia 141: 171–178.
Warfe, D. M. & L. A. Barmuta, 2006. Habitat structural complexity mediates food web dynamics in a freshwater macrophyte community. Oecologia 150: 141–154.
Warfe, D. M., L. A. Barmuta & S. Wotherspoon, 2008. Quantifying habitat structure: surface convolution and living space for species in complex environments. Oikos 117: 1764–1773.
Williamson, M. & J. Lawton, 1991. Fractal geometry of ecological habitats. In Bell, S., E. McCoy & H. Mushinsky (eds), Habitat Structure: The Physical Arrangement of the Objects in Space. Chapman and Hall, London: 69–86.
Wilzbach, M. A., K. W. Cummins & R. A. Knapp, 1988. Toward a functional classification of stream invertebrate drift. Verh Internat Verein Limnol 23: 1244–1254.
Acknowledgments
The authors are grateful to the landowner and the manager of Santa María del Arroyo farm for providing access to the study site. We thank the editor and two anonymous reviewers for their careful reading and constructive comments, who greatly improved this manuscript. This project was supported by the Universidad Nacional de Luján.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Katya E. Kovalenko
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Photographs of Elodea, a) control and b) treated to simplify architecture. (TIFF 994 kb)
Online Resource 2
ANOVA results for macroinvertebrate density (N g−1) in all the experiments. (DOC 35 kb)
Online Resource 3
Relationship between log N and log L of macroinvertebrates from each species and predation level. (EPS 2083 kb)
Rights and permissions
About this article
Cite this article
Ferreiro, N., Feijoó, C., Giorgi, A. et al. Macroinvertebrates select complex macrophytes independently of their body size and fish predation risk in a Pampean stream. Hydrobiologia 740, 191–205 (2014). https://doi.org/10.1007/s10750-014-1953-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-014-1953-y