Abstract
The study tests whether diurnal microhabitat use by Hatcheria macraei depends upon specific environmental parameters and/or the abundance of other fish. We carried out a 1-year field study in a low-order river of northern Patagonia, Pichileufu River, and used experimental trials to determine substrate preferences. Fishes were captured during daylight and physicochemical environmental variables were recorded. Headwater zones were dominated by rainbow trout (Oncorhynchus mykiss) and brown trout (Salmo trutta), while native fishes, H. macraei and the creole perch (Percichthys trucha) were more abundant downstream. H. macraei inhabited mostly shallow microhabitats with fast water velocity and substrates having significant interstitial spaces, independently of the abundance of other fishes. Experimental trials pointed out that H. macraei preferred mostly coarser substrates (>6 cm), avoiding fine ones. This study highlights the importance of erosional zones with high water velocity, large substrates, and suitable interstitial space in the microhabitat selection of H. macraei. The knowledge of microhabitat use by native fish populations is critical for management and conservation strategies and should be taken into account before any river modification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish distribution within a river has been related to microhabitat preferences (Onoda et al., 2009), and it is strongly influenced by predation risk (Clavero et al., 2005), food resources (Tyler & Clapp, 1995), and intra- (Petty & Grossman, 2007) or inter-specific interactions (McIntosh et al., 1992; Hesthagen & Heggenes, 2003; Clavero et al., 2009). The main abiotic microhabitat variables are related to hydraulic factors, such as depth, water velocity, and substrate (e.g., Van Liefferinge et al., 2005). However, the importance of each variable depends upon the fish ecomorphological adaptation to the environment (Wootton, 1998). Pelagic fishes are more affected by water velocity, while bottom dwelling fishes are more likely to be influenced by substrate composition (Hlohowskyj & Wissing, 1986; Onoda et al., 2009). For example, a high degree of heterogeneity and patchiness in rivers due to variation in the composition and arrangement of available substrates are the major causes of the distribution of benthic fish, either by substrate selectivity (e.g., Dixon & Vokoun, 2009) or indirectly by selecting patches with higher prey densities (e.g., Petty & Grossman, 1996).
The benthic catfish, Hatcheria macraei (Girard 1855), belongs to the family Trichomycteridae and is widely distributed in Argentina and Chile. It has a fairly continuous distribution from the northern Colorado River system (Grande River, La Rioja Province, 28°33′S, 67°9′W) south to the Baker River system (Blanco River, Santa Cruz Province, 47°34′S, 71°38′W), being found in both Atlantic and Pacific basins (Menni, 2004; Unmack et al., 2009, 2012). Despite this broad distribution, the biology of this species remains poorly known (Pascual et al., 2007). This is primarily because the majority of studies dealing with the Patagonian ichthyofauna have been carried out in lakes and limited efforts have been applied to the study of rivers. In addition, H. macraei are not frequently captured in traditional net surveys because it buries itself in the substrate (Unmack et al., 2012).
Hatcheria macraei has a generalized diet based on benthic invertebrates (Di Prinzio & Casaux, 2012). It is a rheophilic and negatively phototactic species (Menni, 2004), characterized by living in cold and well-oxygenated waters with sandy and rocky substrates (Ringuelet et al., 1967). Arratia & Menu-Marque (1981) mentioned a size-related habitat preference, mostly associated to the type of substrate and the water depth. Individuals up to 20 mm total length (TL) prefer shallow quiet pools with a substrate of sand or small pebbles near shore (<5 cm depth). Larger individuals, from 20 to 60 mm TL are found among plants and stones (<20 cm depth); and the largest individuals choose stony (medium- to large-sized stones) and sandy substrates (<50 cm depth). In addition, Barriga & Battini (2009) determined the indirect ontogeny (sensu Balon, 1990) of this species and related its morphological constraints to habitat and feeding preferences. A complete development of fins allow juvenile colonize deeper and faster water habitats while a bigger mouth gape permitted them to prey on new items and on a larger size prey range. Despite these observations, no specific work has been done to evaluate quantitatively the microhabitat preferences of this species. In the present study, some aspects of the diurnal microhabitat use by H. macraei and accompanying species were analyzed. In addition, H. macraei substrate preferences were evaluated using experiments. The main objectives of this study were (i) identify the main microhabitat variables that influence the presence and density of H. macraei, (ii) characterize diurnal microhabitat and density data of the accompanying species, both native and exotic fishes, and (iii) evaluate the substrate preference of H. macraei using trials performed in experimental channels.
Materials and methods
Study site
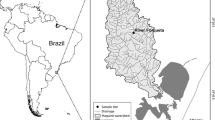
Field work was conducted in the Pichileufu River, Río Negro Province, Argentina. This river begins in the Carrera Mountains and flows north for around 150 km, through the Patagonian steppe before draining into Piedra del Águila Reservoir on the Limay River, a major tributary of the Negro River (Fig. 1). Sampling was performed at three localities, from October 2008 to December 2009. The three localities from upstream to downstream were: Pilila (41°17′50″S, 71°02′30″W, 1088 m a.s.l.); Pilcaniyeu (41°05′24″S, 70°49′42″W, 926 m a.s.l.); and Corralito (40°43′59″S, 70°41′14″W, 658 m a.s.l.). The river distance from Pilila to Pilcaniyeu is ~40 km, from Pilcaniyeu to Corralito is ~75 km, and from Corralito to Piedra del Águila reservoir is ~20 km (Fig. 1). Sampling dates by localities are indicated in Table 1. Pilcaniyeu was also sampled in October 6 and November 17, 2008, and February 9, May 4, June 8, and August 10, 2009.
Fish collection and habitat characterization
Fishes were captured using a 24 V DC backpack electrofishing unit, model 12-B (Smith-Root, Inc., Vancouver, WA, USA) fitted with a diamond shaped anode (diagonals of 36.8 and 19.7 cm). Sampling units consisted of 1 m2 areas. Once the area was chosen, two dip nets (50 cm wide) were carefully juxtaposed by two people on the bottom to block the area while the electrofisher operated the unit along 1 m upstream of the nets. Fish affected by the electricity, but not in the sampling area, were ignored. Stunned fishes were captured using hand nets (10 × 10 cm2) and deposited into the dip nets or released downstream according to their location, inside or outside the sampling area, respectively. Next, substrate was disturbed within the area to find stunned fishes among the interstitial space. Distance between units was at least 7 m to avoid electrical perturbation among sampling areas. A total of 295 units were sampled during daytime (from 10:00 to 15:00 h) throughout the sampling period, always starting downstream and working in an upstream direction at each sampling location. We tried to sample all different available mesohabitats [i.e., riffle, glide, run, backwater, edgewater, and pool (see Maddock, 1999 for a physical description)]. As mesohabitats had different areas, the number of sampling units was not equal in each type, and the number of units per locality varied from 14 to 22 by date. H. macraei individuals captured were transported to the laboratory for further analysis, while other fish species were counted, measured, and then released. At the laboratory, catfish were euthanized using an overdose of benzocaine and then weighed (to the nearest 0.01 g), measured (standard length, SL, to the nearest 1 mm), and sexed through examination of their gonads.
Habitat variables were recorded in each of these units. Water velocity of the water column (mean and maximum) and mean water velocity at the bottom were measured with a flowmeter, model FP101 (Global Water, Gold River, California, USA) with an accuracy of 0.03 m s−1. To measure mean and maximum water velocity of the water column the probe was moved slowly and smoothly throughout the flow, between the surface and bottom, over at least 1 min always inside the quadrant. Because the flowmeter takes one reading per second, both values were obtained from at least 60 readings. A similar procedure was carried out to record the mean water velocity at the bottom, with the difference that the probe was moved slowly perpendicularly to the flow direction above the bottom of the sampled area. Temperature, oxygen concentration, and pH were registered with a Water Quality Meter, model 850081 (Sper Scientific Ltd., Scottsdale, Arizona, USA) with an accuracy of 0.8°C, 0.4 mg l−1, and 0.2, respectively. Mean depth of each square was calculated as the average between the minimum and maximum depths registered with a meter stick, within the sampling area. Substrate size composition was visually estimated as percentage composition by at least two observers, classifying in five diameter size categories as: boulders (>180 mm), cobbles (>64 and <180 mm), gravel (>4 and <64 mm), sand (>1 and <4 mm), and silt (<1 mm). All cutoffs correspond to Wentworth scale with the exception of 180 mm. The mean classification error of each substrate category was less than 5%. This error was calculated comparing 30 sampling units visually ranked with the same units measured with an image analyzer (Digimizer version 4.0, Mariakerke, Belgium) on digital images. This misclassification error was considered negligible due to the codification of data performed previously to the statistic analysis (detailed below).
The interstitial space (or the inverse of embeddedness) as the degree of big substrate particles (>64 mm) surrounded or covered by fine sediment (<4 mm) was coded as: 1, when no interstitial space was detected among particles (they were completely surrounded or covered by the sediment); 2, when more than 50% of the area was embedded by fine sediments; and 3, when less than 50% of the area was embedded by fine sediments and there was substantial interstitial space (many interparticle voids). We also measured the presence or absence (1 or 0, respectively) of submerged macrophytes, filamentous algae, and riparian vegetation. Individual H. macraei were separated into two groups, juveniles and adults, according to their SL, sensu Barriga & Battini (2009). Individuals larger than 61.4 and 64.4 mm SL were considered adults, for males and females, respectively. Fish between these values and 25.9 mm SL were classified as juveniles.
Experimental design of trials
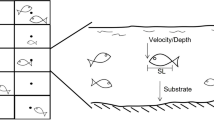
To isolate the effect of great heterogeneity in substrate sizes and food availability in rivers, experiment trails were performed. In this way, the hypothesis that microhabitat selection by H. macraei depends on the size of the substrate particles, which was tested. Fish captured at the middle location (Pilcaniyeu) were transported to facilities of the salmonid hatchery belonging to the Centro de Ecología Aplicada de Neuquén, Argentina (CEAN) to let them adapt to captive conditions for at least 1 week. Substrate selection experiments were conducted in four flow-regulated channels (5 m long × 0.30 m wide × 0.40 m high). Each channel was subdivided in four equal size sections and contained four different size substrate categories as those defined for the river (boulders, cobbles, gravel, and sand). The relative position of the substrates varied from channel to channel in a way that each substrate was placed in the four possible positions with respect to the water entrance (Fig. 2). These channels were placed outside and exposed to natural photoperiod. Four fish were introduced in each channel and left to acclimatize for 2 days before the experiment. Catfish density in each channel was 3.1 fish m−2, based on preliminary sampling data in Pichileufu River considering only those sampling units, where H. macraei was present. Both acclimation and the experiment were carried out in the absence of prey organisms to avoid the effect of non-random distribution of prey. The location of each fish was recorded eight times during the experiment, four times per day (i.e., dawn, midday, dusk, and midnight) during two consecutive days. As no statistical differences were detected between both days in substrate selection (see “Results”), data generated from the same group of experimental fish (n = 4) were pooled by time of the day. Thus, we investigated whether the utilization of different substrates was related to the time of the day during 24 h cycle. These experiments were performed four times, using a total of 64 individuals ranging from 4.2 to 10.5 cm SL (mean ± SD, 7.64 ± 1.44 cm), and from 0.36 to 6.09 g total mass (mean ± SD, 2.37 ± 1.20 g).
Experimental design used in substrate preference trials of H. macraei. a Dimensions of channels (Ch 1–4), substrate size and relative position of each substrate (1–4) to water flow (arrow) are indicated. 1 boulders (>180 mm), 2 cobbles (>64 and <180 mm), 3 gravel (>4 and <64 mm), and 4 sand (>1 and <4 mm). b Transversal channel section
Trials were performed during 2009. The first series of experiments were run from March 1 to 5, the second from April 12 to 16, the third from June 2 to 6, and the last from August 1 to 5. After 2 days acclimatization, each trial began with the dusk observation and finished with the mid-day observation, approximately 40 h after the experiment began (depending on the change in natural photoperiod). The experimental water supply came from the Chimehuín River (Neuquén Province) which has similar temperatures, mean water velocities, and mean depths to the Pichileufu River (Table 2). Water discharge of each channel during acclimatization and experiment was maintained constant at 0.2 l s−1.
Experimental channels were laterally covered with an opaque nylon of 2 m height to avoid fish disturbance. Before counting, each substrate was isolated using a mobile sluice that prevented fish moving from one section to another as a result of the presence of the observer. This sluice was removed after each observation. Substrate preference was evaluated as the percentage of H. macraei present in each substrate category.
Data analyses
The hypothesis that fish density vary along the river was analyzed using a non-parametric ANOVA, Kruskal–Wallis analysis, and Mann–Whitney test as the data were not normally distributed. A multiple comparison procedure (Dunn’s method) was used to detect differences between each pairwise comparison. For this analysis, we compared the seasonal samplings from Pilila and Corralito sites, and similar sampling dates from Pilcaniyeu (Table 1).
To test the hypothesis that microhabitat selection by H. macraei depends upon specific environmental parameters, which was performed using principal component analysis (PCA). The PCA was carried out to summarize the main habitat gradients in the Pichileufu River. Environmental variables were transformed for statistical analysis. Cover proportion variables were coded in a 0–5 semi-quantitative scale, where: 0, 0%; 1, 1–10%; 2, 11–25%; 3, 26–50%; 4, 51–75%, and 5, 76–100% (Clavero et al., 2009). Continuous variables were log10 (X + 1) transformed. Components that had eigenvalues >1 were retained (Grossman & Freeman, 1987). First, microhabitat characteristics of sites where H. macraei was present were compared with sites where it was absent. A one way ANOVA was conducted using the factor scores of the PCA. The three sampling sites on the Pichileufu River were introduced as co-variables in the analysis, as well as months, in order to analyze microhabitat variation in the whole river taking into account variation due to seasonal conditions (e.g., physicochemical variables).
To assess the dependence of H. macraei density, we evaluated different models using the information-theoretic approach (Burnham & Anderson, 2002). For this analysis only those areas with H. macraei presences (n = 127) were used. Density of each fishes species [i.e., H. macraei, creole perch Percichthys trucha (Valenciennes 1833), rainbow trout Oncorhynchus mykiss (Walbaum 1792), and brown trout Salmo trutta L. 1758] was previously transformed as log10 (density + 1). We constructed linear regression models by incorporating all possible combinations of the following independent variables: P. trucha, O. mykiss, and S. trutta densities; and the three main environmental gradients, principal component (PC) 1, PC2, and PC3 (see “Results”). We constructed individual global regression models with all variables. Models were fit to the data using linear regression and then the Akaike’s Information Criterion adjusted for small sample size (AICc; Burnham & Anderson, 2002; Johnson & Omland, 2004) was applied to evaluate the models. First, we calculated the difference between model with the lowest AICc and the other models as: ΔAICci = AICci − min AICc, where ΔAICci is the difference between the AICc of the best-fitting model and that of model i. AICci is AICc for model i, and min AICc is the minimum AICc value among all models. Then, we normalized the relative likelihood values as: w i = [exp (−0.5 ΔAICci)] [∑ exp (−0.5 ΔAICcn)]−1, where w i is known as Akaike weight for model i and the denominator is simply the sum of the relative likelihoods for all candidate models. Values of w i range from 0 (complete information loss) to 1 (no information loss). The w i can be interpreted as the probability that i is the best model, given the data and set of candidate models (Burnham & Anderson, 2002). We incorporated model selection uncertainty in analyses of the predictor variables of interpretable models (i.e., those with w i 10% of the best model) by calculating model-averaged estimates based on the unconditional variance of an estimate (Burnham & Anderson, 2002). We estimated the relative importance of predictor variables by summing their w i over all interpretable models. For interpretation, the precision of model-averaged parameter estimates were reported with the aid of 95% confidence intervals (CI) using a t statistic with n − 1 degrees of freedom.
Finally, a partial canonical correspondence analysis (CCA) was performed to identify the habitat preferences of each fish species present in Pichileufu River. In this direct gradient analysis (ter Braak & Šmilauer, 1998), the density of each fish species can be directly related to environmental data by performing both ordination and multiple regression techniques. Thus, CCA is used to explore species data in relation to the environmental data, and also the relationship among species. The species matrix considered in the CCA consisted of 165 sites (only sites with fish presence) × the log10 (density + 1) of 5 groups (H. macraei juveniles, H. macraei adults, P. trucha, O. mykiss, and S. trutta). Variables highly correlated (i.e., correlation level greater than 0.75), with high variance of inflation factor (VIF > 10) were removed from this analysis, such as mean water velocity at bottom and maximum water velocity in the water column. Environmental variables were selected employing manual forward selection, where significance of each variable was tested by Monte Carlo permutations (n = 499), and only those variables with P < 0.05 were retained (ter Braak & Šmilauer, 1998). The three sampling locations on Pichileufu River were incorporated as co-variables as well as the month of sampling. As advised by ter Braak & Šmilauer (1998), a biplot scaling and a downweighing of rare species were used in this analysis. Statistical analyses were performed using SPSS 15.0 and CANOCO 4.5.
Differences in H. macraei substrate preference during experiments were assessed using Mann–Whitney and Kruskal–Wallis tests as normality and homoscedasticity assumptions of the data failed. A multiple comparison procedure (Tukey test) was used to detect differences between each pairwise comparison.
Results
Fish assemblage variation along the river
Four fish species were captured during the sampling period, two native, H. macraei and P. trucha; and two introduced salmonids, O. mykiss and S. trutta. Fishes, other than H. macraei, were all juveniles within a size range from ~20 to ~100 mm SL. Fish assemblage varied along the longitudinal axis of the river (Fig. 3). Native species abundances varied among sampling sites (Kruskal–Wallis, H = 31.88, df = 2, P < 0.001), but not the abundance of salmonid species (Kruskal–Wallis, H = 4.91, df = 2, P > 0.05). Native species were less abundant at Pilila with 0.25 ± 0.59 fish m−2, and differed from Pilcaniyeu and Corralito (Dunn, P < 0.05, Table 1). Natives species were more abundant than salmonid species in Corralito (Mann–Whitney, U = 1,116.00, P < 0.001) and Pilcaniyeu (Mann–Whitney, U = 1,185.00, P < 0.001); however, in Pilila no difference was found between both groups (Mann–Whitney U = 4,388.50, P > 0.05, Table 1).
Intra-specific differences in density among sites were found for H. macraei (Kruskal–Wallis, H = 21.84, df = 2, P < 0.001) and P. trucha (Kruskal–Wallis, H = 9.11, df = 2, P < 0.05), but not for O. mykiss (Kruskal–Wallis, H = 5.16, df = 2, P > 0.05) and S. trutta (Kruskal–Wallis, H = 2.34, df = 2, P > 0.05). Both native species were more abundant in Pilcaniyeu, with a mean density 2.73 ± 4.61 and 0.31 ± 1.25 fish m−2 for H. macraei and P. trucha, respectively. A similar pattern was found in the percentage of occupancy (Table 1).
Microhabitat selection
The 17 environmental variables registered in 295 sampling units along the Pichileufu River showed a high degree of variation due to the great diversity of microhabitats sampled. Non-transformed data grouped by the presence or absence of H. macraei per sampling site are indicated in Table 3. The PCA including these environmental variables (transformed) produced six components (i.e., habitat gradients) that explained 73.2% of the total variance. PC1 (27% variance explained) represented a gradient from depositional to erosional habitats. Positive values on PC1 indicated high water velocity, with medium to large substrate and without silt, denoting more interstitial space. Negative values indicated depositional microhabitat with opposite characteristics. PC2 (13% variance explained) described mainly physicochemical water quality, such as pH, temperature, and dissolved oxygen. PC3 (11% variance explained) appeared to represent microhabitats in a depth gradient associated inversely to medium size particle substrates (i.e., gravel). The remaining components (PC4, PC5, and PC6) were not ecologically interpretable and therefore excluded from further analyses (Table 4). There was a significant difference between areas occupied and unoccupied by H. macraei along PC1 (ANOVA, F 1,158 = 54.01, P < 0.001) and PC2 (ANOVA, F 1,158 = 6.76, P < 0.01, Table 5). H. macraei utilized those habitats with fast water velocities, with more interstitial space and without submerged macrophytes. The occupied habitats were also characterized by low pH, low temperature, and by less proportion of cobbles (Table 4). Months incorporated as covariable proved to be significant for PC2 (ANCOVA, F 1,156 = 25.56, P < 0.001), and PC3 (ANCOVA, F 1,156 = 18.72, P < 0.001); similarly sampling site was significant for PC2 (ANCOVA, F 1,156 = 7.38, P < 0.05) and PC3 (ANCOVA, F 1,156 = 6.80, P < 0.05).
The information theory approach evidenced that the best significant fitting model to explain H. macraei density in Pichileufu River took into account PC1, PC2, and PC3, but not densities of the other fish species (Table 6). The best model had the greatest explanatory power, and was almost 3 times more likely to be true than when incorporating densities of the other fishes (Table 6). The components more explicative for the most plausible models were PC1 and PC2 based on their w i . Only environmental gradients (i.e., PC1, PC2, and PC3) estimates and model intercept did not include zero into the 95% CI (Table 7). In consequence, despite the fish density of other species was incorporated to plausible models, H. macraei density was better explained by environmental gradients, specifically PC1 and PC2 rather than the interaction with other fish species.
Eleven of a total of 17 environmental variables were retained in the final solution of the CCA. The covariables (month and site) included in the analysis explained 4.8% of the variation in the species data. The first CCA axis (eigenvalue = 0.394, 64.8% of the species-environment relationship) was primarily related to habitat features modeled by the water velocity. On the left (negative scores of x axis), habitats with fast water velocity were characterized by having different size of rocks, shallow waters, and a significant interstitial space among the substrate. On the right (positive scores), deeper habitats had very low water velocities in depositional zones with high proportion of fine sediment (silt) and submerged macrophytes. The second CCA axis (eigenvalue = 0.112, 18.3% of the species–environment relationship) was directly related with the presence of bedrocks, boulders, riparian vegetation, and depth. The availability of different microhabitats was similar among sampling localities (Fig. 4a).
Three groups of fishes were clustered by the CCA: H. macraei (juveniles and adults), P. trucha, and salmonids (O. mykiss and S. trutta) (Fig. 4b). H. macraei occupied habitats with high water velocity characterized by larger interstitial spaces among median to large size substrate. Differences between juveniles and adults H. macraei in their utilized habitat was negligible. A subtle difference in substrate size could be associated with fish size. Juveniles preferred cobbles and gravel while adults were more associated with boulders. With respect to depth preference, juveniles occupied shallower habitats than adults. Juvenile individuals of P. trucha were captured in depositional zones, where water velocity was the lowest. These habitats were characterized by submerged macrophytes rooted on a silty substrate or filamentous algae. The third group, exotic salmonids, was associated with bedrock zones where riparian trees were present. Salmonid sites were the deepest and had an intermediate water velocity (Fig. 4b).
Partial CCA relating a 165 sampling units from Corralito (white circle), Pilcaniyeu (gray circle), and Pilila (black circle); and b fish species (gray diamond) to environmental variables (arrows) in the Pichileufu River. Eleven variables were selected through a forward selection procedure from 17 original variables (see Table 4). The percentage of the explained variance of the species–environment relationship is indicated in each axis. Hm_ju Hatcheria macraei juveniles, Hm_ad H. macraei adults, Pt Percichthys trucha, Om Oncorhynchus mykiss, St Salmo trutta
Substrate experiments
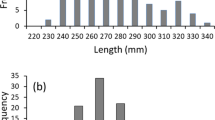
No differences were found between the first and second day of experiment in H. macraei substrate selection, for any series of experiments (Mann–Whitney, U > 100.00, P > 0.05). Thus, data from both days were pooled for later analyses. There were differences in substrate selection (Kruskal–Wallis, H = 126.20, df = 3, P < 0.001, n = 256). H. macraei showed a marked preference for substrates of large size. Boulder was significantly selected (mean ± SD, 52 ± 26) more than the other substrates followed by cobbles (35 ± 27) (Tukey, P < 0.05). Gravel (8 ± 17) and sand (6 ± 10) were scarcely selected and no difference were found between them (Tukey, P > 0.05). The same pattern was observed when analyzing the substrate preference within each of the four periods analyzed during the 24-h cycle (Kruskal–Wallis, 462.20 > H > 206.90, P < 0.001, n = 128). Both larger substrates were preferred than the two smaller ones (Fig. 5). Although there were some differences (Tukey, P < 0.05) in the substrate preference among positions with respect to water inflow, preference for larger substrates was maintained (Fig. 6).
Substrate selection of H. macraei measured as percentage of individuals present in each substrate. Median, quartiles and data outside 10th and 90th percentiles are indicated for boulders (white), cobbles (gray), gravel (white stripped), and sand (gray stripped). Significant differences between substrates within each time of the day (dawn, midday, dusk, and midnight) are indicated by different letters (Tukey, P < 0.05)
Substrate selection of H. macraei measured as percentage of individuals present in each substrate. Median, quartiles and data outside 10th and 90th percentiles are indicated for boulders (white), cobbles (gray), gravel (white stripped), and sand (gray stripped). Significant differences between substrates within each channel position with respect to water inflow (direction from 1 to 4) are indicated by different letters (Tukey, P < 0.05)
Discussion
Density of fish species was variable along the longitudinal axis of the Pichileufu River. Headwater zones were dominated by salmonids while native fishes were more abundant downstream. This pattern has been found in nearby rivers in Chile (Habit et al., 2007) and the central region of Argentina (Bistoni & Hued, 2002). Changes in richness, composition, and diversity of fish assemblages along the longitudinal river axis have been attributed to gradual changes of environmental variables (Ibanez et al., 2007; Kouamé et al., 2008; McGarvey, 2011). Headwater sections of a river are characterized by cold water temperature, fast water velocity, and substrate of large size. In downstream sections the flow is slow. In terms of food availability, lower values of aquatic invertebrate biomass (and density) have been recorded in the headwater than in downstream sections of Pichileufu River (Navone, 2006). Fish density showed the same pattern as invertebrates. Even in this river, where Pilila is located in headwater zone and both Pilcaniyeu and Corralito in a transition section between headwater and downstream sections, differences in fish assemblages were evident.
Both juvenile and adult H. macraei mostly inhabit microhabitats with faster water velocity and larger interstitial space among substrate, such as those present in riffles or runs. This agrees with Ringuelet et al. (1967), who said that H. macraei inhabits fast flowing well-oxygenated waters. This species, as do all Trichomycteridae catfishes, has a highly specialized morphological system for anchoring to the substratum (Adriaens et al., 2010). The presence of spines or odontodes in both opercular and interopercular bones helps H. macraei to remain fixed on the substrate in fast flowing waters. Furthermore, this morphological system permits an “elbowing move”, allowing this catfish to move between rocks (Adriaens et al., 2010). Also, H. macraei is negatively phototactic (Menni, 2004), and they use the interstitial space to rest or hide during daylight. Similar results were found for Trichomycterus corduvensis Weyenbergh 1877 by Hued & Bistoni (2006), where the microhabitats preferred were associated with faster water velocities from 0.465 to 0.650 m s−1 and substrates between 15 and 30 cm size. At odds, others related species of Trichomycterus, such as T. chapmani (Eigenmann 1912) and T. caliensis (Eigenmann 1912) from Andean streams of Colombia, occurred over sand and mud substrates with slow water flow (Chará et al., 2006).
The spatial niche segregation related to fish length, and probably with ontogeny, could be a frequent pattern found within Trichomycteridae. Species mentioned previously (i.e., T. corduvensis, T. chapmani, and T. caliensis), and also T. chiltoni (Eigenmann 1928) (Arratia, 1983), T. areolatus Valenciennes 1846 (Arratia, 1983; Manriquez et al., 1988), and T. maracaya Bockmann & Sazima 2004 prefer mostly shallow areas as juveniles, while adults choose deeper habitats. However, in H. macraei, differences between juveniles and adults microhabitat use were not strikingly different. However, small individuals did occupy gravel and cobbles while bigger catfish utilized boulders and deeper zones (Fig. 4). The main difference in H. macraei may primarily be between larvae and both juveniles and adults, with larvae inhabiting shallow marginal pools (<10 cm depth, Barriga & Battini, 2009). Larvae have not developed their swimming and feeding structures, thus restricting both their microhabitat and food use during early life of H. macraei.
By contrast, P. trucha was associated to low water velocity habitats, depositional zones with aquatic vegetation on silty substrates, i.e., macrophytes or filamentous algae. Juveniles of P. trucha were caught among vegetation probably because they used it as shelter. Similarly, juveniles of this species have been found mostly associated with macrophytes in the littoral zone of lakes (Lattuca et al., 2008). Other ecologically similar species, such as Perca flavescens (Mitchil 1814) (Fullerton & Lamberti, 2006) or Perca fluviatilis Linnaeus 1758 (Byström et al., 2003; Lewin et al., 2004) in the northern hemisphere, also use vegetated areas as an anti-predatory strategy during their early life stages (Snickars et al., 2004).
Exotic salmonids (O. mykiss and S. trutta) were mainly associated with bedrock zones and the presence of riparian trees. This pattern of trout occurrence was directly related to sampling locations, as the riparian vegetation increased from downstream (Corralito = 0%) to the headwaters (Pilila = 100%). Salmonid habitats were also the deepest, characterized by having an intermediate water velocity. It is widely known that both O. mykiss and S. trutta are drift feeders, preying on aquatic and terrestrial invertebrates (Bridcut, 2000; Baxter et al., 2004). Results would indicate that both salmonid species in Pichileufu River primarily utilized those areas where the terrestrial subsidies of the allochthonous prey would be higher, i.e., sites near to the tree canopy. Similarly, Kawaguchi et al. (2003) have found a direct relationship between riparian vegetation and salmonid abundance.
The AIC analysis always included PC1 and PC2 parameters in the more explicative models of H. macraei density, and with the highest explanatory power (Table 7). This, in agreement with the ANOVA results, points out that H. macraei utilized erosional habitats with low pH and temperature. Model of diurnal H. macraei density at microhabitat level was better explained by these environmental gradients rather than incorporating density of the other fishes. However, Penaluna et al. (2009) using a before–after control-impact manipulation in rivers of Chile, found a change of mesohabitat in the closely related catfish species T. areolatus due to the presence of salmonids. After both O. mykiss and S. trutta were excluded from experimental segments of the river, T. areolatus showed more preference for runs than riffles.
The experimental results showed that H. macraei preferred coarser substrates, larger than 6 cm. The selection was independent of the substrate position in the channel and the time of the day. This substrate selection pattern was not modified with low light intensities despite of the high swimming activity performed by H. macraei above the substrate during dusk and night (N. Espinós, unpublished data). Thus, the present results confirm the importance of substrate size and suggest that it was actively selected by H. macraei rather than a chance association. Other benthic stream fishes have also been associated with coarse substrates, such as Cottus spp., Etheostoma rufilineatum (Cope 1870), Percina sp. (Greenberg & Stiles 1993), and Catostomus santaanae (Snyder 1908) (Thompson et al., 2010). We should note that H. macraei utilize erosional sites (without fine sediment) with substantial interstitial space, places associated with fast waters. However, as this catfish lives among the substrate during daylight, water velocity is much lower than the mean water column velocity, even zero. Similarly Chun et al. (2011) found for Mylopharodon conocephalus (Baird & Girard 1854), O. mykiss, and Catostomus occidentalis Ayres 1854 that fish used the substrate to occupy locations with lower velocities than the average. Further experimental trials to test the importance of water velocity in the microhabitat selection are needed.
Substrate selection was not directly associated with food availability because the experiments were performed without aquatic invertebrates. However, taking into account the positive relationship between substrate size and invertebrate abundance found in nature (Velásquez & Miserendino, 2003; Brooks et al., 2005; Pan et al., 2011) we cannot rule out that the selectivity of larger substrate particles by H. macraei may be used as a cue for finding prey. Prey abundance has been frequently related to microhabitat selection by small benthic stream fishes [e.g., Cottus sp. (Grossman et al., 2006), Rhinichthys cataractae (Valenciennes 1842) (Thompson et al. 2001), Etheostoma olmstedi Storer 1842 (Henry & Grossman, 2007) or Trichomycterus sp. (Chará et al., 2006)]. Furthermore, due to highly heterogeneous conditions within streams prey availability is patchily distributed. In this context, territorial behavior adds complexity to models of fish distribution because the patch utilization is strongly influenced by the intra-specific individual hierarchy (Petty & Grossman, 2007). Nevertheless, H. macraei has not been characterized as a territorial fish (Arratia & Menu-Marque, 1981). In this sense, within the dichotomy of the ideal free or ideal pre-emptive distributions (see Petty & Grossman, 2010) the habitat use of this catfish should be better predicted by the former model. Future works must be focused on the effect of food distribution on microhabitat choice of this stream benthic catfish.
Conclusion
This study provides the first quantifiable baseline information of microhabitat use of H. macraei. Our results highlight the importance of erosional zones with high water velocity, large substrates, and suitable interstitial space in the microhabitat selection of this species. The microhabitat use during daytime in the Pichileufu River is strongly influenced by the innate hiding behavior of this species in suitable shelters. This response could be an adaptive behavior not only for diminishing predation risk but also to increase the chance of encountering prey. The association with coarse substrate in lotic systems (i.e., runs and riffles) suggests that H. macraei may be vulnerable in situations of loss of this habitat type, such as river damming. Furthering the knowledge of microhabitat preferences will increase the ability to protect habitat for H. macraei populations and will allow the development of management strategies to improve the conservation status of the species.
References
Adriaens, D., J. N. Baskin & H. Coppens, 2010. Evolutionary morphology of trichomycterid catfishes: about hanging on and digging in. In Nelson, J. S., H.-P. Schultze & M. V. H. Wilson (eds), Origin and Phylogenetic Interrelationships of Teleosts. Verlag Dr. Friedrich Pfeil, Munich: 337–362.
Arratia, G., 1983. Preferencias de habitat de peces Siluriformes de aguas continentales de Chile (Fam. Diplomystidae y Trichomycteridae). Studies on Neotropical Fauna and Environment 18: 217–237.
Arratia, G. & S. Menu-Marque, 1981. Revision of the freshwater catfishes of the genus Hatcheria (Siluriformes, Trichomycteridae) with commentaries on ecology and biogeography. Zoologischer Anzeiger 207: 88–111.
Balon, E. K., 1990. Epigenesis of an epigeneticist: the development of some alternative concepts on the early ontogeny and evolution of fishes. Guelph Ichthyology Reviews 1: 1–48.
Barriga, J. P. & M. A. Battini, 2009. Ecological significances of ontogenetic shifts in the stream-dwelling catfish, Hatcheria macraei (Siluriformes, Trichomycteridae), in a Patagonian river. Ecology of Freshwater Fish 18: 395–405.
Baxter, C. V., K. D. Fausch, M. Murakami & P. L. Chapman, 2004. Fish invasion restructures stream and forest food webs by interrupting reciprocal prey subsidies. Ecology 85: 2656–2663.
Bistoni, M. A. & A. C. Hued, 2002. Patterns of fish species richness in rivers of the central region of Argentina. Brazilian Journal of Biology 62: 753–764.
Bridcut, E. E., 2000. A study of terrestrial and aerial macroinvertebrates on river banks and their contribution to drifting fauna and Salmonid diets in a Scottish catchment. Hydrobiologia 427: 83–100.
Brooks, A. J., T. Haeusler, I. Reinfelds & S. Williams, 2005. Hydraulic microhabitats and the distribution of macroinvertebrate assemblages in riffles. Freshwater Biology 50: 331–344.
Burnham, K. P. & D. R. Anderson, 2002. Model Selection and Inference: A Practical Information—Theoretic Approach. Second edition. Springer, New York.
Byström, P., L. Persson, E. Wahlström & E. Westman, 2003. Size- and density-dependent habitat use in predators: consequences for habitat shifts in young fish. Journal of Animal Ecology 72: 156–168.
Chará, J. D., D. J. Baird, T. C. Telfer & E. A. Rubio, 2006. Feeding ecology and habitat preferences of the catfish genus Trichomycterus in low-order streams of the Colombian Andes. Journal of Fish Biology 68: 1026–1040.
Chun, S. N., S. A. Cocherell, D. E. Cocherell, J. B. Miranda, G. J. Jones, J. Graham, A. P. Klimley, L. C. Thompson & J. J. Cech Jr, 2011. Displacement, velocity preference, and substrate use of three native California stream fishes in simulated pulsed flows. Environmental Biology of Fishes 90: 43–52.
Clavero, M., F. Blanco-Garrido, L. Zamora & J. Prenda, 2005. Size-related and diel variations in microhabitat use of three endangered small fishes in a Mediterranean coastal stream. Journal of Fish Biology 67: 72–85.
Clavero, M., Q. Pou-Rovira & L. Zamora, 2009. Biology and habitat use of three-spined stickleback (Gasterosteus aculeatus) in intermittent Mediterranean streams. Ecology of Freshwater Fish 18: 550–559.
Di Prinzio, C. Y. & R. J. Casaux, 2012. Dietary overlap among native and non-native fish in Patagonian low-order streams. International Journal of Limnology 48: 21–30.
Dixon, C. J. & J. C. Vokoun, 2009. Burbot resource selection in small streams near the southern extent of the species range. Ecology of Freshwater Fish 18: 234–246.
Fullerton, A. H. & G. A. Lamberti, 2006. A comparison of habitat use and habitat-specific feeding efficiency by Eurasian ruffe (Gymnocephalus cernuus) and yellow perch (Perca flavescens). Ecology of Freshwater Fish 15: 1–9.
Grossman, G. D. & M. C. Freeman, 1987. Microhabitat use in a stream fish assemblage. Journal of Zoology 212: 151–176.
Grossman, G. D., R. E. Ratajczak Jr., J. T. Petty, M. D. Hunter & G. Grenouillet, 2006. Population dynamics of mottled sculpin in a variable environment: an information theoretic approach. Ecological Monographs 76: 217–234.
Habit, E., M. Belk, P. Victoriano & E. Jaque, 2007. Spatio-temporal distribution patterns and conservation of fish assemblages in a Chilean coastal river. Biodiversity and Conservation 16: 3179–3191.
Henry, B. E. & G. D. Grossman, 2007. Microhabitat use by blackbanded (Percina nigrofasciata), turquoise (Etheostoma inscriptum), and tessellated (E. olmstedi) darters during drought in a Georgia piedmont stream. Environmental Biology of Fishes 83: 171–182.
Hesthagen, T. & J. Heggenes, 2003. Competitive habitat displacement of brown trout by Siberian sculpin: the role of size and density. Journal of Fish Biology 62: 222–236.
Hlohowskyj, I. & T. E. Wissing, 1986. Substrate selection by faintail (Etheostoma flabellare), greenside (E. blennioides), and rainbow (E. caeruleum) darters. The Ohio Journal of Science 86: 124–129.
Hued, A. & M. A. Bistoni, 2006. Microhabitat use by two silurid species in the Anizacate River (central Argentina). Folia Zoologica 55: 175–182.
Ibanez, C., T. Oberdorff, G. Teugels, V. Mamononekene, S. Lavoué, Y. Fermon, D. Paugy & A. K. Toham, 2007. Fish assemblages structure and function along environmental gradients in rivers of Gabon (Africa). Ecology of Freshwater Fish 16: 315–334.
Johnson, J. B. & K. S. Omland, 2004. Model selection in ecology and evolution. Trends in Ecology & Evolution 19: 101–108.
Kawaguchi, Y., Y. Taniguchi & S. Nakano, 2003. Terrestrial invertebrate input determine the local abundance of stream fishes in a forested stream. Ecology 84: 701–708.
Kouamé, K. A., S. S. Yao, G. G. Bi, E. P. Kouamélan, V. N’Douba & N. J. Kouassi, 2008. Influential environmental gradients and patterns of fish assemblages in a West African basin. Hydrobiologia 603: 159–169.
Lattuca, M. E., M. A. Battini & J. P. Macchi, 2008. Trophic interactions among native and introduced fishes in a northern Patagonian oligotrophic lake. Journal of Fish Biology 72: 1306–1320.
Lewin, W.-C., N. Okun & T. Mehner, 2004. Determinants of the distribution of juvenile fish in the littoral area of a shallow lake. Freshwater Biology 49: 410–424.
Maddock, I., 1999. The importance of physical habitat assessment for evaluating river health. Freshwater Biology 41: 373–391.
Manriquez, A., L. Huaquin, M. Arellano & G. Arratia, 1988. Aspectos reproductivos de Trichomycterus areolatus Valenciennes, 1846 (Pisces: Teleostei: Siluriformes) en Río Angostura, Chile. Studies on Neotropical Fauna and Environment 23: 89–102.
McGarvey, D. J., 2011. Quantifying ichthyofaunal zonation and species richness along a 2800-km reach of the Rio Chama and Rio Grande (USA). Ecology of Freshwater Fish 20: 231–242.
McIntosh, A. R., C. R. Townsend & T. A. Crowl, 1992. Competition for space between introduced Brown trout (Salmo trutta L.) and a native galaxiid (Galaxias vulgaris Stokell) in a New-Zealand stream. Journal of Fish Biology 41: 63–81.
Menni, R. C., 2004. Peces & ambientes en la Argentina continental. Monografías del Museo Argentino de Ciencias Naturales 5: 1–316.
Navone, G., 2006. Distribución y uso de hábitat de la ictiofauna en el río Pichi leufu. Lincenciate Thesis. Universidad Nacional del Comahue.
Onoda, Y., A. Maruyama, Y. Kohmatsu & M. Yuma, 2009. The relative importance of substrate conditions as microhabitat determinants of a riverine benthic goby, Rhinogobius sp. OR (orange form) in runs. Limnology 10: 57–61.
Pan, B., Z. Wang, M. Xu & L. Xing, 2011. Relation between stream habitat conditions and macroinvertebrate assemblages in three Chinese rivers. Quaternary International. doi:10.1016/j.quaint.2011.06.008.
Pascual, M. A., V. E. Cussac, B. Dyer, D. Soto, P. Vigliano, S. Ortubay & P. Macchi, 2007. Freshwater fishes of Patagonia in the 21st century after a hundred years of human settlement, species introductions, and environmental change. Aquatic Ecosystem Health & Management 10: 1–16.
Penaluna, B. E., I. Arismendi & D. Soto, 2009. Evidence of interactive segregation between introduced trout and native fishes in Northern Patagonian Rivers, Chile. Transactions of the American Fisheries Society 138: 839–845.
Petty, T. & G. Grossman, 1996. Patch selection by mottled sculpin (Pisces: Cottidae) in a southern Appalachian stream. Freshwater Biology 35: 261–276.
Petty, T. & G. Grossman, 2007. Size-dependent territoriality of mottled sculpin in a southern Appalachian stream. Transactions of the American Fisheries Society 136: 1750–1761.
Petty, T. & G. Grossman, 2010. Giving-up densities and ideal pre-emptive patch use in a predatory benthic stream fish. Freshwater Biology 55: 780–793.
Ringuelet, R. A., R. H. Arámburu & M. A. de Arámburu, 1967. Los peces argentinos de agua dulce. La Plata, Argentina, Comisión de Investigación Científica de la Provincia de Buenos Aires: 602.
Snickars, M., A. Sandström & J. Mattila, 2004. Antipredator behaviour of 0 + year Perca fluviatilis: effect of vegetation density and turbidity. Journal of Fish Biology 65: 1604–1613.
ter Braak, C. J. F. & P. Šmilauer, 1998. CANOCO Reference manual and user’s guide to Canoco for Windows: software for Canonical Community Ordination (version 4). Ithaca: Microcomputer Power.
Thompson, A. R., J. T. Petty & G. D. Grossman, 2001. Multi-scale effects of resource patchiness on foraging behaviour and habitat use by longnose dace, Rhinichthys cataractae. Freshwater Biology 46: 145–161.
Thompson, A. R., J. N. Baskin, C. C. Swift, T. R. Haglund & R. J. Nagel, 2010. Influence of habitat dynamics on the distribution and abundance of the federally threatened Santa Ana Sucker, Catostomus santaanae, in the Santa Ana River. Environmental Biology of Fishes 87: 321–332.
Tyler, J. A. & D. P. Clapp, 1995. Perceptual constraints on stream fish habitat selection: effects of food availability and water velocity. Ecology of Freshwater Fish 4: 9–16.
Unmack, P. J., E. M. Habit & J. B. Johnson, 2009. New records of Hatcheria macraei (siluriformes, trichomycteridae) from Chilean province. Gayana 73: 102–110.
Unmack, P. J., J. P. Barriga, M. A. Battini, E. M. Habit & J. B. Johnson, 2012. Phylogeography of the catfish Hatcheria macraei reveals a negligible role of drainage divides in structuring populations. Molecular Ecology 21: 942–959.
Van Liefferinge, C., P. Seeuws, P. Meire & R. F. Verheyen, 2005. Microhabitat use and preferences of the endangered Cottus gobio in the River Voer, Belgium. Journal of Fish Biology 67: 897–909.
Velásquez, S. M. & M. L. Miserendino, 2003. Habitat type and macroinvertebrate assemblages in low order Patagonian streams. Archiv Für Hydrobiologie 158: 461–483.
Wootton, R. J., 1998. Ecology of Teleost Fishes. Kluwer Academic Publishers, London.
Acknowledgments
We thank CEAN for allowing us to use the facilities for the experimental work, and Dirección de Pesca Continental of the Río Negro Province for permission to collect native fishes. We are grateful to P. J. Unmack for comments, suggestions, and detailed revision of our English. We also greatly appreciate the field work assistance of J. Allison, J. C. Barriga, L. Battini, N. Battini, J. Chawarski, C. González-Minguez, J. Hill, and J. Smith. The manuscript benefited from the comments and suggestions from the anonymous reviewers. This study was funded by Agencia Nacional de Promoción Científica y Tecnológica, Argentina (ANPCyT, PICT No. 262), by Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina (CONICET, PIP No. 282) and by the US National Science Foundation (NSF) PIRE program (OISE 0530267) for collaborative research on Patagonian Biodiversity to support collaboration among the following institutions (listed alphabetically): Brigham Young University, Centro Nacional Patagónico, Dalhousie University, Instituto Botánico Darwinion, Universidad Austral de Chile, Universidad Nacional del Comahue, Universidad de Concepción, and University of Nebraska.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: M. Power
Rights and permissions
About this article
Cite this article
Barriga, J.P., Espinós, N.A., Chiarello-Sosa, J.M. et al. The importance of substrate size and interstitial space in the microhabitat selection by the stream-dwelling catfish Hatcheria macraei (Actinopterygii, Trichomycteridae). Hydrobiologia 705, 191–206 (2013). https://doi.org/10.1007/s10750-012-1398-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1398-0