Abstract
Different architectures in aquatic plants with different levels of morphological complexity provide environmental heterogeneity in freshwater ecosystems, and consequently influence invertebrate assemblages. We investigated the relative importance of the structural complexity of macrophytes and environmental variables on the abundance and richness of the macroinvertebrate assemblages associated with aquatic plants across the Esteros del Iberá. This protected wetland system located in Corrientes (Argentina) is fed by rain. Macrophyte habitat complexity was quantified by measuring fractal geometry dimensions of area and perimeter and plant biomass. We sampled macroinvertebrates associated with five species of macrophyte (Egeria najas, Cabomba caroliniana, Potamogeton gayi, Eichhornia azurea and Salvinia biloba) in five shallow lakes during two different seasons (dry and rainy) between 2007 and 2008. Regression analyses revealed that macrophyte structural complexity was an important factor on macroinvertebrate assemblages, whereas explanatory power of environmental variables was low. In both seasons, the fractal dimension of area was the variable with the highest explanatory power on richness, and plant biomass was in the case of macroinvertebrate abundance. To conserve macroinvertebrate diversity in Esteros del Iberá, it would be necessary to maintain the natural heterogeneity indicated by the different structural complexities of the macrophytes across the wetland.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Macrophytes play an important role in structuring communities in aquatic ecosystems. Vegetation type, and in particular, its growth forms, with a particular architecture and structural complexity, increase habitat heterogeneity and influence the composition and trophic structure of invertebrates occurring in wetlands (Batzer and Wissinger 1996; Tessier et al. 2004) and have a cascading effect on other communities (Meerhoff et al. 2003). Plants exhibiting different morphology provide shade and spatial complexity that may be important for young fishes (Dibble et al. 1996).
Invertebrates play a crucial role in wetland food webs. In many cases, they are the primary trophic link between plants and fishes, amphibians or birds. Under the same limnological conditions, the abundance of invertebrates is related to a suite of factors, including plant morphology (Vieira et al. 2007; Warfe et al. 2008; Monção et al. 2012; Walker et al. 2013), habitat complexity quantified by the fractal index (Thomaz et al. 2008; Dibble and Thomaz 2009) and food availability indicated by epiphytic biomass (Ferreiro et al. 2011). Differences in plant morphology and complexity at different scales determine the architecture (Dibble et al. 2006) which can influence the macroinvertebrate community. Previous studies have observed a significant relation between macrophyte complexity and structural parameters of the invertebrate assemblages (McAbendroth et al. 2005; Thomaz et al. 2008; Ferreiro et al. 2011; Mormul et al. 2011; Walker et al. 2013). Habitats with high space-size heterogeneity support a greater number of macroinvertebrate taxa (St. Pierre and Kovalenko 2014).
Cross-landscape studies have found a weak association between invertebrate structure and emergent plant community habitat (Batzer et al. 2004; Kratzer and Batzer 2007). Submerged aquatic vegetation however together with environmental variables, have been shown to be an important factor influencing zooplankton community structure and functional diversity of temperate lakes (Bolduc et al. 2016). An intensive survey of submerged aquatic plants in temperate lakes (Cyr and Downing 1988) revealed that the abundance of phytophilous invertebrates is related to the biomass of macrophytes and the average plant biomass per lake area.
We, however, have limited understanding of the influence of habitat complexity on biotic assemblages in wetlands, and even less knowledge of its effects on community and ecosystem attributes (Kovalenko et al. 2012). One of the major reasons for this gap in our knowledge is the difficulty in quantifying habitat structure in a way that allows for comparison between different habitats and is relevant to the associated fauna (Downes et al. 1998; Beck 2000). Further, sampling methods are limited in their ability to generate accurate quantitative estimates of invertebrate abundance; collection, processing, and quantification of samples are also laborious (Downing and Cyr 1985). Finally, classification of tropical and subtropical invertebrate species is difficult for nonspecialists, and descriptions of immature stages at the regional level are very scarce (Jacobsen et al. 2008; Clarke et al. 2017).
In this study, we analyzed the changes in the richness and abundance of macroinvertebrate assemblages associated with five macrophytes with different structural complexity within the Esteros del Iberá wetland across five vegetated lakes during two seasons (dry and rainy) in the 2007-2008 period. We tested for the relative importance of the structural complexity of macrophyte versus environmental variables on invertebrate assemblages in two contrasting seasons.
We hypothesized that macroinvertebrate richness and abundance might 1) differ among macrophytes that represent a different range of fractal dimensions and biomass, and 2) are affected by complexity to a greater degree than by other environmental factors.
Methods
Study Site
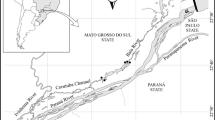
The study was conducted in Esteros del Iberá, one of the most pristine and largest inland wetlands of South America (12,000 km2). This subtropical wetland is located in Corrientes Province (northeast Argentina, Fig. 1), between the Paraná and Uruguay Rivers (27°30′-29°00’S and 56°25′-58°00’W) although the wetland does not have superficial connections with either of these rivers. The whole area is protected as a provincial reserve and national park and, one part of the wetland is recognized by the Ramsar Convention as a habitat of international significance for breeding and overwintering of birds, reptiles and mammals. The wetland consists of a vast mosaic of marshes, swamps and shallow lakes interconnected by streams of slow-flowing water. The basin is fed by rain and limited drainage occurs through Corriente River in the south of the system (Fig. 1), due to the very flat slope (gradient around 1: 10,000) and the large amount of vegetation accumulated in the basin. Changes in the water level (between dry and rainy periods) are the main vectors of biological changes in the Iberá wetland (Poi et al. 2017). A previous study, using climate projections coupled to hydrological models, predicted a reduction in the extension of the wetland (Úbeda et al. 2013) with potential effects on flora and fauna driven by water level fluctuations.
Esteros del Iberá wetland. Black thick lines indicate the wetland border. The location of the shallow lakes of the present study are indicated (adapted from Neiff et al. 2011)
In the lakes of Esteros del Iberá the number of species of aquatic plants is high (161), but only a few of them occupy extensive areas (Neiff et al. 2011). Macrophyte species sampled were the rooted submerged: Egeria najas Planch, Cabomba caroliniana A. Gray and Potamogeton gavi A. Benn, the free-floating Salvinia biloba Raddi, and the rooted with floating leaves Eichhornia azurea Kunth. C. caroliniana and E. najas formed extensive beds in both seasons while S. biloba and E. azurea were found in some lakes during the rainy season and P. gavi in the dry season. The macrophytes selected in this study represented species that dominated extensive areas larger than 100 m2 in the lakes and were chosen because they exhibited different levels of structural plant complexity.
We selected five shallow lakes (Galarza, Luna, Iberá, Itatí and Paraná) based on accessibility (other lakes are accessible only by air). Mean depths of the studied lakes ranged from 1.2 to 3.2 m, and areas varied from 15 to 86 km2 (Cózar et al. 2005, Table 1). The bottom of the waterbodies is quite flat, even in open water areas, and were made up of medium and fine sands covered by different thicknesses of organic debris. The rainy season occurred from September to October 2007 (295.6 and 170.5 mm, respectively), whereas the dry season was concentrated in January-February 2008 (22 and 43.5 mm, respectively). The annual rainfall was similar in both years (1033 and 1019 mm, respectively). All lakes were sampled in both sampling campaigns, except Paraná Lake, which due to bad weather conditions and difficulties in access could only be sampled in February 2008.
Environmental Variables
Physical-chemical characteristics were measured at each sampling site between 10 and 12 h. Water temperature, electrical conductivity and dissolved oxygen concentration were measured with an YSI 54A Water Quality probe (YSI Incorporated, Yellow Springs, USA) and pH was recorded using a WTW 330/SET-1 digital pH-meter. A Secchi disk was used to measure water transparency. Water samples taken subsurface were collected for nutrient and chlorophyll analysis. In the laboratory, these samples (l L) were filtered through pre-washed Gelman DM-450 membrane filters (0.45 μm pore) for nutrient analyses within 1-2 h of collection. Spectrophotometric methods were used for determination of NH4+1 (indophenol blue method), NO3− + NO2− (called NO3−) by Cd reduction and orthophosphate called Phosphate (molybdenum blue method) with persulfate oxidation (APHA l995). Total nitrogen was calculated as the sum of NH4+1, NO3− and NO2−. The chlorophyll-a concentration was determined by filtration of 0.5 L onto Whatman GF/C filters (0.7 μm, 47 mm). Filters were stored frozen at −20 °C until extraction with 90% acetone solution in darkness for 24 h at 4 °C. The extract was then measured by the fluorometric method (APHA 1975).
Determination of Macrophyte Fractal Dimension
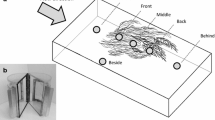
To determine fractal dimensions, four representative portions of each macrophyte species were submerged in a container with clear water and photographed with a SONY DSC-HX1 (20 megapixels, zoom lens G 5.0-100.0 mm) digital camera, capturing an area of 100.47 × 150.71 cm2. For E. azurea, only the roots were considered because they represented the dominant structure in the water column (Thomaz et al. 2008; Dibble and Thomaz 2009), which provides substrate for aquatic invertebrates. Selected TIFF images were transferred to grayscale and bit maps to produce black and white images using Adobe Photoshop CS6 Version 13 × 64, and then were modified to adjust background shades to improve the resolution of plant features (Dibble and Thomaz 2009). Because the fractal dimension of area (DA) and perimeter (DP) may represent different properties of macrophytes (McAbendroth et al. 2005), we calculated both estimators of the fractal dimensions with the program ImageJ 1.49 v (Rasband 2015) using the box counting method (Sugihara and May 1990) with a series of grid sizes of 2, 4, 6, 8, 12, 16, 32, 64, 128 and 256 pixel widths (Thomaz et al. 2008).
Macrophyte and Macroinvertebrate Sampling
Samples were collected within each macrophyte bed using a frame net of 35 cm diameter (962 cm2) and 500 μm mesh size (Poi de Neiff and Carignan 1997; USEPA 2002). The net with a 1.5 m long handle, operated from a boat, was vertically immersed in the water between the water surface to 50 cm deep and then lifting it into a horizontal position capturing invertebrates and macrophytes alike. In the bed of submerged macrophytes it was necessary to trim the stems.
We collected three replicate samples of 10 lake/macrophyte combinations (Table 2) for each sampling date (October 2007 and February 2008) which represent a diverse range of environmental variables and macrophyte complexities, for a total of 60 samples. All samples were collected within 50 cm of the surface to reduce variation due to differences in water quality and amount of radiation between the surface and bottom.
The invertebrates were separated from the plants by washing the sample on sieves, and the animals retained by a 500 μm sieve were preserved in 80% ethanol and then identified and counted under a stereoscopic microscope. The searching though detritus and plant material for invertebrates took the most time during the counts. The plants (free of macroinvertebrates and detritus) were drained on a sieve for 24 hs and afterwards, oven-dried at 105 °C for 72 h to determine plant biomass. Prior to drying, macrophytes were carefully examined under a watchmaker’s magnifying glass. This procedure allowed to find invertebrates attached to the plants (funnel building midge larvae of Rheotanytarsus, larval cases of some Trichoptera and pupae of Ceratopogonidae), these stages and features provided additional information for generic classification. For Odonata, the nymph of some genera were identified by rearing out the nymphs in the laboratory. Taxonomic determinations of macroinvertebrates were carried out at genus level following Angrisano (1992); Lopretto and Tell (1995); Trivinho-Strixino and Strixino (1995); Michat et al. (2008); Domínguez and Fernández (2009); Libonatti et al. (2011) and other taxonomic references given in Ramírez (2010). The invertebrate assemblages were quantified as abundance (number of individuals per m2) and richness, which was calculated as the total number of genera encountered in a sample (962 cm2). To facilitate comparisons with other studies, the Shannon–Wiener diversity was also calculated.
Statistical Analysis
To characterize complexity provided by the different architectures of macrophytes we used the fractal dimensions of area (DA) and perimeter (DP), and the plant biomass (Table 4). A nonparametric analysis of variance (Kruskal-Wallis test) was used to detect significant differences in the environmental variables among the lakes and in the abundance and richness of macroinvertebrates among 10 lake/macrophyte combinations for each season. Single-factor analysis of variance with post hoc Tukey test was used to assess differences in the fractal dimensions of area (DA) and perimeter (DP), and the plant biomass among the five species of macrophytes.
Simple linear regressions were used to test the relationships between DA, DP and plant biomass on macroinvertebrate abundance and richness. Multiple regression analyses were performed for each season to relate macroinvertebrate abundance and richness to the measured complexity attributes, including: DA, DP, plant biomass and physical-chemical variables in the lakes. To avoid multicollinearity, only explanatory variables with a correlation factor below 0.7 were kept in the multiple regression analyses. For this reason, the effect of DA and DP on abundance and richness was measured separately in the multiple regressions of the dry period (February). Prior to this analysis, macroinvertebrate abundance (ind./m2) data were standardized through logarithmic transformation (log x + 1). All statistical analyses were performed using InfoStat (Di Rienzo et al. 2018) and PAST 2.08 (Hammer et al. 2001) and statistical significance was evaluated (p < 0.05).
Beta diversity was estimated for each season as an integrator of habitat heterogeneity across the Esteros del Iberá wetland using the Whittaker index with the modification introduced by Harrison (Magurran 2004):
where: S = total number of species recorded, α = mean species richness, and N = number of sites. The measure ranges from 0 (no turnover) to 100 (every sample has a unique set of species).
The similarities among the macroinvertebrates from the different macrophyte complexities and seasons were measured by the Euclidean distance using Hellinger transformation (square root of the abundance), which is appropriate for beta diversity assessment (Legendre and De Cáceres 2013). The Hellinger transformation allows the reduction of the relative weight of abundant species in the analysis.
Results
Environmental Variables
Water temperature was generally high (24.6 - 32.1 °C) across the lakes, and the pH was slightly acidic or neutral. Both variables did not show significant differences among lakes in October or February (Table 3). Conductivity (range between 8.6 and 62.7 μS.cm−1) was significantly different among the lakes, and had the lowest values during the rainy period (October, Table 3). The dissolved oxygen concentration varied between 3.8 and 8.5 mg/L in the rainy period, and only showed significant differences among lakes in the dry period (February, Table 3). Nutrients varied little among the lakes, except for total nitrogen in Iberá Lake, which registered the highest nitrogen concentration, being an order of magnitude higher than in the rest of the lakes (Table 3). This lake also showed the highest chlorophyll-a concentrations, and low water transparency during the dry season. The water transparency showed significant differences among lakes in both seasons (Table 3).
Macrophyte Structural Complexity
The morphological traits of the selected macrophytes (E. najas, C. caroliniana, P. gayi, E. azurea and S. biloba) are summarized in Table 4. The fractal dimension of the area (DA, Table 4) decreased following the sequence S. biloba > E. najas > C. caroliniana > E. azurea roots > P. gayi. The differences in DA among the selected macrophytes were significant (ANOVA, Tukey’s post hoc test, F = 20.81, P > 0.0001, n = 20), although E. najas and C. caroliniana did not differ significantly from each other (Table 4). When the fractal dimension of the perimeter was considered (DP, Table 4), both S. biloba, E. azurea and C. caroliniana had similar plant architecture, whereas DP value of E. najas and P. gayi differed significantly of the other macrophytes (ANOVA, Tukey’s post hoc test, F = 21.70, P > 0.0001, n = 20). The three rooted submerged species have different leaf features (whorled in E. najas, palmatisects in C. caroliniana and taped sheet leaves in P. gayi). Of all macrophytes studied, P. gayi had the simplest structure and the lowest fractal dimension value of both area and perimeter, whereas S. biloba had the highest values (Table 4). The linear regression between DP and DA was not significant (r2 = 0.47, P = 0.118), when the data of all studied species were pooled.
The rooted emergent plant E. azurea, with long floating stems and secondary submerged roots coming from stem nodes, accounted for the highest mean plant biomass per m2, whereas P. gayi showed the lowest (Table 4). The biomass of both species differed significantly (ANOVA, Tukey’s post hoc test, F = 2.85, P > 0.05). The means of E. najas, C. caroliniana and S. biloba were not significant different (Table 4). However, the biomass of each macrophyte was highly variable across lakes and seasons, which could be related to both the degree of plant cohesion and the effect of wind.
Abundance and Richness of Macroinvertebrate Assemblages
The total number of individuals collected was 83,129, distributed among a total of 40 families and 62 genera of macroinvertebrates (SI Table 1) during the two study periods: 43 genera in February (dry season) and 56 genera in October (rainy season). Only few genera were abundant (SI Table 1), while many (27) comprised less than 1% of the total macroinvertebrates.
The mean macroinvertebrate abundance of 10 lake/macrophyte combinations (Table 2) for each sampling date varied between 3062 and 23,222 ind./m2 (February) and between 11,320 and 40,031 ind./m2 (October). Macroinvertebrates richness ranged between 16 and 33 taxa per sample in the dry season and between 20 and 37 taxa in the rainy season. The linear regression between abundance and richness (Fig. 2) was weak in February (R2 = 0.23, F = 8.05, P = 0.07, n = 30) and October (R2 = 0.01, F = 0.38, P = 0.543, n = 30). The Shannon–Wiener diversity index ranged from 1.91 to 2.33 in February and from 0.96 to 2.49 in October.
The fractal dimension of area was correlated with richness in February (R2 = 0.59, F = 40.86, P < 0.0001, n = 30) and October (R2 = 0.41, F = 19.4, P < 0001, n = 30; Fig. 3). In both seasons, the linear regression showed similar trends; therefore, we present the results pooled in Fig. 3 (R2 = 0.49, F = 55.6, P < 0.001, n = 60) to allow a better coverage of the complexity gradient. At the highest complexity, the mean richness was 40 and at the lowest complexity was 18.1; the difference in the complexity gradient was 45%.
The fractal dimension of perimeter was weakly correlated with richness in the dry season (R2 = 0.20, F = 7.19, P < 0.01) and in the rainy season (R2 = 0.04, F = 1.5, P < 0.314). During the dry season C. caroliniana (highest Dp) had lower taxa richness than E. najas in lakes where the beds of both species coexisted. S. biloba had the highest taxa richness during the rainy season but E. azurea (with similar Dp) did not. Both measures of fractal dimension (DA and Dp) were unrelated to invertebrate abundance. Not surprisingly, the correlation between plant biomass and macroinvertebrate richness was weak and not significant in February (R2 = 0.05, F = 1.51, P = 0.22) and October (R2 = 0.08, F = 2.48, P = 0.126).
Plant biomass was significantly correlated with macroinvertebrates abundance (Fig. 4) in February (R2 = 0.41, F = 19.43, P = 0.0001) and October (R2 = 0.37, F = 5.91, P = 0.002). The multiple regression with DA alone (Table 5a) indicated that the model explained 69% of the variability in macroinvertebrate abundance (R2 adjusted = 0.626, F = 10.4, P < 0.0001, n = 30) in February, with plant biomass as the variable with greatest explanatory power. The coefficient of determination (R2) for transparency, nitrogen and dissolved oxygen was high and significant (Table 5a). The multiple regression model with Dp as fractal dimension alone, explained 61.2% (R2 adjusted = 0.531, F = 7.57, P < 0.0001, n = 30) of the variability, and the selected environmental variables were the same as in DA (Table 5b). In October (Table 6), the model explained 47% of the variability (R2 adjusted = 0.364, F = 4.31, P < 0.006, n = 30), and just one variable (plant biomass) was significantly related to macroinvertebrate abundance. The environmental variables and DA had no effect on the abundance of macroinvertebrates in the rainy season (Table 6).
The multiple regression relationship between macroinvertebrate richness and environmental variables and macrophyte complexities was significant and indicated that DA was the variable with greatest explanatory power in both seasons. In February (Table 5 a and b) the model explained 69.1% of the variability with DA as fractal dimension alone (R2 adjusted = 0.626, F = 10.72, P < 0.0001) and decreased to 36.9% with Dp alone. (R2 adjusted = 0.237, F = 2.80, P < 0.01). In October, DA, transparency and Dp explained 72% the variability in macroinvertebrates richness with greatest explanatory power of DA (R2 adjusted = 0.663, F = 12.42, P < 0.0001, Table 6). The correlation matrix of the multiple regression analysis were in SI Tables 2, 3 and 4.
The Shannon–Wiener diversity index showed no relationship with the macrophyte complexities variables (DA, Dp and plant biomass) in February. Only DA was correlated with diversity (R2 = 0.53, F = 8.91, P < 0.01) in October. The turnover rate of macroinvertebrate richness estimated with the Whittaker index was relatively low in February (β = 9.2%) and October (β = 13.19%). A higher value was recorded in October due to the greater number of plant life forms considered (submerged, free floating and rooted with floating leaves plants) in this season.
The dendrogram derived from the cluster analysis of the relative abundance of macroinvertebrates genera identified five groups (Fig. 5). Grouping provided a good interpretation (Cophenetic correlation coefficient = 0.928) of the relative effect of macrophyte structural complexity and seasonality. The macroinvertebrates living on S. biloba from different lakes showed a high degree of similarity. This free-floating plant had a high proportion of Ceratopogonidae larvae (SI Table 1) and high richness of Coleoptera and Hemiptera genera (SI Table 1). Assemblages associated with E. azurea were dominated by Reotanytarsus larvae (Chironomidae) and Hyalella sp. (SI Table 1) and were segregated from the rest (Fig. 5). Macroinvertebrates associated with P. gayi in the different lakes were segregated from the rest with lower affinity than those associated with E. azurea, C. caroliniana and S. biloba (Fig. 5). Assemblages for the rest of submerged plants were grouped according to seasonality.
Cluster analysis based on Euclidean distance (UPGMA method) of the relative abundances of macroinvertebrate genera in the different lakes and macrophyte in both seasons (rainy and dry). Where: 1 is Paraná Lake, 2 is Iberá Lake, 3 is Luna Lake, 4 is Itatí Lake, 5 is Galarza Lake. Gray color: February. White color: October
Discussion
Our results showed that macrophyte structural complexity is an important factor influencing not only macroinvertebrate richness but also macroinvertebrate abundance. The fractal dimension of area (DA) was the variable with greatest explanatory power on taxa richness and plant biomass on macroinvertebrate abundance. The most important finding was that these variables explained the structure of the macroinvertebrate assemblages in both seasons, so it was not affected by seasonal change.
Previous studies suggest that aquatic plants with more complex architectures support a higher abundance and different array of invertebrates than plants with simpler shapes, but not a greater number of taxa (Dibble and Thomaz 2009; Ferreiro et al. 2011; Walker et al. 2013). Thomaz et al. (2008) found that structural complexity measured by fractal geometry in some species of macrophytes included in this study affected both abundance and richness of invertebrates. Similar results were obtained by Mormul et al. (2011) in an experiment with artificial substrates simulating submersed macrophytes.
In this study, we used the fractal dimensions, discriminated as DA and Dp, and the plant biomass to characterize complexity provided by the different architectures of macrophytes that grew in monospecific stands. We found that values of DA were major than those of Dp, a fact that also observed by Ferreiro et al. (2011) and McAbendroth et al. (2005). During the rainy season, the highest DA was found in the free floating plant S. biloba, which also presents a greater ranges of microhabitats for invertebrates (submerged and floating aerial fronds) than submerged plants or E. azurea roots. In the dry season, E. najas had the highest fractal dimension of area. The shape of the leaves in the case of E. najas (leaf entirely arranged in whorls) allows a greater retention of organic matter than other submerged macrophytes; in fact, this was observed when the samples were sieved (unmeasured data). This detritus, together with periphyton biomass, is an important source of food for invertebrates and could contribute to explaining the differences found in terms of the richness of invertebrates, especially in systems with a high concentration of organic matter, such as the Iberá wetland. S. biloba and E. najas have a high taxa richness in the studied lakes, therefore it is not surprising that DA had positive relationship with taxa richness in both seasons. In another rain feed lake of Northeast Argentina (Gallardo et al. 2017), S. biloba supported a greater number of taxa and higher number of macroinvertebrates per plant dry weight than E. najas.
Because we not found correlation between macroinvertebrate richness and abundance, we conclude that the effects of fractal dimensions on richness measured in our investigation are not related to effects of organism abundance.
Our results indicate that the impact of DA on macroinvertebrate richness was higher than Dp. A high significant correlation and a difference of 45% in the complexity gradient DA–richness support this conclusion. Furthermore, the difference between the relationships richness-DA alone and richness-Dp alone was 32.1% in the multiple regression model of the dry period. In other words, submerged plants with finely dissected leaves (C. caroliniana) and high Dp did not support greater taxa richness than other submerged plants. A bulk fractal, of area occupancy (DA) and a boundary complexity fractal (Dp) provide subtly different information and potentially meaningful for macroinvertebrates associated with macrophytes. Both fractal measurements may indicate differences in the size of the gaps and spaces within plant beds for refuge of the invertebrates as well as in the colonization by the periphyton and in the retention of organic matter, which have an effect on the richness of taxa.
McAbendroth et al. (2005), who explicitly discriminated the effect of DA and Dp, indicated that both complexity measures in mixed macrophyte stands were unrelated to invertebrate taxa richness. Other authors found a relationship between Dp and the total number of individuals expressed per unit dry weight of plants (Ferreiro et al. 2011), and the densities of Annelida and Odonata (Dibble and Thomaz 2009). However, in our study, abundances of macroinvertebrates were not related to fractal dimension measures.
In contrast to plant complexity, plant biomass was a significant predictor of macroinvertebrate abundance in the Esteros del Iberá wetland. Previous studies demonstrated that the abundance of phytophilous invertebrates is related to the biomass of submerged plants (Cyr and Downing 1988). In floodplain lakes of the studied area, taxa richness of macroinvertebrates of seven plants with different architecture were related to plant biomass (Poi de Neiff and Neiff 2006). More recently, other studies indicated that macrophyte biomass affects macroinvertebrate richness primarily through increasing organism abundance rather than by providing distinct habitats for more taxa (St. Pierre and Kovalenko 2014).
Plant complexity measures (DA, Dp and plant biomass) had no effect on the diversity of taxa, except for DA in the rainy season. The Shannon diversity index was not useful for the measurement of the changes in the studied lakes, as the macroinvertebrate assemblages had few dominant taxa, and the rest were present in smaller proportions.
The explanatory power of environmental variables on richness was low. Water transparency, nitrogen content and dissolved oxygen had an effect on the abundance of macroinvertebrates in the dry season. The homogenization effect on environmental variables during the rainy season by the dilution of the water after the rainfall has been described for Esteros del Iberá lakes (Poi et al. 2017). This fact is especially apparent in dissolved oxygen concentration and conductivity, which in some lakes are among the lowest for freshwater environments in South America.
The low beta diversity, measured across seasons, indicated that macroinvertebrate taxa richness changed little across Iberá. However, the relative abundance of some genera differed between macrophyte complexities and seasons, which is reflected by the similarities indicated by cluster analysis from quantitative data. Macroinvertebrate assemblages of S. biloba and E. azurea were differentiated from that of other aquatic plants, regardless of the environmental variables. These results revealed the importance of the structural macrophyte complexity on the relative abundance of macroinvertebrates. The macroinvertebrate assemblages of C. caroliniana were grouped only in the dry period. Segregation of submerged plants according to seasonality is not surprising because different taxa were found in February and October.
The results of this study revealed that the structural complexities of macrophytes that have extensive monospecific beds across the Esteros del Iberá were the main factors explaining the structure the macroinvertebrate assemblages. These conclusions were not affected by the seasonal change studied. More complex beds had greater invertebrate richness, this being particularly true when structural complexity was estimated using a bulk fractal (DA). Macrophyte complexity affected macroinvertebrate richness to a greater degree than other environmental factors, thus the hypothesis two is accepted. Environmental variables had an effect on macroinvertebrates abundance only in the dry period. To conserve macroinvertebrate diversity in Esteros del Iberá, it would be necessary to maintain the natural heterogeneity indicated by the different structural complexities of the macrophytes across the wetland.
The present study only refers to some aspects of the macrophyte structural complexity that may not be the most important for the organisms that use the habitat. Future studies could incorporate other aspects of habitat complexity and heterogeneity such as leaf and stem configuration or space-size heterogeneity, which could be related to how animals perceive and use their environment (Dibble et al. 2006; St. Pierre and Kovalenko 2014).
Data Availability
All data will be available at CONICET repository after acceptation of the manuscript.
Code Availability
Not applicable.
References
Angrisano EB (1992) El orden Trichoptera en la Argentina y países limítrofes. Physis 50:118–119
APHA (1975) Standard methods for the examination of water and wastewater. APHA (American public health association), Washington D.C.
APHA (1995) Standard methods for the examination of water and wastewater. APHA (American public health association), Washington D.C.
Batzer DP, Wissinger SA (1996) Ecology of insect communities in nontidal wetlands. Annual Review of Entomology. https://doi.org/10.1146/annurev.en.41.010196.000451
Batzer DP, Palik BJ, Buech R (2004) Relationships between environmental characteristics and macroinvertebrate communities in seasonal woodland ponds of Minnesota. Journal of the North American Benthological Society 23(1):50–68
Beck MW (2000) Separating the elements of habitat structure: independent effects of habitat complexity and structural components on rocky intertidal gastropods. Journal of Experimental Marine Biology and Ecology. https://doi.org/10.1016/s0022-0981(00)00171-4
Bolduc P, Bertolo A, Pinel-Alloul B (2016) Does submerged aquatic vegetation shape zooplankton community structure and functional diversity? A test with a shallow fluvial lake system. Hydrobiologia. https://doi.org/10.1007/s10750-016-2663-4
Clarke DA, York PH, Rasheed MA, Northfield TD (2017) Does biodiversity-ecosystem function literature neglect tropical ecosystems? Trends in Ecology & Evolution. https://doi.org/10.1016/j.tree.2017.02.012
Cózar A, García CM, Gálvez JA, Loiselle SA, Bracchini L, Cognetta A (2005) Remote sensing imagery analysis of the lacustrine system of Iberá wetland (Argentina). Ecological Modelling. https://doi.org/10.1016/j.ecolmodel.2005.01.029
Cyr H, Downing JA (1988) Empirical relationships of phytomacrofaunal abundance to plant biomass and macrophyte bed characteristics. Canadian Journal of Fisheries and Aquatic Sciences 45:976–984
Di Rienzo JA, Casanoves F, Balzarini MG, González L, Tablada M, Robledo CV (2018) InfoStat. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. URL http://www.infostat.com.ar
Dibble ED, Thomaz SM (2009) Use of fractal dimension to assess habitat complexity and its influence on dominant invertebrates inhabiting tropical and temperate macrophytes. Journal of Freshwater Ecology. https://doi.org/10.1080/02705060.2009.9664269
Dibble ED, Killgore KJ, Dick GO (1996) Measurement of plant architecture in seven aquatic plants. Journal of Freshwater Ecology. https://doi.org/10.1080/02705060.1996.9664453
Dibble ED, Thomaz SM, Padial AA (2006) Spatial complexity measured at a multi-scale in three aquatic plant species. Journal of Freshwater Ecology. https://doi.org/10.1080/02705060.2006.9664992
Domínguez E, Fernández HR (2009) Macroinvertebrados bentónicos sudamericanos. Sistemática y Biología. Fundación Miguel Lillio, San Miguel de Tucumán
Downes BJ, Lake PS, Schreiber ESG, Glaister A (1998) Habitat structure and regulation of local species diversity in a stony upland stream. Ecological Monographs. https://doi.org/10.1890/0012-9615(1998)068[0237:HSAROL]2.0.CO;2
Downing JA, Cyr H (1985) Quantitative estimation of epiphytic invertebrate populations. Canadian Journal of Fisheries and Aquatic Sciences. https://doi.org/10.1139/f85-197
Ferreiro N, Feijoó C, Giorgi A, Leggieri L (2011) Effects of macrophyte heterogeneity and food availability on structural parameters of the macroinvertebrate community in a Pampean stream. Hydrobiologia. https://doi.org/10.1007/s10750-010-0599-7
Gallardo LI, Carnevali RP, Porcel EA, Poi ASG (2017) Does the effect of aquatic plant types on invertebrate assemblages change across seasons in a subtropical wetland? Limnetica. https://doi.org/10.23818/limn.36.07
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis, Palaeontologia electronica. http://palaeo-electronica.org
Jacobsen D, Cressa C, Mathooko JM, Dudgeon D (2008) Macroinvertebrates: composition, life histories and production. In: Dudgeon D (ed) Tropical streams ecology. Elsevier, Amsterdam, pp 65–105
Kovalenko KE, Thomaz SM, Warfe DM (2012) Habitat complexity: approaches and future directions. Hydrobiologia. https://doi.org/10.1007/s10750-011-0974-z
Kratzer EB, Batzer DP (2007) Spatial and temporal variation in aquatic macroinvertebrates in the Okefenokee swamp, Georgia, USA. Wetlands. https://doi.org/10.1672/0277-5212(2007)27[127:SATVIA]2.0.CO;2
Legendre P, De Cáceres M (2013) Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecology Letters. https://doi.org/10.1111/ele.12141
Libonatti ML, Michat MC, Torres PLM (2011) Key to the subfamilies, tribes and genera of adult Dytiscidae of Argentina (Coleoptera: Adephaga). Revista de la Sociedad Entomológica Argentina 70:317–336
Lopretto EC, Tell G (1995) Ecosistemas de aguas continentales. Metodología para su estudio. Ediciones Sur, La Plata
Magurran AE (2004) Measuring biological diversity. Blackell Publishing, Oxford
McAbendroth L, Ramsay PM, Foggo A, Rundle SD, Bilton DT (2005) Does macrophyte fractal complexity drive invertebrate diversity, biomass and body size distributions? Oikos. https://doi.org/10.1111/j.0030-1299.2005.13804.x
Meerhoff M, Mazzeo N, Moss B, Rodríguez-Gallego L (2003) The structuring role of free-floating versus submerged plants in a subtropical shallow lake. Aquatic Ecology. https://doi.org/10.1023/B:AECO.0000007041.57843.0b
Michat MC, Archangelsky M, Bachmann AO (2008) Generic keys for the identification of larval Dytiscidae from Argentina (Coleoptera: Adephaga). Revista de la Sociedad Entomológica Argentina 67:17–36
Monção F, Medeiros dos Santos A, Bini LM (2012) Aquatic macrophyte traits and habitat utilization in the upper Paraná River. Aquatic Botany. https://doi.org/10.1016/j.aquabot.2012.04.008
Mormul RP, Thomaz SM, Takeda AM, Behrend RD (2011) Structural complexity and distance from source habitat determine invertebrate abundance and diversity. Biotropica. https://doi.org/10.1111/j.1744-7429.2011.00762.x
Neiff JJ, Casco SL, Cózar A, Poi de Neiff A, Ubeda B (2011) Vegetation diversity in a large Neotropical wetland during two different climatic scenarios. Biodiversity and Conservation. https://doi.org/10.1007/s10531-011-0071-7
Poi de Neiff A, Carignan R (1997) Macroinvertebrates on Eichhornia crassipes roots in two lakes of the Paraná River floodplain. Hydrobiologia. https://doi.org/10.1023/A:1002949528887
Poi de Neiff A, Neiff JJ (2006) Riqueza de especies y similaridad de los invertebrados que viven en plantas flotantes de la planicie de inundación del río Paraná. Interciencia 3:220–225
Poi ASG, Neiff JJ, Casco SL, Úbeda B, Cózar A (2017) El agua de los esteros, lagunas y ríos. In: Poi A (comp) Biodiversidad en las aguas del Iberá. EUDENE, Corrientes, pp 21–39
Ramírez A (2010) Odonata. Revista de Biología Tropical 58:97–136
Rasband WS (1997-2015) ImageJ 1.49v. U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/
St. Pierre JI, Kovalenko KE (2014) Effect of habitat complexity attributes on species richness. Ecosphere. https://doi.org/10.1890/ES13-00323.1
Sugihara G, May RM (1990) Applications of fractals in ecology. Trends in Ecology & Evolution. https://doi.org/10.1016/0169-5347(90)90235-6
Tessier C, Cattaneo A, Pinel-alloul P, Galanti G, Morabito G (2004) Biomass, composition and size structure of invertebrate communities associated to different types of aquatic vegetation during summer in Lago di Candia (Italy). Journal of Limnology. https://doi.org/10.4081/jlimnol.2004.190
Thomaz SM, Dibble ED, Evangelista LR, Higuti J, Bini LM (2008) Influence of aquatic macrophyte habitat complexity on invertebrate abundance and richness in tropical lagoons. Freshwater Biology. https://doi.org/10.1111/j.1365-2427.2007.01898.x
Trivinho-Strixino S, Strixino G (1995) Larvas de Chironomidae (Diptera) do estado de São Paulo: guia de identifiçacao e diagnose dos géneros. Universidade Federal de São Carlos, São Carlos
Úbeda B, Di Giacomo AS, Neiff JJ, Loiselle SA, Poi ASG, Gálvez JA, Casco SL, Cózar A (2013) Potential effects of climate change on the water level, flora and macro-fauna of a large neotropical wetland. PLoS One. https://doi.org/10.1371/journal.pone.0067787
USEPA (2002) Methods for evaluating wetland conditions: #9 developing and invertebrate index of biological integrity for wetlands. USEPA (United States Environmental Protection Agency), Washington, DC
Vieira LCG, Bini LM, Velho FM, Mazãro GR (2007) Influence of spatial complexity on the density and diversity of periphytic rotifers, microcrustaceans and testate amoebae. Fundamental and Applied Limnology. https://doi.org/10.1127/1863-9135/2007/0170-0077
Walker PD, Wijnhoven S, van der Velde G (2013) Macrophyte presence and growth form influence macroinvertebrate community structure. Aquatic Botany. https://doi.org/10.1016/j.aquabot.2012.09.003
Warfe DM, Barmuta LA, Wotherspoon S (2008) Quantifying habitat structure: surface convolution and living space for species in complex environments. Oikos. https://doi.org/10.1111/j.1600-0706.2008.16836.x
Acknowledgments
We are grateful to Juan José Neiff for his valuable critical review. Since the creation of the provincial reserve Iberá, many specialists collaborated with the systematic identification of invertebrates A. Bachmann (in memorian), M. Archangelsky, Ch. O’Brien, O. Flint (in memorian), W. Peters, E. Domínguez, ME, Varela, A. Paggi, C. Armúa, P. Collins, whom we thank for their work and for sending the reference material. We also thanks to staff and park rangers to “Reserva del Iberá” for their valuable support during the surveys and C. Giese for your statistic support.
We thank to the associated editor, one anonymous reviewer and K. Kovalenko for their constructive suggestions.
Funding
This study was funded by the BBVA Foundation Bilbao under projects IBERAQUA (ref. BIOCON 04-100/05) and PI 18Q004 (Secretaría General de Ciencia y Técnica, Universidad Nacional del Nordeste).
Author information
Authors and Affiliations
Contributions
A.S.G. Poi, S.L. Casco and B. Úbeda participated equally in the conception or design of this study, as well as in the acquisition, analysis and interpretation of the data. L.I. Gallardo and L.M. Sabater contributed substantially to invertebrate identification and statistical processing.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 57 kb)
Rights and permissions
About this article
Cite this article
Poi, A.S.G., Gallardo, L.I., Casco, S.L. et al. Influence of Macrophyte Complexity and Environmental Variables on Macroinvertebrate Assemblages Across a Subtropical Wetland System. Wetlands 41, 105 (2021). https://doi.org/10.1007/s13157-021-01508-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-021-01508-4