Abstract
We inventoried 10 ha of late-successional and seasonally inundated black-water floodplain (igapó) forest along four river sections of the Negro River, Central Amazonia, Brazil. The aim of the study was to test if tree species composition and diversity changes along the river course, and whether these changes reflect the different geological formations of the Negro River. On a continental-wide scale, we assessed alpha-diversity patterns of black-water flooded forests across the Amazon and Orinoco basins. Phytosociological analyses include family and species importance, species similarity, and Fisher’s alpha-diversity, as well as Detrended Correspondence Analysis. A total of 6.126 individuals were recorded, belonging to 243 tree species. Only few tree species occurred in more than one river section, and floristic composition changed abruptly from one section to the other. Tree species richness ranged from 57 to 79 species ha−1, and alpha-diversity was highest (27.24) in the lower river section upon sediments of Pliocene–Pleistocene origin. We found a gradual decrease in species diversity with increasing age of the geological formations. The igapó forest is relatively species-poor, which we interpret to be the result of general low nutrient availability in alluvial substrates of the Negro River.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Negro River flows 2,500 km from its headwaters in Colombia before discharging into the Solimões River near the city of Manaus (Goulding et al., 2003). It incorporates the two largest fluvial archipelagos in the world, Mariuá and Anavilhanas located in the middle and lower reaches of the river, respectively. Inundation forests (igapó) occur throughout the main river channel and most of its tributaries. These are considered the largest nutrient-poor black-water forests of the Amazon basin (Montero, 2011).
The waters of the Negro River carry low levels of suspended matter, but contain a high concentration of humic acids that characterize the distinctive dark brownish color (Sioli, 1956). The water pH values range from 3.8 to 5, depending on the site and time of the year (Furch, 1997; Junk et al., 2011). The floodplains of the black-water rivers are characterized by impoverished substrates and low primary productivity (Furch, 1997). Due to seasonal variation in rainfall and generally flat topography of the majority of the catchment area, the Negro River is governed by a predictable, monomodal flood-pulse (Junk et al., 2011).
Flood amplitudes range from 3.6 m at the upper reaches to 9.3 m near the lower reaches of the river (Agência Nacional de Águas—ANA) and subject the floodplain vegetation to seasonal flooding, lasting from 50 to 230 days per year (Junk, 1989). The variable flooding regime and differences in water and sediment chemistry determine tree species composition and diversity patterns along the river channel and are considered important factors influencing the spatial distribution of tree communities (Junk et al., 1989; Rosales et al., 1999; Wittmann et al., 2010; Junk et al., 2011).
To document and understand floristic patterns of forests across landscape scales, inventories usually consist of either long transects (Duque et al., 2003; Tuomisto et al., 2003) or on series of scattered plots (ter Steege et al., 2006; Pitman et al., 2008; Toledo et al., 2010) and record either just location (i.e., longitude, latitude) or environmental variables (i.e., precipitation, nutrients). This approach allows researchers to determine if changes in floristic components (i.e., species composition and richness) or in evolutionary traits (e.g., seed dispersal mechanisms, growth rates, etc.) are related to environmental gradients and niche characteristics at each site. Using this approach, several studies along riverine systems along temperate forests (e.g., Sabo et al., 2005) and Amazonian floodplain forest have described high spatial heterogeneity across riverbanks (Rosales et al., 1999; Wittmann et al., 2006; Albernaz et al., 2012).
The assemblages of biological communities along river-floodplain systems may be explained by the flood pulse concept (Junk et al., 1989). These authors argued that the major driving force affecting the biota and biogeochemical processes within a floodplain is the pulsing of rivers, which result in defined wet and dry periods. Thus, the flood pulse determines species composition and diversity patterns along floodplains and is a decisive factor influencing the spatial distribution of tree communities (Rosales et al., 1999; Wittmann et al., 2010). Most studies in seasonally flooded Amazonian forests have been carried out along white-water rivers (várzea forests sensu Prance, 1979) and have focused on changes in species composition and diversity patterns along vertical gradients (Worbes, 1997; Ferreira, 2000; Rosales et al., 2001; Wittmann et al., 2002; Piedade et al., 2005) rather than spatial gradients along river corridors. Wittmann et al. (2006) provided an extensive dataset showing gradients of species diversity across multiple sites in Amazonian várzea forests. The few studies emphasizing spatial gradients of floristic variation across river corridors found abrupt changes in species composition (Rodrigues, 2007; Rosales et al., 2001; Albernaz et al., 2012).
Despite the considerable extent of the Negro River, only few quantitative floristic inventories have been carried out in igapó forests (Keel & Prance, 1979; Revilla, 1981; Worbes, 1986; Parolin et al., 2003; Parolin et al., 2004; Ferreira & Almeida, 2005a, b; Piedade et al., 2005 and Scudeller & de Souza, 2009). Most of these studies concentrated on the lower Negro River, and inventoried small plots (<0.5 ha). Exceptions are the forest inventories at the National Park Jaú located approximately 200 km NW of Manaus conducted by Ferreira (1997, 2000) and Ferreira & Stohlgren (1999), who analyzed the effect of flooding on igapó forest of different habitats using three 1-ha plots. Overall, less than 10 ha of floristic inventories are available for igapó forests along the Negro River. Hence, there are still important gaps in the floristic data coverage, especially with regard to the middle and remote upper sections of the Negro River.
In the following, we present a quantitative floristic inventory of a total area of 10 ha in late-successional igapó forest distributed across a 600-km long corridor of the Negro River in Brazil. The aim of this study is to describe the tree species composition, species richness, and diversity at each site and to investigate how these parameters vary along the river course. In addition, supported by available literature, we assess continental-wide scale alpha-diversity patterns of black-water seasonally flooded forests across the Amazon and Orinoco basins. Specifically, we address the following questions: (a) How do tree species composition, species richness, and diversity vary within and between sites? (b) Are there alpha-diversity gradients of igapó forests across geographical locations and geological formations?
Methods
Study area
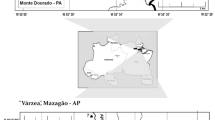
We focused our study on four areas along a 600-km stretch of the Negro River in the Brazilian Amazon, running from the city of Santa Isabel to the Anavilhanas: (1) Santa Isabel (ST, upper section, 00º27′S, 64º46′W), (2) Barcelos (BA, middle section 00°38′S, 63°15′W), (3) the northern tributary lower Jufaris River (JU, middle section, 00°46′S, 62°29′W), and (4) Anavilhanas (AN, lower section, 02°46′S, 60°45′W, lower section) (Fig. 1).
Annual rainfall in the Brazilian part of the Negro River basin averages 2000–2,200 mm, with a maximum rainfall of over 3,000 mm in the upper section. Mean monthly temperatures vary little over the year and range between 25 and 28°C (Sombroek, 2000). Due to the general high rainfall along the upper Negro River and in its large catchment with an area of approximately 700,000 km2 (Latrubesse, 2008), the water levels of the Negro River undergo seasonal variations, with flood amplitudes ranging from 3.6 m (upper Negro) to 9.2 m (lower Negro) (Fig. 2). While the flood pulse in the lower section is strongly monomodal, with highest water levels occurring from May–August, it is slightly bimodal in the upper section, where a second, smaller high-water period frequently occurs from February–April (Fig. 2).
The substrates in ST, BA, and also along the lower tributary JU consist mostly of nutrient-poor sand which originates from the western extension of the Guyana Shield, and the alluvial landscape is characterized by rocky islands and the presence of granite inselbergs close to the river channel (Goulding et al., 1996; Latrubesse & Franzinelli, 2005). The alluvial substrate in the lower section is influenced by the Branco River with its source located in the northern Roraima massif, transporting silt and clayish sediments to the Negro. Below the confluence of the Branco and Negro Rivers, the Anavilhanas archipelago has been formed from substrates characterized by intermediate fertility (Junk et al., 2011). This complex and dynamic network of islands acts as a fine sediment trap and is interpreted as the result of relatively recent, Holocene deposition (Irion et al., 2010).
Floristic inventories
Field work was carried out between September 2008 and March 2010 during the low-water periods. In the four study areas, we established 160 quadratic 25 × 25 m (625 m2) plots in late-successional igapó forest totaling an area of 10 ha: (1) Santa Isabel (ST, 48 plots), (2) Barcelos (BA, 36 plots), (3) Jufaris (JU, 28 plots) and Anavilhanas (AN, 48 plots). Due to varying accessibility, the number of plots at each study area differed.
In ST, BA, and AN, we distributed the plots among three sites (e.g., ST1, ST2, and ST3), while in JU plots were distributed to two sites (Fig. 1). There were a maximum of 16 plots established per site, corresponding to an area of 1 ha. The upper and lower areas are separated by approximately 600 km, whereas within areas, the sites are separated by a distance of between 5 and 50 km.
All trees ≥10 cm diameter at breast height (dbh) were labeled, numbered and their dbh measured. Species were identified in the field, and when this was not possible, identified provisionally as morpho-species with voucher specimens collected for later identification at the herbarium of the National Institute for Amazon Research—INPA, Manaus.
Data analysis
To summarize the spatial heterogeneity of community composition, we performed a Detrended Correspondence Analysis (DCA) on tree species abundance (Jongman et al., 1995). The DCA permits evaluation of beta diversity in terms of standard deviation (length of the gradient) (Duivenvoorden & Lips, 1998; Svenning et al., 2004) and is recommended in the absence of abiotic parameters (Jongman et al., 1995). Analyses were performed using (1) the entire dataset, (2) separately in each of the four sites, and (3) excluding the JU plots to assess the influence of the Jufaris River’s species pool on the whole tree community. The log-transformed number of individuals per plot was used as a measure of abundance. In order to avoid the over-proportional influence of rare species, only species that occurred in at least two plots were included in the DCA.
The floristic composition was evaluated by examining species and family importance using the Importance Value Index (IVI, Curtis & McIntosh, 1951) and the Family Importance Value (FIV, Mori et al., 1983), which summarizes relative species (or family) density, dominance, and frequency. Importance values are not influenced either by large numbers of small trees and unequal individual and species numbers per plot (McCune & Grace, 2002; Wittmann et al., 2006).
Species richness and Fisher’s alpha-diversity coefficient (Fisher et al., 1943) were calculated for each plot and river section. Fisher’s index is widely used and is relatively insensitive to sample size thus performing well on tropical forest plots (ter Steege et al., 2000; Chave, 2008). Differences of mean values were compared using the Welch F-test and a post-hoc pair-wise comparison using Tukey test. The spatial variation of richness along the river course was investigated by performing a species-area curve. The curve expresses the number of new species in each plot from the lower to the upper river section against the cumulative area of those plots. Total species richness was estimated using the Bootstrap incidence-based and Chao 1 abundance-based richness estimators (Magurran, 2004; Chao et al., 2005).
The degree of floristic similarity and shared species between river sections and plots was examined by calculating the Chao-Sørensen index for raw data (Chao et al., 2005). This index is based on abundance information and it is appropriate and recommended for data from rapid inventories because it is able more precisely to assess compositional similarity between sites that were under-sampled and/or contain many rare species (Chao et al., 2005).
The diversity gradients along geographical regions and geological zones were assessed by plotting Fisher’s alpha coefficient against plot location. Thus, in addition to our results, we compiled available literature on black-water inundation forest across the Amazon and Orinoco basins for which we calculated Fisher’s coefficient (Online Resource 1). The plots were classified as those located in equatorial western Amazonia (WAe), southern western Amazonia (WAs), central Amazonia (CA), northern Amazonia (NA), Orinoco basin (OR), and eastern Amazonia (EA). Similarly, to evaluate the relationship between alpha-diversity and geological zones, we overlapped Fisher’s alpha coefficient of each plot over the geological zone in which they occurred. Thus, following the geological map of the Amazon basins by Irion & Morais (2011), plots were sorted in three geological formations (Pre-Cambrian, Tertiary, and Pleistocene-Pliocene) representing different bedrock ages. Differences in mean values were compared by using Kruskall–Wallis and t test. Pair-wise comparisons were further assessed using the Mann–Whitney U test.
Species richness and diversity were computed with the program PAST v. 2.04 “Paleontological Statistics Software Package for Education and Data Analysis” (http://palaeoelectronica.org/2001_1/past/issue1_01.htm) (Hammer et al., 2001). The number of shared species and similarity matrix was processed using EstimateS (Version 8.2, R. K. Colwell, 2009, http://purl.oclc.org/estimates). Ordination analysis (DCA) was performed using the software package PC-ORD version 5.0 (McCune & Mefford 1999). All statistical analyses were carried out with SPSS 16.0 and PAST v.2.04.
Results
A total of 6.126 trees were recorded, belonging to 243 species, 136 genera, and 48 families. At the plot level (625 m2), the results showed a high variability in families (3–17), genera (5–24), and species (5–25). Values averaged to 1-ha scale showed 612 individuals ha−1, 63 species ha−1, 28 families ha−1, and a basal area of 31.83 m2 ha−1.
Floristic gradients
The first axis of the DCA (67 % of explained variation) using the entire dataset shows a length of gradient of 5.44 sd of species turnover (Online Resource 2). There are few species shared by both ends of the gradient, suggesting high beta-diversity (Hill 1979). Thus, JU and BA have few species in common and occupy the extremes of axis 1 (Fig. 3a). When JU plots were excluded, the gradient length of the first axis (64 % of explained variation) decreased to 4.16 sd (Fig. 3b, Online Resource 2). In both scatter plots, the second axes have long length of gradients with 5.87 and 4.78 standard deviation units, respectively (Fig. 3).
Looking at each river section separately, the DCA shows that the JU and BA exert the highest variation, thus increasing beta-diversity of the whole dataset. BA had a gradient length of 4.33 and ST and AN of 2.78 and 2.38, respectively, thus reflecting lower beta-diversity (Online Resource 2). The floristic discontinuity at the middle reach of the river as showed by the DCA is confirmed with the species-area curve (Fig. 4), which shows an abrupt change in species numbers. This abruptness is due to the progressive addition of new species from JU plots (plots 85–112) and especially plots 81–84 at BA. However, the relative position of the species-curve at the end of AN and ST sections tends to stabilize; indicating a low shift in floristic composition.
Preliminary ordinations showed that plots BA 81–BA 84 at Barcelos and plots JU 17–JU 28 at the Jufaris River had outlying position due to divergent tree species composition; therefore, these plots were excluded in subsequent analyses.
Floristic composition
A complete list of all recorded species and their respective families organized by river sections is given in Online Resource 3. Most important species in each river section are presented in Table 1. Overall, Fabaceae was the most important family, which along with Lecythidaceae, Chrysobalanaceae, Euphorbiaceae, and Sapotaceae accounted for >50 % of total importance. The most species-rich genera were Swartzia (11 species), Pouteria (8 species), Eschweilera (7 species), Ocotea (6 species), and Ormosia and Zygia (5 species each). These genera accounted for 14.2 % of all recorded species.
The importance value index (IVI) of the species indicated a high floristic variation between river sections (Table 1). Only few species, including Gustavia augusta L., Heterostemon mimosoides Desf., Hevea brasiliensis (Willd. ex A. Juss.) Müll. Arg., and Aldina heterophylla Spruce ex Benth. occurred in more than one river section. The floristic composition at JU was distinct. The most important species was Mouriri angulicosta Morley, followed by Sclerolobium chrysophyllum Poepp. and Sacoglottis guianensis Benth. These species accounted for 16.38 % of the importance, but did not occur in the other river sections.
Tree species richness and diversity
Species richness estimations for the whole dataset are presented in Fig. 4. The estimators Bootstrap (incidence data) and Chao 1 (abundance data) accounted for 263 and 255 species, indicating that the sampled species represent 92 % and 95 % of the estimated species richness, respectively. Both richness estimators reached an asymptote at 250 species representing an inflection at an area of 6 ha which corresponds to 96 plots (Fig. 4).
Comparing species richness between river sections the Welch F test shows a significant difference (F = 7.46, P < 0.001). A Welch F test (F = 7.52, P < 0.001) and the post hoc pair-wise comparison (Tukey test) detected significant differences between BA compared with JU (P ≪ 0.001) and AN (P = 0.007). The Tukey test further revealed highly significant differences between the site AN 2 compared to ST 2, BA 1, BA 2, and AN 1 (all with P < 0.001). Although both JU and BA plots are located in the same river section the Tukey post hoc test revealed significant differences in species richness between these sites (JU compared with BA 1 P = 0.03; BA 2, P = 0.02). There were no statistically significant differences between ST and BA plots (P > 0.05).
Comparing alpha diversity between river sections we found a statistically significant difference (Welch test, F = 6.28, P < 0.001). A Tukey post hoc test revealed significant differences between JU and ST (P = 0.01) and especially between JU and AN (P = 0.001). However, values did not differ significantly between ST and AN (P = 0.88). When performing intra-site comparison the Welch F test detected significant differences (F = 9.42, P < 0.001). However, pair-wise comparisons determined by the Tukey post hoc test revealed that except for ST 1 (P = 0.198), the ST 2 and AN 1 sites presented a significant difference compared with the other sites (P < 0.001). These two sites have the highest diversity values averaged per hectare (as indicated by the Fisher’s alpha coefficient, Table 2).
For better comparisons species richness and alpha-diversity were standardized to 1 ha (Table 2). Thus, at this level, species richness did not show much variation; values ranged from 57 species ha−1 at BA 2 and JU to 79 species ha−1 at AN 1 (Table 2). The average value for the whole sample was 63 tree species ha−1. The Fisher’s alpha coefficient confirmed that AN 1 and ST 2 were overall the most diverse (P < 0.001) (Table 2). Although species composition was highly variable between river sections, species richness, and alpha-diversity at 1-ha scale was nearly constant, with a slight increase in the upper and lower sections (ST and AN, respectively).
Floristic resemblance between river sections
Floristic similarity (Chao-Sørensen) between river sections averaged 0.35 (0.26–0.63). AN and ST were the most similar forest communities (63 % of similarity, 49 shared species), whereas JU and AN were less similar (26 % of similarity, 14 shared species) (Table 3). JU was the most distinct forest community. At this site, the average floristic similarity between plots amounted to 0.24 (range 0.19–0.42).
The floristic resemblance within river sections showed high similarity. Thus, at AN, BA, and ST similarity averaged 62 % (35 species) (Table 3). These findings suggest that geographical distance may be an important factor determining floristic similarity within river sections. However, we also found that closer sites are not always more floristically similar. There is less floristic similarity between JU and BA, which are ca. 100 km apart (35 % of similarity, 12 shared species) than similarity between AN 1 and ST 2 (51 % of similarity, 27 shared species) which are located approximately 600 km apart.
Diversity gradients of igapó across geographical regions and geological zones
When quantifying spatial gradients of alpha-diversity by comparing Fisher’s alpha of all igapó studies, the Kruskal–Wallis test was not significant (P = 0.06). Nevertheless, pair-wise comparisons (Mann–Whitney test) indicated significant differences between some regions (Fig. 5). Highly significant difference (P = 0.01) was found between plots in Southwestern Amazonia (n: 5, WAs) and northern Amazonia (n: 5, NA).
Fisher’s alpha diversity in different regions across the Amazon. WAs southern western Amazonia, WAe equatorial western Amazonia, CA central Amazonia, OR Orinoco basin, NA northern Amazonia and EA eastern Amazonia. The Mann–Whitney test found highly significant difference between WAs and NA (P value = 0.01). The OR and WAe are also significant different
Although alpha-diversity among the three geological formations was different, it was not significant (P = 0.30). Mean values of Fisher’s α increased from oldest to youngest geological formations from 18.04 on the Pre-Cambrian Shield (n: 12) to 20.38 upon Tertiary Lowlands (n: 9) and 25.60 upon the Pleistocene-Pliocene formation (n: 23) (Fig. 6).
Fisher’s alpha diversity plotted against geological formations. Tertiary: tertiary lowlands, Pleistocene-Pliocene: youngest bedrocks and Pre-Cambrian: oldest bedrocks including Brazilian and Guyana Shields. Data of geological formations derived from Irion & Morais (2011)
Discussion
Spatial floristic patterns
In agreement with other studies, we consistently found evidence for floristic discontinuity and heterogeneity in species composition along the river course (Rosales et al., 1999; Albernaz et al., 2012). The most important species at each river section is characterized by a unique species pool while no significant differences in species richness and Fisher’s alpha between sites were detected. Moreover, species composition at different sites appears to change abruptly rather than gradually from one river section to the next. These results are in accordance with the hypotheses of homogeneity and ecological determinism, which are two of the most debated hypotheses describing spatial tree distribution at landscape scales in Amazonian rain forests. Although our objectives are not to test these two hypotheses, in what follows we focus the discussion on these two approaches.
According to the homogeneity hypothesis, forests are in essence uniform and dominated by a small proportion of species, forming predictable associations or “species oligarchies” which are relatively constant over huge geographical areas (Pitman et al., 2001; Terborgh et al., 2002). This is reflected in our results by the fact that 62.3 % of individuals belong to the 30 most abundant species. Likewise, at each river section, above 60 % of relative abundance values are composed by only 15 species. The presence of species oligarchies for trees and other life forms has been well documented in terra firme forests (Pitman et al., 2001; Vormisto et al., 2004; Macía & Svenning, 2005; Macía, 2011; but see Toledo et al., 2012 for criticism). For várzea forests, Wittmann et al. (2010) suggested that species oligarchies are even more evident than in terra firme due to the high connectivity of riparian corridors, leading to similar ecological niches across landscapes. Based on the occurrence and distribution of the 186 most common Amazonian várzea tree species, Wittmann et al. (2010) oligarchies are mainly composed of generalist species (90 %) which occur in other neotropical ecosystems, while the remaining 10 % are endemic species (sensu Ricklefs, 1990) that dominate large areas along river courses.
However, despite the river connectivity between river sections our study exhibited a unique set of few species that dominates each river section, indicating a marked geographical/environmental gradient and high species turnover. This leads us to discuss the second view that explains the spatial distribution of trees across the Amazon. Tree communities appear to be assemblages related to local ecological determinism. This hypothesis argues that forests are a mosaic in which species distributions change continuously along environmental gradients. Thus, the environment (e.g., soil texture or height and duration of flooding) constitutes strong filters, determining which and how many species are present in the community (Gentry, 1988, Tuomisto et al., 2003, Higgins et al., 2011). These predictions are consistent with our results in three aspects: (1) the clear assemblage of plots into four river sections exhibiting highly significant differences in floristic composition; (2) the comparatively high beta-diversity detected (Hill, 1979); and (3) the lack of overall dominant species with occurrence in all river sections.
A few studies on neotropical floodplain forests have shown a significant floristic discontinuity along the course of water bodies. In a recent floristic study on várzea along ca. 2800-km corridor of the main course of the Solimões-Amazon River, Albernaz et al. (2012) identified three main biogeographic regions defined by significant compositional differences along the course of the river. The authors further concluded that these regions were delimitated by a strong floristic variation caused mainly by the effect of geographical position on species composition. In the Orinoco basin, Rosales et al. (2001) found an abrupt change in species composition along a 260 km corridor of the lower Caura River (black-water River) with floristic components from both várzea and igapó forests. This pattern was interpreted by the authors as a response of the influence of the rich nutritional status of the Orinoco on the lower Caura. The biogeochemical gradient with the input of fertile sediments may control the occurrence of igapó and várzea tree species. Similarly, in central Amazonia Rodrigues (2007) found a marked floristic gradient between igapó and várzea along 45 km of the Amanã Lake, concluding that the soil chemistry is the main driver influencing tree species distribution.
Although our study areas are located along the same riverine system, we did not observe a transition of tree composition between sites at the lower reach and others upstream. In addition to the large geographic distances between study areas, this abruptness might be a response to the variable flooding regime along the course of the Negro River. Thus, flood amplitudes increase from the upper (3.6 m) to the lower (9.3 m) sections of the River, subjecting igapó trees to periodic flooding lasting from 50 to 230 days year−1, respectively. Furthermore, spatial floristic patterns may be determined by the status of alluvial soils (i.e., chemistry and granulometry), which may change in function of the position of the river and the influence of tributaries along the entire course (Prance, 1979; Kubitzki, 1989, Junk et al., 2011). Finally, channel dynamics and geomorphology may also exert certain influence on the floristic variation along the course of the Negro River (Salo et al., 1986; Latrubesse & Franzinelli, 2005; Wittmann et al., 2004; Peixoto et al., 2009).
Tree species composition
At the family level, Fabaceae, Lecythidaceae, Chrysobalanaceae, Euphorbiaceae, and Sapotaceae are the most important families at each river section. While Fabaceae is widely distributed across different forest types in the Amazon, Lecythidaceae, and Chrysobalanaceae are particularly abundant and relatively richer in species in the eastern Amazonia and the Guianas (Gentry, 1990; ter Steege et al., 2000; Honorio-Coronado et al., 2009). Mori (2001) points out the huge diversity of species of Lecythidaceae in black-water systems. This author revealed that 38 species of Lecythidaceae are present in the Negro River basin, suggesting that this area is perhaps an endemism and distribution center for this family. Other important families are Annonaceae, Malvaceae, Rubiacae, and Sapotaceae, the latter is ranked among the top most important families along the Jufaris River and seems to be most abundant family in Central Amazonia (ter Steege et al., 2000; Honorio-Coronado et al., 2009). At the generic level, with the exception of Ocotea, the most diverse in our dataset are originated in the Neotropics and are widely distributed in lowland rainforest (Gentry, 1990; ter Steege et al., 2006; Pitman et al. 1999). The influence of the Guyana Shield may be revealed by the presence of the genera Henriquezia, Micrandra, Macrolobium, Heterostemon, Mollia, Swartzia, Cynometra, Simaba, and Peltogyne.
The floristic composition at the Jufaris River (JU) varies markedly from the other river sections. This is a black-water northern tributary that flows through savanna-like white-sand flooded vegetation thus determining its particular tree composition. Altogether, the 57 % of species occurring at Jufaris were only reported at this site (Online Resource 3). In fact, most important species (Mouriri angulicosta, Sclerolobium chrysophyllum, and Sacoglottis guianensis) also occur on white-sand and terra firme forests (Stropp, 2011) and they were not recorded in other river sections of this study or other studies along the Negro River. According to Kubitzki (1989), resemblance of flooded forests by black-water Rivers with savanna vegetation on oligotrophic white sand is caused by the water chemistry which is the most important factor driving floristic differentiation in Amazonian igapó.
Species richness and diversity patterns
The results indicate that species richness and alpha-diversity tend to be slightly higher at Anavilhanas than in the other river sections. This is mainly due to the influence of the plot AN 1 with 79 species ha−1 and a Fisher’s alpha coefficient of 27.24. The tendency of a higher richness and diversity at the lower reach of the Negro River has also been reported by other studies at Tarumã-Mirim (Worbes 1986, Parolin et al., 2004) and also at Anavilhanas (Parolin et al., 2003; Piedade, 1985; Piedade et al., 2005). That this region is characterized by high species richness and diversity may be partially explained by the presence of finer sediments, which are slightly more fertile than those upstream (Junk et al., 2011). Increased soil fertility associated with a geomorphically dynamic landscape generates conditions for the development of higher beta-diversity, resulting in higher species richness and alpha-diversity. This explanation, however, is challenged by a study on the Jaú River (north-western tributary, upstream from Anavilhanas) which recorded the highest species richness (≥10 cm dbh) reported for Amazonian black waters (Ferreira, 1997). Along three different habitats (lake, river margin, and stream) this author recorded 44, 103, and 137 species ha−1, respectively. The author further attributed the difference in species richness between habitats to the variation of flooding duration, without emphasizing possible causes for the high species richness in this sector of the Negro River.
Although Barcelos (BA) and Jufaris (JU) have the highest number of individuals per hectare, they also presented the lowest alpha-diversity indicating that the number of individuals is not the proximate driver of diversity at these river sections. This pattern has also been reported by other studies in igapó (Ferreira 1997, Gribel et al., 2009) and várzea (Campbell et al., 1992). A reasonable explanation for this pattern can be found in the characteristics of forest succession. Although we focused on mature forest, there are no studies that determine tree ages and addresses successional stages in our sampling areas. The common factor in these forests types is probably the limited availability of resources, which may lead to an interspecific competition resulting in a low alpha-diversity (Tilman, 1982). Thus, spatial heterogeneity in the availability of limiting resources can generate a mosaic of species composition (Terborgh et al., 2002). Therefore, in contrast to Condit et al. (1996) and besides other possible causes (i.e., succession), our results suggest that the number of tree species per unit area in the nutrient-poor igapó forest tends to decrease with increasing number of individuals present in a sample.
At a regional scale, non-flooded rainforests adjacent to the Negro River host one of the richest tree communities in Amazonia. A single hectare of terra firme in this region can hold up to 285 tree species with dbh ≥10 cm (Oliveira & Mori, 1999), while várzea forests can reach up to 142 species ha−1 (Wittmann et al., 2002) and white-sand forest around 100 species ha−1 (Stropp et al., 2011). Our findings, however, show an average of 63 species ha−1 confirming that the late-successional igapó forests along the Negro River is one of the poorest mature forest types per unit area in the Amazon. This is also confirmed by the Fisher’s alpha coefficient calculated for each river section which ranged between 13.99 (Barcelos) and 27.24 (Anavilhanas).
The key to understanding the variation of diversity patterns along the course of the river lies in processes that act on a regional and local level. Thus, at regional scale (along the river) the driving forces shaping diversity may be geographic distance and the influence of adjacent forest types, while at local scale (lateral gradient) environmental filters such as flooding and soil fertility may play a key role in determining diversity patterns. The former may help to explain the slight increase in species richness and diversity in the lower Negro River, indicating that the species pool at the Anavilhanas sites can draw from a larger regional species pool. Thus, due to the proximity and connectivity with the Solimões-Amazon River, the resemblance of our species pool at Anavilhanas with várzea may increase while decreasing at sites further upstream. A recent study comparing tree communities on white-sand forest between upper and lower Negro basin (Stropp et al., 2011) also found a strong variation and a highest local tree alpha diversity at the lower Negro river basin. Similarly, gradients of tree alpha diversity of terra firme forests increase along the Negro River from northwest (upper) to southeast (lower) (Amazon Tree Diversity Network: collective authors ATDN, 2011).
Diversity gradients at continental scale
Data on terra firme and várzea forests suggest a trend of increasing alpha-diversity from east to west (ter Steege et al., 2006; Wittmann et al., 2006). For igapó forests, our comparisons shows that plots at equatorial western Amazonia (WAe) and Orinoco (OR) present a slight tendency to have higher alpha-diversity than in the other regions. The two opposite extremes of the region, southern western Amazonia (WAs) and eastern Amazonia (EA), present the lowest alpha-diversity, whereas central (CA) and north Amazonia (NA) plots have intermediate values. When considering alpha-diversity in relation to the age of the bedrock, plots occurred on older formations (Guyana and Brazilian Pre-Cambrian Shields) show the lowest alpha-diversity. Plots occurring on younger bedrocks mostly influenced by Andean sediments (Sombroek, 2000) present the highest alpha-diversity.
The fact that plots on Pre-Cambrian bedrocks are less diverse than younger geological formations may be partly explained by the nutrient status of the soils. In agreement with earlier studies, our comparisons indicate a gradual decrease of alpha-diversity while increasing the age the geological formations. This pattern may reflect a transition from recently deposited and nutrient-rich sediments of Andes origin in the west to older weathered and nutrient-poor soils in the East (Irion, 1984; ter Steege et al., 2006, Pitman et al., 2008, but see Higgins et al., 2011 for critics). In this dynamic, the uplift of the Andes and its effect on regional climate greatly reconfigured the drainage patterns creating a vast influx of sediments into the basin (Hoorn et al., 2010), which spread over hundreds or thousands of kilometers toward the West (Higgins et al., 2011).
In this scenario, the flooded landscape of the lower reaches of the lower Negro has been formed by very young Holocene deposits, in which its floodplains have experienced a more dynamic fluvial geomorphology than middle and upper sections. For example, in the Holocene 10-m thick fine sediment was deposited in large parts of the lower Negro valley during high sea-level stages (Irion et al., 2010). In contrast, the middle and upper sections are characterized by a more stable landscape (E. Latrubesse, pers.comm.), where the bed-load is mostly composed of white supermature quartz sand (Latrubesse & Franzinelli, 2005).
Preliminary results of the Hybam project (Hydro-geodynamics of the Amazon Basin) across the Amazon basin revealed that at Santa Isabel region (upper Negro River) at the Serrinha station, the Silicon concentrations have the lowest values compared to other places in the Amazon basin. This is probably due to the high proportion of podzols and arenosols covering this region, which are mature and highly weathered soils, therefore with a very low capacity to release dissolved materials into the river (Moquet, 2011). With all, the lower section of the Negro River has continuously received fine-grained sediments and its hydro-geomorphology dynamic is recent, while the middle and upper sections are more stable environments. This dynamic played a crucial role in determining the current pattern of tree diversity of igapó and ruled the distribution and establishment of endemic species.
Conclusion
Our results suggest that although mean alpha measures of diversity may not consistently differ between river sections, species turnover (beta-diversity) is consistently high and significant. The significant floristic contribution of tributary rivers (e.g., Jufaris River) to the overall species pool suggests that beta-diversity of igapó forests along the Negro River tends to increase as a function of regional species pools. Species richness and alpha-diversity revealed that the late-successional igapó forest of the Negro River is one of the most species-poor floodplain forest types in the Amazon. The effect of spatial distance combined with the variable flooding regime, status of the alluvial soil and fluvial geomorphology along the course of the River may control the floristic variation of the igapó forests. On a continental scale, our data show a trend of increasing diversity along an east to west gradient. Plots situated in the western equatorial Amazonia and the Orinoco show the higher alpha-diversity, whereas plots located on the eastern edges of the Amazon region have the lowest values of alpha-diversity. When overlapping plots on geological zones we found a gradual decrease of alpha-diversity while increasing the age the geological formations, indicating the strong influence of bedrocks and derived soils as controlling factors of diversity patterns across the Amazon.
The floristic data presented in this study are the first of their kind on black-water flooded forest which are based on an extensive quantitative dataset, thus substantially increasing the floristic knowledge of igapó forests. This information constitutes the first attempt to describe the floristic variation in terms of species composition and diversity of the igapó forest along the course of the Negro River thus filling a research gap on Amazonian floodplains. We suggest caution with the results as these reveal the interpretations of the late-successional stage of the igapó forests without considering early successional stages or tree communities along the border zone on the river banks. The results contribute essential data for the classification and management recommendations of Amazonian wetlands, which is an initiative lead by the wetland monitoring working group at the Brazilian National Institute of Amazon Research (INPA). Similarly, our main findings constitute the starting point to seek answers to for example why the igapó is so poor in tree species and what are the causes that determine the abrupt changes in species composition along the course of the river.
References
Albernaz, A. N., R. L. Pressey, L. R. F. Costa, M. P. Moreira, J. F. Ramos, P. A. Assunção & C. H. Franciscon, 2012. Tree species compositional change and conservation implications in the white-water flooded forests of the Brazilian Amazon. Journal of Biogeography 39(5): 869–883.
ATDN, 2011. Amazon tree diversity network. http://www.bio.uu.nl/~herba/Guyana/ATDN/. Retrieved 09/2011.
Ayres, J. M., 1993. As matas de várzea do Mamirauá. In Sociedade civil Mamirauá (ed.), Estudos de Mamirauá, vol. 1. Sociedade civil Mamirauá.
Campbell, D. G., J. L. Stone & A. Rosas, 1992. A comparison of the phytosociology and dynamics of three floodplain (Várzea) forests of known ages, Rio Juruá, western Brazilian Amazon. Biological Journal of the Linnean Society 108: 213–237.
Campbell, D. G., D. C. Daly, G. T. Prance & U. N. Maciel, 1986. Quantitative ecological inventory of terra firme and várzea tropical forest on the Rio Xingu, Brazilian Amazon. Brittoniana 38: 369–393.
Castellanos, H. G., 1998. Floristic composition and structure, tree diversity, and the relationship between floristic distribution and soil factors in El Caura Forest Reserve, southern Venezuela. In Dallmeier, F. & J. A. Comiskey (eds), Forest Biodiversity in North, Central and South America, and the Caribbean. Man and the Biosphere Series. Smithsonian Institution, Washington, DC: 507–533.
Cerón, C. E. & C. I. Reyes, 2003. Composición y estructura de una hectárea de bosque aluvial en la Reserva Biológica Limoncocha. Cinchonia 4(1): 35–46.
Cerón, C. E., D. M. Fernández, E. D. Jiménez & I. Pillajo, 2000. Composicion y estrutura de un igapó ecuatoriano. Cinchonia 1(1): 41–70.
Cerón, C. E., C. Montalvo & C. I. Reyes, 2003. El bosque de tierra firme, moretal, igapó y ripario en la cuenca del Río Güeppi, Sucumbios-Ecuador. Cinchonia 4(1): 80–110.
Chao, A., R. L. Chazdon, R. K. Colwell & T. J. Shen, 2005. A new statistical approach for assessing compositional similarity based on incidence and abundance data. Ecology Letters 8: 148–159.
Chave, J., 2008. Spatial variation in tree species composition across tropical forests: pattern and process. In Carson, W. P. & S. A. Schnitzer (eds), Tropical Forest Community Ecology. Blackwell Scientific Publications, Oxford: 11–30.
Colwell, R. K. 2009. EstimateS: statistical estimation of species richness and shared species from samples. Version 8.2. User’s Guide and application published at: http://purl.oclc.org/estimates.
Comiskey, J. A., F. Dallmeier & R. Foster, 1998. Forest structure and diversity in managed and unmanaged rainforest of Beni, Bolivia. In Dallmeier, F. & J. A. Comiskey (eds), Forest Biodiversity in North, Central and South America, and the Caribbean. Man and the Biosphere Series, Smithsonian Institution, Washington, DC: 663–680.
Condit, R., S. P. Hubbell & R. B. Foster, 1996. Changes in tree species abundance in a neotropical forest: impact of climate change. Journal of Tropical Ecology 12: 231–256.
Coronado, E. N. H., T. R. Baker, O. L. Phillips, N. C. A. Pitman, R. T. Pennington, R. V. Martınez, A. Monteagudo, H. Mogollón, N. D. Cardozo, M. Ríos, R. García-Villacorta, E. Valderrama, M. Ahuite, I. Huamantupa, D. A. Neill, W. F. Laurance, H. E. M. Nascimento, S. S. de Almeida, T. J. Killeen, L. Arroyo, P. Núnez & L. F. Alvarado, 2009. Integrating regional and continental scale comparisons of tree composition in Amazonian terra firme forests. Biogeosciences 6: 2719–2731.
Curtis, J. T. & R. P. McIntosh, 1951. An upland forest continuum in the prairie-forest border region of Wisconsin. Ecology 32: 476–496.
Duivenvoorden, J. & J. Lips, 1993. Ecología del paisaje del medio Caquetá. Memoria explicativa de los mapas, Tropenbos- Colombia, Santa Fé de Bogotá, Colombia
Duivenvoorden, J. F. & J. M. Lips, 1998. Mesoscale patterns of tree species diversity in Colombian Amazonia. In Dallmeier, F. & J. A. Comiskey (eds), Forest Biodiversity in North, Central and South America, and the Caribbean: Research and Monitoring. MAB Series Vol. 21. UNESCO, Paris: 535–549.
Duque, A., J. Cavelier & A. Posada, 2003. Strategies of tree occupation at the local scale in terra firme forests in the Colombian Amazon. Biotropica 35: 20–27.
Ferreira, L. V., 1997. Effects of the duration of flooding on species richness and floristic in three hectares in the Jaú National Park in floodplain in central Amazonia. Biodiversity Conservation 6: 1353–1363.
Ferreira, L. V., 2000. Effect of flooding duration on species richness, floristic composition and forest structure in river margin habitats in Amazonian blackwater floodplain forests: Implications for future design of protected areas. Biodiversity and Conservation 9: 1–14.
Ferreira, L. V. & S. S. de Almeida, 2005. Relação entre altura de inundação, riqueza específica de plantas e o tamanho de clareiras naturais em uma floresta inundável de igapó na Amazônia central. Revista Árvore 29(3): 445–453.
Ferreira, L. V. & G. T. Prance, 1998. Structure and species richness of low-diversity floodplain forest on the Rio Tapajós, Eastern Amazonia, Brazil. Biodiversity and Conservation 7: 585–596.
Ferreira, L. V. & T. J. Stohlgren, 1999. Effects of river level fluctuation on plant species richness, diversity, and distribution in a floodplain forest in Central Amazonia. Oecologia 120: 582–587.
Ferreira, L. V., S. S. de Almeida & P. Parolin, 2010. Amazonian white and blackwater floodplain forests in Brazil: Large differences on a small scale. ECOTROPICA 16: 31–41.
Ferreira, L. V., S. S. de Almeida, D. D. Amaral & P. Parolin, 2005. Riqueza e composição de espécies da floresta de igapó e várzea da estação científica Ferreira Penna: Subsídios para o plano de manejo da Floresta Nacional Caxiuanã. Pesquisas Botânica 56: 103–116.
Fisher, A. A., A. S. Corbet & C. B. Williams, 1943. The relation between the number of species and the number of individuals in a random sample of an animal population. Journal of Animal Ecology 12: 42–58.
Furch, K., 1997. Chemistry of várzea and igapó soils and nutrient inventory of their floodplain forests. In Junk, W. J. (ed.), The Central Amazon Floodplain: Ecology of a Pulsing System. Ecological Studies 126, Springer, Berlin: 47–68.
Gentry, A. H., 1988. Changes in plant community diversity and floristic composition on environmental and geographical gradients. Annals of the Missouri Botanical Garden 75: 1–34.
Gentry, A. H., 1990. Floristic similarities and differences between southern Central America and upper and central Amazonia. In Gentry, A. H. (ed.), Four neotropical rain forests. Yale University Press, New Haven: 141–157.
Goulding, M., N. Smith & D. Mahar, 1996. Floods of fortune: Ecology and economy along the Amazon. Columbia University Press, New York.
Goulding, M., R. Barthem & R. E. Ferreira, 2003. The Smithsonian atlas of the Amazon. Smithsonian Institute, Princeton Editorial Associates.
Gribel, R., C. A. Cid Ferreira, L. S. Coelho, J. Lima, J. F. Ramos & K. Â. Farias da Silva, 2009. Vegetacão do parque nacional do Viruá-Roraima. Technical report, INPA, Manaus.
Hammer, O., D. Harper & P. Ryan, 2001. PAST: Paleontological statistics software for education and data analysis. V. 1.92. Paleontología Electrónica 4: 1–9.
Haugaasen, T. & C. A. Peres, 2006. Floristic, edaphic, and structural characteristics of flooded and unflooded forests in the lower Rio Purús region of central Amazonia, Brazil. Acta Amazonica 36(1): 25–36.
Higgins, M. A., K. Ruokolainen, H. Tuomisto, N. Llerena, G. Cardenas, O. L. Phillips, R. Vásquez & M. Räsänen, 2011. Geological control of floristic composition in Amazonian forests. Journal of Biogeography 38: 2136–2149.
Hill, M. O., 1979. DECORANA: A FORTRAN program for detrended correspondence analysis and reciprocal averaging. Ecology and Systematics, Cornell Ecology Programs, Cornell University, Ithaca, NY.
Hoorn, C., F. P. Wesselingh, H. ter Steege, M. A. Bermudez, A. Mora, J. Sevink, I. Sanmartín, A. Sanchez-Meseguer, C. L. Anderson, J. P. Figueiredo, C. Jaramillo, D. Riff, F. R. Negri, H. Hooghiemstra, J. Lundberg, T. Stadler, T. Särkinen & A. Antonelli, 2010. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 330: 927–931.
Inuma, J. J., 2006. Comparação na diversidade e estrutura das comunidades de plantas lenhosas da terra firme, várzea e igapó do Amaná. Amazônia Central, PhD Thesis, Instituto Nacional de Pesquisas da Amazônia, Manaus.
Irion, G., 1984. Sedimentation and sediments of Amazonian rivers and evolution of the Amazonian landscape since Pliocene times. In Sioli, H. (ed.), The Amazon: Limnology and landscape ecology of a mighty tropical river and its basin. Dr. W. Junk Publishers, Dordrecht: 201–214.
Irion, G. & J. O. Morais, 2011. Sócio-economia da Amazônia. In Morais, J. O. & I. S. Pinheiro (eds), Gestão integrada das zona costeiras: Desafios e perspectivas. Universidade Estadual do Ceará, Fortaleza.
Irion, G., J. A. S. N de Mello, J. Morais, M. T. F. Piedade, W. J. Junk & L. Garming, 2010. Development of the Amazon valley during the Middle to Late Quaternary: sedimentological and climatological observations. In Junk, W. J., M. T. F. Piedade, F. Wittmann, J. Schöngart & P. Parolin (eds), Ecology and management of Amazonian floodplain forests. Ecological Series 210, Springer, Berlin: 27–42.
Jongman, R. H. G., C. J. F. ter Braak & O. F. R. van Tongeren (eds), 1995. Data analysis in community and landscape ecology. Cambridge University Press, Cambridge.
Junk, W. J., 1989. Flood tolerance and tree distribution in central Amazonian floodplains. In. Holm-Nielsen, L. B., I. C. Nielsen & H. Balslev (eds), Tropical forests: botanical dynamics, speciation and diversity. Academic Press, London: 47–64.
Junk, W. J., P. B. Bayley & R. E. Sparks, 1989. The Flood pulse concept in river-floodplain systems. In Dodge, D. (ed.), Proceedings of the International Large River Symposium, Ottawa. Canadian Special Publications of Fisheries and Aquatic Sciences 106: 110–127.
Junk, W. J., M. T. F. Piedade, J. Schöngart, M. Cohn-Haft, J. M. Adeney & F. Wittmann, 2011. A classification of major naturally occurring Amazonian lowland wetlands. Wetlands 31(4): 623–640.
Knab-Vispo, C., P. Berry & G. Rodríguez, 1999. Floristic and structural characterization of a lowland rain forest in the lower Caura watershed, Venezuelan Guayana. Acta Botánica Venezuélica 22(2): 325–359.
Keel, S. H. K. & G. T. Prance, 1979. Studies of the vegetation of a white-sand black water igapó (Rio Negro, Brazil). Acta Amazonica. 9(4): 645–655.
Kubitzki, K., 1989. The ecogeographical differentiation of Amazonian inundation forests. Plant Systematics and Evolution 163: 285–304.
Latrubesse, E.M., 2008. Patterns of anabranching channels: The ultimate end-member adjustment of mega rivers. Geomorphology 101: 130–145.
Latrubesse, E. M. & E. Franzinelli, 2005. The late quaternary evolution of the Negro River. Amazon, Brazil: Implications for island and floodplain formation in large anabranching tropical systems. Geomorphology 70: 372–397.
Macía, M. J., 2011. Spatial distribution and floristic composition of trees and lianas in different forest types of an Amazonian rainforest. Plant Ecology 7: 1159–1177.
Macía, M. J. & J. C. Svenning, 2005. Oligarchic dominance in western Amazonian plant communities. Journal of Tropical Ecology 21: 613–626.
Magurran, A. E., 2004. Measuring biological diversity. Blackwell Publishing, Oxford.
McCune, B. & J. B. Grace, 2002. Analysis of ecological communities. MjM Software Design, Oregon.
McCune, B. & M. J. Mefford, 1999. Multivariate analysis of ecological data: Version 5.0. MjM Software, Gleneden Beach, Oregon.
Montero, J. C., 2002. Estructura y composición florística de los bosques de várzea e igapó en la Amazonia boliviana. Bachelor thesis, Universidad Gabriel René Moreno. Santa de la Sierra, Bolivia.
Montero, J. C., 2011. Der Igapó des Rio Negro. Senckenberg—natur., forschung., Museum, 141 (9/10): 264–273
Moquet, J. S., 2011. Caractérisation des flux d’altération des contextes orogéniques en milieu tropical—Cas des bassins andins et d’avant pays de l’Amazone. PhD thesis. University Toulouse III: Paul Sabatier. Toulouse, France.
Mori, S., 2001. A família da Castanha-do-Pará: Símbolo do Rio Negro. In Oliveira, A. A. & D. C. Daly (eds), Florestas do Rio Negro. UNIP, NYBG e Companhia das Letras. Sao Paulo.
Mori, S. A., B. M. Boom, A. M. de Carvalho & T. S. dos Santos, 1983. Southern Bahian moist forests. Botanical Review 49(2): 155–232.
Oliveira, A. A. & S. A. Mori, 1999. A central Amazonian terra firme forest. I. High tree species richness on poor soils. Biodiversity Conservation 8: 1219–1244.
Pacheco, M. A., 2010. Fisionomía y estructura de un bosque en zona inundable en Puerto Almendras, San Juan, Perú. Bachelor thesis, Universidad Nacional de la Amazonía Peruana. Iquitos.
Parolin, P., J. Adis, M. F. da Silva, I. L. do Amaral, L. Schmidt & M. T. F. Piedade, 2003. Floristic composition of a floodplain forest in the Anavilhanas archipelago, Brazilian Amazonia. Amazoniana 17 (3/4): 399–411.
Parolin, P., J. Adis, W. A. Rodrigues, I, Amaral & M. T. F. Piedade, 2004. Floristic study of an igapó floodplain forest in Central Amazonia, Brazil (Tarumã-Mirim, Rio Negro). Amazoniana 18(1/2): 29–47.
Peixoto, J. M. A., B. W. Nelson & F. Wittmann, 2009. Spatial and temporal dynamics of river channel migration and vegetation in central Amazonian white-water floodplains by remote-sensing techniques. Remote Sensing of Environment 113: 2258–2266.
Piedade, M. T. F., 1985. Ecologia e biologia reprodutiva de Astrocaryum jauari Mart. (Palmae) como exemplo de população adaptada às áreas inundáveis do Rio Negro (igapós). MSc-Thesis. Istituto nacional de Pesquisas da Amazônia, Manaus
Piedade, M. T. F., W. J. Junk, J. Adis & P. Parolin, 2005. Ecologia, zonação e colonização de vegetação arbórea das ilhas Anavilhanas. Pesquisa Botânica 56: 117–144.
Pitman, N. C. A., J. Terborgh, M. R. Silman & P. V. Núñez, 1999. Tree species distributions in an upper Amazonian forest. Ecology 80: 2651–2661.
Pitman, N. C. A., J. Terborgh, M. R. Silman, D. A. Neill, P. V. Núñez, C. E. Cerón, et al. 2001. Dominance and distribution of tree species in upper Amazonian terra firme forests. Ecology 82: 2101–2117.
Pitman, N., H. Mogollón, N. Dávila, M. Ríos, R. García-Villacorta, J. Guevara, T. Baker, A, Monteagudo, O. Phillips, R. Vásquez-Martínez, M. Ahuite, M. Aulestia, D. Cardenas, C. Cerón, P.A. Loizeau, D. Neill, P. Nuñez, W. Palacios, R. Spichiger & E. Valderrama, 2008. Tree community change across 700 km of lowland Amazonian forest from the Andean foothills to Brazil, Biotropica, 40: 525–535.
Prance, G. T., 1979. Notes on the vegetation of Amazonia III. The terminology of Amazonian forest types subject to inundation. Brittonia 3(1): 26–38.
Revilla, J. D. C., 1981. Aspectos florísticos e fitosociólogicos do igapó de Praia Grande, Rio Negro, Amazonas. MSc Thesis, Instituto Nacional de Pesquisas da Amazônia. Manaus, Brazil.
Ricklefs, R. E., 1990. Ecology. W.H Freeman, New York.
Rodrigues, R., 2007. Diversidade florística, estrutura da comunidade arbórea e suas relações com variáveis ambientais ao longo do lago Amanã (RDSA), Amazônia Central. MSc Thesis, INPA/UFAM, Manaus, Brazil.
Rosales, J., G. Petts & J. Salo, 1999. Riparian flooded forests of the Orinoco and Amazon basins: a comparative review. Biodiversity Conservation 8: 551–586.
Rosales, J., G. Petts & C. Knab-Vispo, 2001. Ecological gradients in riparian forests of the lower Caura River, Venezuela. Plant Ecology 152(1): 101–118.
Sabo, J. L., R. Sponseller, M. Dixon, K. Gade, T. Harms, J. Heffernan, A. Jani, G. Katz, C. Soykan, J. Watts & J. Welter, 2005. Riparian zones increase regional species richness by harboring different, not more, species. Ecology 86: 56–62.
Sioli, H., 1956. Über Natur und Mensch im brasilianischen Amazonasgebiet. Erdkunde 10(2): 89–109.
Salo, J., R. Kalliola, I. Häkkinen, Y. Mäkinen, P. Niemelä, M. Puhakka & P. D. Coley, 1986. River dynamics and the diversity of the Amazon lowland forest. Nature 322: 254–258.
Scudeller, V. V. & A. M. G. de Souza, 2009. Florística da mata de igapó na Amazônia Central. In Santos-Silva, E. N. & V. V. Scudeller (eds), Diversidade Biológica e Sociocultural do Baixo Rio Negro. Amazônia Central, UAE Edições: 97–108.
Sombroek, W., 2000. Amazon landforms and soils in relation to biological diversity. Acta Amazonica 30: 81–100.
Svenning, J. C., D. A. Kinner, R. F. Stallard, B. M. J. Engelbrecht & S. J. Wright, 2004. Ecological determinism in plant community structure across a tropical forest landscape. Ecology 85: 2526–2538.
Stropp, J., 2011. Towards an Understanding of Tree Diversity in Amazonian Forests. PhD Thesis, Utrecht University, Utrecht.
Stropp, J., L. L do Amaral, G. Aymard, O. Banki, C. V de Castillo, Cid Fereira, T. Henkel, D. Lopez, W. Magnuson, F. D. de Alemeida Matos, W. Milliken, A. Oliveira, D. Pauletto, O. P. Phillips, R. Thomas & H. Ter Steege, 2011. Comparing tree communities of white-sand and terra-firme forests across three Amazonian regions. In Stropp, J. (eds), Towards an Understanding of Tree Diversity in Amazonian Forests. PhD Thesis, Utrecht University, Utrecht.
Ter Steege, H., D. Sabatier, H. Castellanos, T. Van Andel, J. Duivenvoorden, A. A. de Oliveira, R. de Ek, R. Lilwah, P. Maas & S. Mori, 2000. An analysis of the floristic composition and diversity of Amazonian forests including those of the Guiana shield. Journal of Tropical Ecology 16: 801–828.
Ter Steege, H., N. Pitman, O. L. Phillips, J. Chave, D. Sabatier, A. Duque, J. F. Molino, M. F. Prévost, R. Spichiger, H. Castellanos, P. Hildebrand & R. Vásquez, 2006. Continental scale patterns of canopy tree composition and function across Amazonia. Nature 443: 444–447.
Terborgh, J., N. Pitman, M. Silman, H. Schichter & P. V. Nuñez, 2002. Maintenance of tree diversity in tropical forests. In Levey, D. J., W. R. Silva & M. Galetti (eds), Seed Dispersal and Frugivory: Ecology, Evolution and Conservation. CAB International, Wallingford, UK: 1–17.
Tilman, D., 1982. Resource competition and community structure. Monographs in Population Biology. Princeton University Press, Princeton.
Toledo, M., L. Poorter, M. Peña-Claros, A. Alarcón, J. Bálcazar, J. Chuviña, J. C. Licona, C. Leaño, H. ter Steege & F. Bongers. 2010. Patterns and determinants of floristic variation along lowland forests of Bolivia. Biotropica 1–9.
Toledo, M., M. Peña-Claros, F. Bongers, A. Alarcón, J. Bálcazar, J. Chuviña, C. Leaño, J. C. Licona & L. Poorter, 2012. Distribution patterns of tropical woody species in response to climatic and edaphic gradients. Journal of Ecology 100(1): 253–263.
Tuomisto, H., K. Ruokolainen, M. Aguilar & A. Sarmiento, 2003. Floristic patterns along a 43-km long transect in an Amazonian rain forest. Journal of Ecology 91: 743–756.
Wittmann, F., D. Anhuf & W. J. Junk, 2002. Tree species distribution and community structure of Central Amazonian várzea forests by remote-sensing techniques. Journal of Tropical Ecology 18: 805–820.
Wittmann, F., W. J. Junk & M. T. F. Piedade, 2004. The várzea forests in Amazonia: flooding and the highly dynamic geomorphology interact with natural forest succession. Forest Ecology and Management 196: 199–212.
Wittmann, F., J. Schöngart, J. C. Montero, T. Motzer, W. J. Junk, M. T. F. Piedade, H. L. Queiroz & M. Worbes, 2006. Tree species composition and diversity gradients in white-water forests across the Amazon basin. Journal of Biogeography 33: 1334–1347.
Wittmann, F., J. Schöngart & W. J. Junk, 2010. Phytogeography, species diversity, community structure and dynamics of Amazonian várzea forests. In Junk, W.J., M. T. F. Piedade, F. Wittmann, J. Schöngart & P. Parolin (eds), Ecology and management of Amazonian floodplain forests. Ecological Series 210, Springer, Berlin: 61–102.
Worbes, M., 1986. Lebensbedingungen und Holzwachstum in zentral-amazonischen Überschwemmungswäldern. Scripta Geobotanica 17, Göttingen.
Worbes, M., 1997. The forest ecosystem of the floodplains. In Junk, W. J. (ed.), The Central Amazon Floodplain: Ecology of a Pulsing System. Ecological Studies 126, Springer, Berlin: 223–265.
Van Andel, T., 2001. Floristic composition and diversity of of mixed primary and secondary forests in northwest Guyana. Biodiversity and Conservation 10: 1645–1682.
Vormisto, J., J. C. Svenning, P. Hall & H. Balslev, 2004. Diversity and dominance in palm (Arecaceae) communities in terra firme forests in western Amazon basin. Journal of Ecology 92: 577–588.
Acknowledgments
Financial support for the field work was provided by PRONEX (MCT-FAPEAM-CNPq) 2007—“Tipologias Alagáveis”. Logistical assistance was organized by the INPA/Max Planck project in Manaus. We also acknowledge the valuable support of field assistants, especial thanks to José Lima and Francisco Quintiliano who greatly contributed to the identification in situ of trees and encouraged discussions about the distribution of Amazonian trees. We thank Mark Bilton for improving the English of a preliminary version and James Johnson for a final revision of the manuscript. Two reviewers are acknowledged for valuable suggestions. The results presented in this article are part of the doctorate thesis carried out by JCM at the Institute of Silviculture, University of Freiburg, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: H. Habersack, S. Muhar & H. Waidbacher / Impact of human activities on biodiversity of large rivers
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Montero, J.C., Piedade, M.T.F. & Wittmann, F. Floristic variation across 600 km of inundation forests (Igapó) along the Negro River, Central Amazonia. Hydrobiologia 729, 229–246 (2014). https://doi.org/10.1007/s10750-012-1381-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1381-9