Abstract
This study investigated the effects of fish farming on phytoplankton and zooplankton structure in a Brazilian tropical reservoir. Samplings were undertaken among 15 net cages and upstream and downstream of the cages over a 120-day period. Soon after the tilapia feeding, we observed the highest density of phytoplankton, with dominance of Cyanobacteria which probably increased due to the increase in predation pressure by cladocerans and copepods on the Cryptophyceae. However, after 30 days from that, a reduction in zooplankton density was registered due to the dominance of Cyanobacteria. The absence of a clear spatial difference in the phytoplankton and zooplankton densities was observed. The effects of the net cages on the nutrients and planktonic communities were small, probably due to the low number of net cages and fish employed, the seasons (autumn/winter) and the hydrodynamics, as lotic traits, the strong influence of the wind, and the large extension of the mixture zone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global growth in the human population has driven a growing requirement for water resources, whether for public supply, industrial purposes, navigation, or food production. There is a corresponding growth in worldwide and Brazilian aquaculture that presents huge developmental possibilities. Like any other productive activity, fish farming in net cages causes environmental impacts, among them the increase in nutrient concentrations originating from the waste that is directly released in the water, changes in the trophic web and the balance of aquatic communities, the introduction of exotic species, a decrease in biodiversity, and the spread of diseases (Naylor et al., 2000; Borghetti et al., 2003; Alves & Baccarin, 2005; Agostinho et al., 2007; Guo et al., 2009).
The great amounts of residuals coming from fish farming in net cages increase the concentrations of nitrogen and phosphorus in the water column and in the sediment, causing eutrophication (Guo & Li, 2003; Alves & Baccarin, 2005; Figueredo & Giani, 2005; Guo et al., 2009). As a function of the changes in the nutrient loads from rivers, lakes, and reservoirs, an increase in phytoplankton proliferation, particularly in Cyanobacteria, has been recorded and represents a growing problem in these ecosystems (Chorus & Bartram, 1999; Codd et al., 2005).
The consumption and consequent reduction in density and biomass of phytoplankton in the presence of zooplankton have been verified in numerous environments (González, 2000; Ventelä et al., 2002; Gosselain et al., 1998). However, some authors do not consider predation by the zooplankton as a controlling factor; on the contrary, many studies have observed the increase in phytoplankton due to the selective consumption made by the zooplankton and the resultant dominance in both density and biomass of Cyanobacteria (Haney, 1987; Gasiunaité & Olenina, 1998; Degans & Meester, 2002; Kozak & Goldyn, 2004).
In general, the zooplankton community is negatively affected by the presence of Cyanobacteria as a food resource. The reduction in the population growth of zooplankton is related to the morphology of these algae, as well as the intracellular production of secondary toxic metabolites and the deficiency in essential nutrients (Lampert, 1987; Haney, 1987; Demott, 1999; Ferrão-Filho et al., 2000; Ghadouani et al., 2003).
In this way, this study aimed to investigate the effects of fish farming (Oreochromis niloticus L.) on phytoplankton and zooplankton structure in a Brazilian tropical reservoir (Rosana Reservoir, Southern Brazil). We tested the hypothesis that the experimental cultivation of tilapia in net cages increases the nutrient concentrations in the environment and, consequently, increases the phytoplankton density, especially the Cyanobacteria, so that the zooplankton predation will have little influence on community control because of its low predation capacity on those algae.
Study area
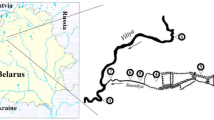
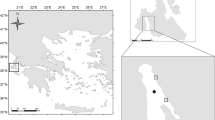
Rosana Reservoir (22°36′ S; 52°49′ W) is located in the inferior stretch of the Paranapanema River (Fig. 1), compounding a border between São Paulo and Paraná States, Brazil. Due to the last position in a cascade of reservoirs, this environment presents higher values of water transparency and alkalinity and low concentrations of total phosphorus and nitrogen, making it classified as oligotrophic by Pagioro et al. (2005).
This study was accomplished in a lateral arm formed by the Guairacá Stream located at the left bank from the lacustrine region of the Rosana Reservoir, which presents the banks with grasses and the predominance of sugar cane cultivation; aquatic macrophyte exists and is strongly influenced by the wind. Although the Rosana Reservoir is oligotrophic, the mean concentrations of TN and TP in the Guairacá Stream before the net cage installation characterize this reservoir compartment as mesotrophic.
Materials and methods
This study was accomplished in an area with the experimental cultivation of Nile tilapia (Oreochromis niloticus L.) within net cages. For this cultivation, 15 net cages (2 × 2 × 1.7 m) were used, each one with 6.0 m3 of volume, which were transversally settled into three sets of five, with different densities of fish storage for each set (50 kg m−3 or 100 fish m−3, 75 kg m−3 or 150 fish m−3, and 100 kg m−3 or 200 fish m−3). The fish were fed with extruded rations twice daily.
The sampling stations were established close to the net cages and at 400 m upstream and downstream from the installation local. The first sampling was performed 1 week before the net cage installation, and the other samplings were performed 15, 30, 60, 90, and 120 days after the installation. This study was carried out between April and August of 2006.
For the analysis of abiotic variables and phytoplankton community, the water samples were taken in triplicate, at subsurface from the pelagic zone using plastic flasks; for the zooplankton, samplings were also carried out at subsurface during the morning (until 12 a.m.), using a motorized pump to filter 200 l of water through a plankton net (68 μm), also in triplicate. Abiotic variables, phytoplankton and zooplankton attributes, and their respective methodologies are listed in Table 1.
Guairacá Stream is a lotic system highly exposed to wind action due to the absence of riparian vegetation in the banks. Algae smaller than 30 μm, like Cryptophyceae species were considered easily edible algae and larger Cyanobacteria colonies were considered less edible for zooplankton, according to several studies such as Haney (1987), Gasiunaité & Olenina (1998), Ventelä et al. (2002), Ghadouani et al. (2003) and Agasild et al. (2007).
An analysis of variance (two-way ANOVA) was carried out in order to test the differences in the densities of phytoplankton and zooplankton, as well as in the nutrient concentrations (TP, SRP, TN, NO3, NH4) in the different treatments (cages, downstream and upstream; Factor 1) and the sampling periods (before and after the beginning of the cultivation; Factor 2). The assumptions of normality and homoscedasticity required by this analysis were previously tested (the Shapiro–Wilk test and Levene test, respectively); when significant differences (P < 0.05) were detected, we also employed Tukey’s test a posteriori. For this analysis, the values of the density from planktonic communities and the values of nutrient concentrations were log-transformed.
Moreover, a Canonical Correspondence Analysis (CCA) (Ter Braak, 1986) was used to reduce the data dimensionality and identify the main variables influencing the structuring of the phytoplankton community. For this analysis, we used the phytoplankton species that contributed with a mean density greater than 5 ind ml−1 and also the values of abiotic variables and the total density of rotifers, cladocerans, and copepods (log-transformed data). The CCA was performed using Pc-Ord 4.0 (McCune & Mefford, 1999).
Results
Higher mean values of precipitation were registered before the net cage installation, as well as higher water temperature and a larger extension of the mixture zone. On the other hand, the light availability in the mixture zone increased over the study period, as well as the N:P ratio. The highest mean value of turbidity was recorded after 15 days, but this variable was also high before the beginning of the cultivation.
Concentrations of TN (F = 10.1; P < 0.05) and NH4 (F = 5.4; P < 0.05) were significantly greater after the beginning of the cultivation, unlike the nitrate (F = 77.4; P < 0.05) and soluble reactive phosphorus (SRP) (F = 18.9; P < 0.05) concentrations, which decreased over time. Regarding the mean values of TP, there were no significant differences considering the periods and treatments (Table 2).
The pH, dissolved oxygen, and electric conductivity presented the lowest variability over time, as expressed in the low values of their variation coefficient. Ninety days after the net cage installation, we registered higher mean values of TN, SRP, the Zeu:Zmix ratio, and dissolved oxygen and the lowest N:P ratio (Table 2).
After the beginning of the cultivation, higher phytoplankton density was observed, with mean values above 1,000 ind ml−1 (Fig. 2a), with significant differences (F = 61.7; P < 0.05) among the periods. The most abundant groups were Cyanobacteria and Cryptophyceae. This latter group presented a greater contribution to the total density before the net cage installation and at the end of this study, unlike the Cyanobacteria, whose higher values were registered 15 and 30 days after the beginning of tilapia cultivation (Fig. 2b, c). The Cyanobacteria density, in number of cells, was also high, with a peak above 180,000 cells ml−1 registered after 90 days of the beginning of the experiment, downstream from the net cages (Fig. 3), and the most abundant species were Radiocystis fernandoi Komárek et Komárková-Legnerová, Microcystis protocystis Crow., Microcystis aeruginosa Kütz and Pseudanabaena muscicola (Hüb.-Pest. & Naum.) Bourr.
Density of the total phytoplankton (a), Cyanobacteria (b), Cryptophyceae (c), other phytoplankton groups (Bacillaryophyceae, Chlorophyceae, Zygnemaphyceae, Euglenophyceae, Chrysophyceae, Dinophyceae) (d); density of the total zooplankton (e), Rotifera (f), Cladocera (g), and Copepoda (h), in the different sampling stations over time in the Guairacá Stream. Mean ± standard error. Notice differences in the scale
The other phytoplankton groups contributed with values less than 300 ind ml−1, presenting a density increase 60 days after the beginning of the experiment (Fig. 2d). It was not possible to detect significant differences in relation to the phytoplankton density among the sampling stations. The concentration of microcystin LR was of 1.94 μg l−1 after 30 days from the beginning of the culture, near the net cages.
The zooplankton also presented a greater density after the beginning of the cultivation, mainly after 15 days (Fig. 2e–h), and a significant difference was recorded among the periods (F = 26.58; P < 0.05). For the copepods and rotifers, the greater density was observed 15 days after the beginning of the experiment; moreover, the rotifers also presented high densities at the end of this study (120 days). A peak in density for the cladocerans occurred 30 days after the beginning of the cultivation (Fig. 2g). Spatially, as observed for the phytoplankton, it was not possible to observe significant differences among the sampling stations (Fig. 2e–h).
The most abundant rotifer species were Synchaeta pectinata Ehrenberg, 1832, S. oblonga Ehrenberg, 1831, Conochilus coenobasis (Skorokov, 1914), C. unicornis Rousselet, 1892 and Polyarthra dolichoptera Idelson, 1924. The most abundant cladocerans were Ceriodaphnia cornuta Sars, 1886, C. silvestrii Daday, 1902, Moina minuta Hansen, 1899 and Bosmina hagmanni Stingelin, 1904, while the most abundant calanoid copepod was Notodiaptomus amazonicus (Wright, 1935). We also observed Thermocyclops decipiens Kiefer, 1929 (cyclopoid copepod) at low abundances.
In general, the temporal variations in phytoplankton and zooplankton densities were not similar during the study period. The mean density of phytoplankton and zooplankton obtained in the first samplings varied concomitantly, with lower values before the beginning of the experiment and an increase in these values in the sampling performed at 15 days and a decrease at 30 days. Thereafter, the phytoplankton density increased until the 90th day after the net cage installation, and afterward, it decreased sharply at the end of the experiment (120 days); on the other hand, the zooplankton density presented an inverse pattern; i.e., the density decreased constantly after 30 days and increased later, 120 days after the beginning of the experiment (Fig. 4).
Cyanobacteria numerically dominated the phytoplankton community soon after the beginning of the experiment (15 and 30 days), and a reduction was observed for Cryptophyceae, whereas the zooplankton was represented by all of the groups during this period, particularly by cladocerans and copepods. After 90 days, there was an increase of Cryptophyceae and a decrease of cladocerans and copepods, and, at the end of this study (120 days), we observed an increase in rotifer density and a decrease of the Cryptophyceae group (Figs. 2, 4).
The CCA showed significant correlations between the density of phytoplanktonic species and abiotic variables, together with zooplankton density. The first two axes were significant (P < 0.05), according to the Monte Carlo Test, and explained 55.2% of the total data variability. This analysis still pointed out differences in the temporal distribution of phytoplankton density in the Guairacá Stream, as well as of the zooplankton groups and abiotic variables, but spatial differences were not verified (Fig. 5a, b).
Score dispersion of locations for sampling time and abiotic variables and zooplankton abundance (a) and the density of principal phytoplanktonic species (b) along the first two CCA axes. Net cages (n), Downstream (d), Upstream (u), cladocerans (Cla), rotifers (Rot), and copepods (Cop). Codes in Table 1
The first CCA axis explained 39.1% of the total data variability, and the principal variables that contribute to the formation of this axis were the density of cladocerans (0.75) and copepods (0.77), the water temperature (0.37), total phosphorus concentration (0.33), N:P ratio (0.29), wind velocity (−0.43), and rotifer density (−0.34). This axis separated to the right of the diagram the sampling units referring to the periods 15 and 30 days after the beginning of the experiment from the other sampling units and periods when the Cyanobacteria were favored (Fig. 5a, b).
The second CCA axis explained 16.1% of the total data variability, and the main related variables were NO3 (0.74), mixture zone (0.55), turbidity (0.35), dissolved oxygen (−0.48), and NH4 (−0.42). The CCA diagram, concerning this axis, highlighted the temporal distinction of the sampling units corresponding to the period before the beginning of cultivation from the sampling units referring to the end of the same, mainly after 90 and 120 days, or the period when the Cryptophyceae species were more abundant in density (Fig. 5a, b).
Discussion
The significant increases in the concentration of total nitrogen and ammonium during the tilapia cultivation in net cages were possibly caused by an unconsumed ration and by the fish excreta released directly into the water column. However, the increase in nutrient concentrations did not present high magnitudes, probably due to the small number of net cages used in this study, the seasons (autumn/winter) when the experiment was performed, the settlement process, the nutrient absorption by aquatic photosynthetic communities and the hydrodynamics of the reservoir compartment, characterized by the large extension of the mixture zone, probably due to the strong influence of the wind.
In regard to the SRP, a significant decrease was observed in the mean values of this nutrient over time, probably due to its fast absorption by the phytoplankton, its settlement in the sediment and its displacement along the course of the Guairacá Stream. According to Alves & Baccarin (2005), the soluble nutrients may have their distribution influenced by the current flow and may not be concentrated just where they are released. Frequently, the turbidity increases after the net cage installation but in this study, the turbidity decreased. The highest values of rainfall and wind velocity probably caused higher turbidity before the net cage installation. The decrease in the rainfall and wind velocity over time contributed to reduce the turbulence, reducing turbidity and the depth of the mixed layer.
The direct release of the wastes generated by the fish farming in net cages into the water results in the deterioration of the environment and promotes alterations in the structure and dynamics of aquatic communities (Guo & Li, 2003; Agostinho et al., 2007). Higher phytoplankton densities recorded after the beginning of the tilapia feeding indicate the probable favoring of this community during the experiment, mainly due to the absorption of nutrients coming from the wastes generated by tilapia production. This fact was evidenced by the significant difference between the periods before and after the net cage installation.
In the case of the reservoirs, the capacity to dilute and minimize the impacts on the communities will depend on the water circulation. Furthermore, over time, the constant release of nutrients may exceed the environmental carrying capacity causing eutrophication, which has a considerable impact on planktonic communities (Guo & Li, 2003; Agostinho et al., 2007; Dias et al., 2010; Guo et al., 2009).
Together with the phytoplankton, the zooplankton is compounded by organisms with high environmental sensitivity and that can be used as bio-indicators of environmental changes caused by anthropogenic impacts (Pinto-Coelho et al., 2005; Eskinazi-Sant’Anna et al., 2007). Zooplankton also presented greater abundances, with a significant difference after the beginning of the experiment, with an increase in all groups soon after the beginning of the cultivation; for the rotifers, we also observed a second peak during the final period of this study.
The density of cladocerans and copepods was related to the water temperature and turbidity, nutrient concentration and food availability—mainly Cryptophyceae—since there was a decrease of these micro-crustaceans when the Cyanobacteria were dominant. Considering the rotifers, the greatest density of this group occurred together with higher values of dissolved oxygen and Cryptophyceae density, and the lowest occurred during the density peak of cladocerans, indicating probable competition between these zooplankton groups.
Cryptophyceae, dominant in density in the period before the beginning of cultivation and at the end of the experiment, were probably favored by higher concentrations of dissolved oxygen, as well as by the lower densities of cladocerans and copepods. The water input from the reservoir to the Guairacá Stream and the influence of the wind and current flow from the stream promote turbulence; several authors have observed that, during periods of water column mixing or after high turbulence, the Cryptophyceae are favored because the turbulence causes the redistribution of nutrients in the water column and reduces the predation pressure (Klaveness, 1988; Reynolds et al., 2002; Train et al., 2005). Prior studies performed in the Rosana Reservoir (Train et al., 2005; Rodrigues et al., 2005) also verified the high contribution of Cryptomonas species under conditions of water column mixing.
The susceptibility of Cryptophyceae to zooplankton predation is well discussed in the literature (Klaveness, 1988; Reynolds, 1997; Ventelä et al., 2002; Reynolds et al., 2002; Padisák et al., 2009) and was also observed in this study, explaining the decrease of this group at the same time of the highest density of the micro-crustaceans. The increase in predation pressure from zooplankton on small-sized phytoplankton species (<40 μm) and the consequent favoring of large-sized Cyanobacteria have been pointed out by several authors (Haney, 1987; Agasild et al., 2007; McCarthy et al., 2007; Bonecker et al., 2007). Therefore, the increase in predation pressure from cladocerans and copepods on the Cryptophyceae soon after the experiment began likely favored the increase of the Cyanobacteria.
Nevertheless, after the establishment of the Cyanobacteria bloom, especially of Radiocystis fernandoi, there was a reduction of micro-crustaceans that is ascribed to the dominance of colonial Cyanobacteria with large size and wide mucilage, less edible and more difficult to digest, and to the presence of toxic strains. The release of cyanotoxins in the water during the blooms of toxic Cyanobacteria causes the intoxication of several species, a reduction in herbivory from the zooplankton, and changes in trophic chains, and, consequently, the whole balance of the ecosystem is modified (Christoffersen, 1996; Chorus & Bartram, 1999; Codd, 2000; Dewes et al., 2007).
Several authors have also verified the negative effect of Cyanobacteria and their toxins on the development and growth of the zooplankton community (Haney, 1987; Lampert, 1987; DeMott, 1999; Ferrão-Filho et al., 2000; Ghadouani et al., 2003; Leflaive & Ten-Hage, 2007). Thus, the maintenance of zooplankton density during the beginning of the Cyanobacteria bloom indicates that, in this period, this community must have used other food resources, such as bacterio-plankton and heterotrophic flagellates (Dias et al., 2010) in addition to Cryptophyceae, as stated above, whose density decreased during this experimental phase.
The densities (as cells ml−1) of Cyanobacteria characterized established blooms (>20,000 cells ml−1) during some periods, and the establishment of alert level 1 (>2,000 < 100,000 cells ml−1) or even alert level 2 (>100,000 cells ml−1, at 90th day), downstream from the net cages), according to the Ordinance from the Brazilian Health Ministry 518/2004 (Brasil, 2004). Besides that, 16% from the analyzed samples presented values higher than the pattern established by the Brazilian Environmental Norm (CONAMA Resolution No. 357/2005) that establishes 50,000 cells ml−1 as the maximum limit of Cyanobacteria density for the water destined for aquiculture (Class 2). Thus, the obtained results evidence the need to claim the obligatoriness of the management and control of Cyanobacteria blooms in fishing farming areas, which must have corrective and preventive character.
Furthermore, the accumulation of microcystin in fish is an important route of exposure for humans regarding the recommendation about the maximum amount for the daily intake of microcystins by the World Health Organization, WHO (Chorus & Bartram, 1999). Thus, it is necessary to monitor these toxins in fish farming systems during Cyanobacteria blooms. It is possibly that closer study of toxin in cyanobacteria-impacted fish farming will permit the development of guidelines for fish consumption that will better protect public health.
Cyanobacteria, besides being favored by the lower predation pressure exerted by micro-crustaceans, are capable of changing their position in the water column and increasing the biogenic turbidity harming the other phytoplankton groups (Scheffer et al., 1997). This competitive advantage for this group explains the correlation with the highest value of turbidity, verified 15 days after the beginning of cultivation. Besides that, the Cyanobacteria’s ability to store phosphorus, already reported by other authors (Chorus & Bartram, 1999; Borges et al., 2008a, b; Bovo-Scomparin & Train, 2008), was probably an important competitive advantage for this group, considering the high N:P ratios (>14) observed during the experiment.
At the end of the experiment, we registered a reduction in the Cyanobacteria bloom, which might have been influenced by the increase in wind velocity, the reduction of water temperature, the predation exerted by the fish community and also by viral diseases that may affect the phytoplankton and influence the susceptibility of this community to zooplankton, since some viruses may cause the Cyanobacteria to break off, becoming edible to the rotifers (Van Hannen et al., 1999). Some authors argue that planktivorous and omnivorous fish, such as tilapia, may directly control phytoplankton through herbivory, and when they feed on larger algae, they allow the fast development of small-sized species (Attayde & Hansson, 2001; Figueredo & Giani, 2005; Silva & Arcifa, 2006). With the decrease of Cyanobacteria, there was an increase in the other phytoplankton groups, mainly Cryptophyceae, and consequently, also of rotifers, since the analyses indicated positive correlations between Cryptophyceae and rotifers. However, the susceptibility of phytoplankton to fish and viruses was not evaluated in this study.
Regarding the spatial scale, we did not detect significant differences for nutrient concentrations or for the density of phytoplankton and zooplankton, especially due to the hydrodynamic traits of the Guairacá Stream, a lotic environment strongly influenced by the wind, with a large extension of the mixture zone. Moreover, the proximity of the local area where the experiment was installed at the mouth of this stream at the Rosana Reservoir propitiated the water input from the Paranapanema River into this lateral arm and favored the homogeneous distribution of aquacultural wastes. This explains the spatial similarity observed for the nutrients and the density of the planktonic communities.
For the density of phytoplankton from the Guairacá Stream, in the influence area of the net cages, both abiotic factors and zooplankton density were important structuring factors. Nevertheless, increasing the number of net cages and fish used in commercial production systems would probably cause a remarkable increase in the nutrient concentrations and, consequently, cause drastic changes regarding the trophic webs.
The relationships between the zooplankton and Cyanobacteria depend on the species considered and on their several sizes and physiological states (Lampert, 1987); some zooplankton species may consume Cyanobacteria and become resistant to cyanotoxins (James & Forsyth, 1990; Turner et al., 1998; Panosso et al., 2003). Thus, we suggest studies about the body size of zooplankton organisms and their consumption rates in relation to the phytoplankton community in this environment, mainly regarding the ingestion of Cyanobacteria and the influence of this relationship for the dominance of this group, in addition to research focusing on predation by the fish community.
The results corroborate the predicted hypothesis since there was an increase in the concentrations of the total nitrogen and ammonium and phytoplankton density, particularly the Cyanobacteria, causing low predation pressure by the zooplankton, especially from the micro-crustaceans on these algae. However, the extent of the effects of the net cages on the nutrients and planktonic communities was small, probably due to the low number of net cages and fish employed, the seasons (autumn/winter) and the environmental hydrodynamic area, as mentioned above.
In view of the cumulative effect of wastes coming from the intensive cultivation of fish, there is a growing need for studies that evaluate the impact of net cages for tilapia cultivation with a greater number of cages installed during the spring/winter, since, in agreement with Alves & Baccarin (2005), the higher the environmental temperature is, the greater the fish metabolism and, as a result, the greater the ration inputs and excreta released in the aquatic environment. High temperatures also favor the development of Cyanobacteria blooms (Padisák, 1998; Chorus & Bartram, 1999; Reynolds et al., 2002).
For the continuous expansion of the aquaculture, it is necessary to ensure healthy freshwater ecosystems; therefore, we suggest that fish cultivation in net cages in reservoirs should be the subject of suitable management and permanent monitoring. There is also a need to apply correctly environmental practices and to execute and improve the laws that regulate aquaculture, aiming at the sustainability of this productive system and the maintenance of multiple uses of water in reservoirs.
References
Agasild, H., P. Zingel, I. Tõnno, J. Haberman & T. Nõges, 2007. Contribution of different zooplankton groups in grazing on phytoplankton in shallow eutrophic Lake Võrtsjärv (Estonia). Hydrobiologia 584: 167–177.
Agostinho, A. A., L. C. Gomes & F. M. Pelicice, 2007. Ecologia e Manejo de Recursos Pesqueiros em Reservatórios do Brasil. Eduem, Maringá.
Alves, R. C. P. & A. E. Baccarin, 2005. Ecologia de Reservatórios: Impactos Potenciais, Ações de Manejo e Sistemas em Cascata. São Carlos, Rima: 329–347.
American Public Health Association, 1995. Standard Methods for the Examination of Water and Wastewater, 19th ed. APHA, Washington DC.
Attayde, J. L. & L. A. Hansson, 2001. The relative importance of fish predation and excretion effects on planktonic communities. Limnology and Oceanography 46: 1001–1012.
Bonecker, C. C., M. Y. Nagae, M. C. M. Bletller, L. F. M. Velho & F. A. Lansac-Tôha, 2007. Zooplankton biomass in tropical reservoirs in southern Brazil. Hydrobiologia 579: 115–123.
Borges, P. A. F., S. Train & L. C. Rodrigues, 2008a. Spatial and temporal variation of phytoplankton in two subtropical Brazilian reservoirs. Hydrobiologia 607: 63–74.
Borges, P. A. F., S. Train & L. C. Rodrigues, 2008b. Estrutura do fitoplâncton, em curto período de tempo, em um braço do reservatório de Rosana (ribeirão do Corvo Paraná, Brasil). Acta Scientiarum Biological Science 30: 57–65.
Borghetti, N. R. B., A. O. Ostrensky & J. R. Borghetti, 2003. Aquicultura: Uma Visão Geral Sobre a Produção de Organismos Aquáticos no Brasil e no Mundo. Grupo integrado de aquicultura e estudos ambientais, Curitiba.
Bottrell, H. H., A. Duncan, Z. M. Gliwicz, E. Grygierek, A. Herzing, A. Hillbricht-Ilkowska, H. Kurasawa, P. Larsson & T. Weglenska, 1976. A review of some problems in zooplankton production studies. Norwegian Journal of Zoology 24: 419–456.
Bovo-Scomparin, V. M. & S. Train, 2008. Long-term variability of the phytoplankton community in an isolated floodplain lake of the Ivinhema River State Park, Brazil. Hydrobiologia 610: 331–344.
Brasil. 2004. Ministério da Saúde. Portaria no. 518. Estabelece os procedimentos e responsabilidades relativos ao controle e vigilância da qualidade da água para consumo humano e seus padrões de potabilidade, e dá outras providências.
Chorus, I. & J. Bartram, 1999. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management. WHO, E & FN Spon, London.
Christoffersen, K., 1996. Ecological implications of cyanobacteria toxins in aquatic food webs. Phycologia 35: 42–50.
Codd, G. A., 2000. Cyanobacterial toxins, the perception of water quality, and the prioritisation of eutrophication control. Ecological engineering 16: 51–60.
Codd, G. A., L. F. Morrison & J. S. Metcalf, 2005. Cyanobacterial toxins: risk management for health protection. Toxicology and Applied Pharmacology 203: 264–272.
Cole, G. A., 1994. Text Book of Limnology. Waveland Press Inc., Prospect Heights, IL.
Degans, H. & L. D. Meester, 2002. Top-down control of natural phyto and bacterioplankton prey communities by Daphnia magna by the natural zooplankton community of the hypertrophic Lake Blankaart. Hydrobiologia 479: 39–49.
DeMott, W. R., 1999. Foraging strategies and growth inhibition in five daphnids feeding on mixture of a toxic cyanobacterium and green alga. Freshwater Biology 42: 263–274.
Dewes, L. J., J. M. Monserrat, P. M. Iharal & J. S. Yunes, 2007. Toxin Accumulation and Biochemical Alterations in the Estuarine Crab Neohelice granulata (Decapoda, Brachyura) After Exposure to Microcystins. Journal of the Brazilian Society of Ecotoxicology 2: 203–209.
Dias, J. D., E. M. Takahashi & C.C. Bonecker, 2010. Impact of fish cage-culture on the community structure of zooplankton in a tropical reservoir. Limnologica (submitted).
Eskinazi-Sant’Anna, E. M., R. Menezes, I. S. Costa, R. F. Panosso, M. Araujo & J. L. Attayde, 2007. Composição da comunidade zooplanctônica em reservatórios eutróficos do semi-árido do Rio Grande do Norte. Oecologia Brasiliensis 11: 410–421.
Ferrão-Filho, A. S., S. M. F. O. Azevedo & W. R. Demott, 2000. Effects of toxic and non-toxic cyanobacteria on the life history of tropical and temperate cladocerans. Freshwater Biology 45: 1–19.
Figueredo, C. C. & A. Giani, 2005. Ecological interactions between Nile Tilapia (Oreochromis niloticus. L.) and the phytoplanktonic community of the Furnas reservoir (Brazil). Freshwater Biology 50: 1391–1403.
Gasiunaité, Z. R. & I. Olenina, 1998. Zooplankton-phytoplankton interactions: a possible explanation of the seasonal succession in the Kuršiu Marios lagoon. Hydrobiologia 363: 333–339.
Ghadouani, A., B. Pinel-Alloul & E. E. Prepas, 2003. Effects of experimentally induced cyanobacterial blooms on crustacean zooplankton communities. Freshwater Biology 48: 363–381.
Giné, M. F., H. Bergamin, E. A. G. Zagatto & B. F. Reis, 1980. Simultaneous determination of nitrite and nitrate by flow injection analysis. Analytica Chimica Acta 114: 191–197.
Golterman, H. L., R. S. Clymo & M. A. M. Ohstad, 1978. Methods for Physical and Chemical Analysis of Freshwater. Blackwell Scientific Publication, Oxford.
González, E. J., 2000. Nutrient enrichment and zooplankton effects on the phytoplankton community in microcosms from El Andino reservoir (Venezuela). Hydrobiologia 434: 81–96.
Gosselain, V., L. Viroux & J. P. Descy, 1998. Can a community of small-bodied grazers control phytoplankton in rivers? Freshwater Biology 39: 9–24.
Guo, L. & Z. Li, 2003. Effects of nitrogen and phosphorus from fish cage-culture on the communities of a shallow lake in middle Yangtze River basin of China. Aquaculture 226: 201–212.
Guo, L., L. Zhongjie, P. Xie & L. Ni, 2009. Assessment effects of cage culture on nitrogen and phosphorus dynamics in relation to fallowing in a shallow lake in China. Aquaculture International 17: 229–241.
Haney, J. F., 1987. Field studies on zooplankton–cyanobacteria interactions. New Zealand Journal of Marine and Freshwater Research 21: 467–475.
James, M. R. & D. J. Forsyth, 1990. Zooplankton–phytoplankton interactions in a eutrophic lake. Journal of Plankton Research 12: 455–472.
Jensen, J. P., E. Jeppesen, K. Olrik & P. Kristensen, 1994. Impact of nutrients and physical factors on a shift from Cyanobacterial to Chlorophyte dominance in shallow Danish lakes. Canadian Journal of Fisheries and Aquatic Science 51: 1692–1699.
Klaveness, D., 1988. Ecology of the Cryptomonadida: a first review. In Sandgren, S. D. (ed.), Growth and Reproductive Strategies of Freshwater Phytoplankton. Cambridge University Press, Cambridge: 105–133.
Koroleff, K., 1976. Determination of ammonia. In Grasshoff, K. & E. Kremling (eds), Methods of Seawater Analysis. Verlag Chemie, Winhein.
Kozak, A. & R. Goldyn, 2004. Zooplankton versus phyto and bacterioplankton in the Maltanski reservoir (Poland) during an extensive biomanipulation experiment. Journal of Plankton Research 26: 37–48.
Lampert, W., 1987. Laboratory studies on zooplankton-cyanobacteria interactions. New Zealand Journal of Marine and Freshwater Research 21: 483–490.
Leflaive, J. & L. Ten-Hage, 2007. Algal and cyanobacterial secondary metabolites in freshwaters: a comparison of allelopathic compounds and toxins. Freshwater Biology 52: 199–214.
Mackereth, F. Y. H., J. R. Heron & J. F. Tailing, 1978. Water analysis: some revised methods for limnologists. Scientific Publication, 36. Freshwater Biological Association.
McCarthy, M. J., P. J. Lavrentyev, L. Yang, L. Zhang, Y. Chen, B. Qin & W. S. Gardner, 2007. Nitrogen dynamics and microbial food web structure during a summer cyanobacterial bloom in a subtropical, shallow, well-mixed, eutrophic lake (Lake Taihu, China). Hydrobiologia 581: 195–207.
McCune, B. & M. J. Mefford, 1999. PC-ORD. Multivariate Analysis of Ecological Data, version 4.0. MjM Software Design. Gleneden Blach, Oregon.
Naylor, R. L., R. J. Goldburg, J. H. Primavera, N. Kautsky, M. C. M. Beveridge, J. Clay, C. Folke, J. Lubchenco, H. Mooney & M. Troell, 2000. Effect of aquaculture on world fish supplies. Nature 405: 1017–1024.
Padisák, J., 1998. Sudden and gradual responses of phytoplankton to global climate change: case studies from two large, shallow lakes (Balaton, Hungary; Neusiedlersee, Austria/Hungary). In George, D. G., J. G. Jones, P. Puncochar, C. S. Reynolds & D. W. Sutcliffe (eds), Management of Lakes and Reservoirs During Global Climate. Kluwer Academic Publish, Dordrecht: 111–125.
Padisák, J., L. C. Crossetti & L. Naselli-Flores, 2009. Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia 621: 1–19.
Pagioro, T. A., M. C. Roberto, S. M. Thomaz, S. A. Pierini & M. Taka, 2005. Zonação longitudinal das variáveis limnológicas abióticas em reservatórios. In Rodrigues, L., S. M. Thomaz, A. A. Agostinho & L. C. Gomes (eds), Biocenoses em Reservatórios: Padrões Espaciais e Temporais. São Carlos, Rima: 39–46.
Panosso, R., P. Carlsson, B. Kozlowsky-Suzuki, S. M. O. Azevedo & A. E. Granéli, 2003. Effect of grazing by a neotropical copepod, Notodiaptomus, on a natural cyanobacterial assemblage and on toxic and non-toxic cyanobacterial strains. Journal of Plankton Research 25: 1169–1175.
Pinto-Coelho, R. M., B. Pinel-Alloul, G. Méthot & K. E. Havens, 2005. Crustacean zooplankton in lakes and reservoirs of temperate and tropical regions: variation with trophic status. Canadian Journal of Fisheries and Aquatic Science 62: 348–361.
Reynolds, C. S., 1997. Vegetation Process in the Pelagic: A Model for Ecosystem Theory. Ecology Institute, Oldendorf.
Reynolds, C. S., V. L. M. Huszar, C. Kruk, L. Naselli-Flores & S. Melo, 2002. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research 24: 417–428.
Rodrigues, L. C., S. Train, B. M. Pivato, V. M. Bovo, P. A. F. Borges & S. Jati, 2005. Assembléias fitoplanctônicas de trinta reservatórios do Estado do Paraná. In Rodrigues, L., S. M. Thomaz, A. A. Agostinho & L. C. Gomes (eds), Biocenoses em Reservatórios: Padrões Espaciais e Temporais. São Carlos, Rima: 57–72.
Scheffer, M., S. Rinaldi, A. Gragnani, L. R. Mur & E. H. V. Nes, 1997. On the dominance of filamentous cyanobacteria in shallow turbid lakes. Ecology 78: 272–282.
Silva, L. H. S. & M. S. Arcifa, 2006. Interação Entre Fitoplâncton, Zooplâncton e Peixe: Herbivoria e Reciclagem de Nutrientes. Boletim da Sociedade Brasileira de Limnologia 35: 82–86.
Ter Braak, C. J. F., 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67: 1167–1179.
Train, S., S. Jati, L. C. Rodrigues & B. M. Pivato, 2005. Distribuição Espacial e Temporal do Fitoplâncton em Três Reservatórios da Bacia do Rio Paraná. In Rodrigues, L., S. M. Thomaz, A. A. Agostinho & L. C. Gomes (eds), Biocenoses em Reservatórios: Padrões Espaciais e Temporais. São Carlos, Rima: 73–85.
Turner, J., R. Hopcroft, J. A. Lincoln, C. S. Huestis, P. A. Tester & J. C. Roff, 1998. Zooplankton feeding ecology: grazing by marine copepods and cladocerans upon phytoplankton and cyanobacteria from Kingston Harbour, Jamaica. Marine Ecology 19: 195–208.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen phytoplankton-methodic. Mitteilungen Internationale Vereinigung für Theoretische und Angewandte Limnologie 9: 1–38.
Van Hannen, E. J., G. Zwart, M. P. Van Agterveld, H. J. Gons, J. Ebert & H. J. Laanbroek, 1999. Changes in bacterial and eukaryotic community structure after mass lysis of filamentous cyanobacteria associated with viruses. Applied and Environmental Microbiology 65: 795–801.
Ventelä, A., K. Wiackowski, M. Moilanen, V. Saarikari, K. Vuorio & J. Sarvala, 2002. The effect of small zooplankton on the microbial loop and edible algae during a cyanobacterial bloom. Freshwater Biology 47: 1807–1819.
Acknowledgments
The authors are grateful to the Núcleo de Pesquisas em Limnologia, Ictiologia e Aqüicultura (Nupélia) at Universidade Estadual de Maringá, for logistical support, to researchers of the Basic Limnology Laboratory of Nupélia for determining water abiotic factors, to Agência Nacional de Águas (ANA), to CNPq/PRONEX for financial support, to CAPES for a scholarship granted to the first author, and to two anonymous referees for their excellent contributions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luigi Naselli-Flores
An erratum to this article can be found at http://dx.doi.org/10.1007/s10750-010-0298-4
Rights and permissions
About this article
Cite this article
Borges, P.A.F., Train, S., Dias, J.D. et al. Effects of fish farming on plankton structure in a Brazilian tropical reservoir. Hydrobiologia 649, 279–291 (2010). https://doi.org/10.1007/s10750-010-0271-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0271-2