Abstract
In winter of 2009/2010, Aphanizomenon flos-aquae bloomed in the ice and snow covered oligo-mesotrophic Lake Stechlin, Germany. The photosynthesis of the natural population was measured at eight temperatures in the range of 2–35°C, at nine different irradiance levels in the range of 0–1,320 μmol m−2 s−1 PAR at each applied temperature. The photoadaptation parameter (I k) and the maximum photosynthetic rate (P max) correlated positively with the temperature between 2 and 30°C, and there was a remarkable drop in both parameters at 35°C. The low I k at low temperatures enabled the active photosynthesis of overwintering populations at low irradiance levels under ice and snow cover. The optimum of the photosynthesis was above 20°C at irradiances above 150 μmol m−2 s−1. At lower irradiance levels (7.5–30 μmol m−2 s−1), the photosynthesis was the most intensive in the temperature range of 2–5°C. The interaction between light and temperature allowed the proliferation of A. flos-aquae in Lake Stechlin resulting in winter water bloom in this oligo-mesotrophic lake. The applied 2°C is the lowest experimental temperature ever in the photosynthesis/growth studies of A. flos-aquae, and the results of the P–I and P–T measurements provide novel information about the tolerance and physiological plasticity of this species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The dynamics of phytoplankton populations are controlled by multiple factors including physiological and evolutionary adaptations, environmental and biological processes. The interactions between the different factors are important in understanding the response of organisms to these variables. The effects of temperature on phytoplankton cellular processes and growth are well known (e.g. Reynolds, 1984), being temperature an important, but not the only factor in determining the occurrence of a particular algal species. The physiological adaptations may contribute to the success of certain phytoplankton taxa to become efficient competitors, as demonstrated on several species of cyanobacteria (e.g. Padisák, 1997; Price et al., 1998). Cyanobacterial dominance commonly occurs in eutrophic water bodies (e.g. Yamamoto & Nakahara, 2009a) at water temperatures above 20°C due to the high temperature optima of their growth (e.g. Reynolds, 1984; Wilhelm & Adrian, 2008), but the success of cyanobacteria is a result of their response to interactions between different environmental constraints (e.g. Dokulil & Teubner, 2000).
Aphanizomenon flos-aquae (L.) Ralfs is a filamentous and heterocytic cyanobacterium, which is capable of N2 fixation (e.g. De Nobel et al., 1998; Reynolds et al., 2002). It produces akinetes that enable survival in unfavourable growth conditions (Yamamoto & Nakahara, 2007). The temperature optimum of its growth is above 20°C (e.g. Uehlinger, 1981; Dokulil & Teubner, 2000), but it depends on the light intensities at which the population was grown (Konopka & Brock, 1978). The species prefers shallow eutrophic freshwaters (e.g. Yamamoto & Nakahara, 2009a, b), and since external load reduction resulted in nitrogen scarcity in many lakes, relative biomass share of N-fixing genera, like Aphanizomenon, increased in many lakes during the last decades (Reynolds et al., 2002; Wagner & Adrian, 2009). Aphanizomenon flos-aquae cells contain gas vesicles, which have a significant role in regulating buoyancy (e.g. Walsby, 1994). It can accumulate in the illuminated layer near the water surface, developing a surface bloom (e.g. Dokulil & Teubner, 2000; Preußel et al., 2009). Temperature and light intensity are key factors in the regulation the growth of Nostocales (Mehnert et al., 2010). The competition between A. flos-aquae and other cyanobacterial species for light at different temperatures was investigated in several studies (e.g. De Nobel et al., 1998; Huisman et al., 1999; Yamamoto & Nakahara, 2006; Wagner & Adrian, 2009). The current knowledge about the temperature- and light-dependent growth of A. flos-aquae in temperature regions >15°C is well documented, but information is scarce about its winter occurrence. Wildman et al. (1975) found all developmental stages of A. flos-aquae in samples from an ice-covered lake in November. In another study, some filaments of the species were present in the phytoplankton throughout the winter months until the onset of exponential growth in mid-May in Kinnego Bay, Ireland (Jones, 1979). The possibility that these ‘overwintering’ filaments may have been the result of continuous germination of akinetes through the winter was discounted, since the observed filaments in winter and early spring were mostly long filaments consisted of many cells (Jones, 1979).
Aphanizomenon flos-aquae appeared in the oligo-mesotrophic, deep Lake Stechlin (Germany) first in 2001. Until 2006, the species was seasonal and sporadic between early summer and the autumnal overturn. Since 2006, it provided biomass peaks in late summers. In 2009, the biomass of the late summer peak was 400–500 μg wet weight L−1. In autumn a drop of the biomass was observed, but after the autumnal overturn the population of A. flos-aquae began to grow, and provided another biomass peak in winter with 915–920 μg wet weight L−1. In December, a macroscopically visible surface bloom of A. flos-aquae developed along the shorelines of the lake. Between December 2009 and January 2010, A. flos-aquae contributed 87–90% to total biomass therefore developed a sufficiently long-lasting winter equilibrium phase (Padisák et al., 2010) which continued in February 2010 when 17 cm thick ice developed on the lake and was covered by 20 cm snow. According to our knowledge about the species, appearance of A. flos-aquae in Lake Stechlin, and its winter bloom under ice cover were unexpected phenomena. They triggered the questions that the filaments of A. flos-aquae were physiologically active or not and that the bloom under winter conditions was a result of its low-temperature tolerance or low-temperature preference.
This study focused on the question of how temperature and irradiance level influence the photosynthetic activity of A. flos-aquae. We assumed that the interactions between temperature and the light availability would
-
(i)
cause corresponding changes in the P–I (photosynthesis–irradiance) parameters of A. flos-aquae,
-
(ii)
determine the occurrence of the species under extreme conditions, and
-
(iii)
due to its physiologically distinctive features it could reach high biomass with active photosynthesis in an ice-covered lake.
To test these hypotheses, the photosynthetic activity of a natural population of A. flos-aquae collected on 26th February 2010 in Lake Stechlin was investigated at eight different temperatures (2–35°C) and at nine different irradiance levels (0–1,320 μmol m−2 s−1) in laboratory.
Materials and methods
Site description, sampling
Lake Stechlin is a medium-sized (4.2 km2), deep (z mean: 23.3 m; z max: 69.5 m) lake without surface inflows at 53°10′N latitude, 13°02′E longitude and 84.5 m a.s.l. in Brandenburg, Germany. The lake has glacial origin, was originally oligotrophic, but during the last decade it turned oligo-mesotrophic. In February 2010, the following concentrations of nutrient were measured: SRP: 8.5 μg L−1; TP: 27.7 μg L−1; NO3 −-N: 58.7 μg L−1 (APHA, 1998).
Phytoplankton samples were taken on 26th February 2010 at a sampling site in front of the Leibniz-Institute of Freshwater Ecology and Inland Fisheries (Fig. 1), where the water depth was 26 m. At the sampling occasion, temperature and photosynthetically active radiation (PAR) (Li 192A Underwater Quantum Sensor, LI-COR Biosciences, Lincoln, Nebraska, USA) profiles were taken in the water column of the lake: the sensors were sank into different depth through a leak, while leak was recovered with ice and snow to get the real in situ temperature and light values during the measurements. The in situ temperature ranged between 0.3 and 2°C in the upper 10 m layer. The euphotic zone expanded to depth of 10–12 m. Samples were taken with a Van Dorn sampler in 2 m increments from the euphotic depths then were mixed (integrated sample). The sampling location and light field (under 17 cm thick ice and 20 cm snow cover) are shown on Fig. 1. Vertical light attenuation coefficient, K d (m−1) of different layers of the water column was calculated with the Lambert–Beer function (Kirk, 1994) from simultaneous measurements of irradiances per metre (0–20 m) in the field at time of the sampling. The K d was in the upper 1 m layer the highest, 1.33 m−1. The average K d of the layers between 1 and 20 m was 0.42 ± 0.15 m−1; the euphotic layer was lying at the depth of 11 m (Fig. 1).
Lake Stechlin and the sampling location in the lake. On the chart right the light field of the water column, covered by 17 cm thick ice and 20 cm snow, was shown at the sampling occasion. On 26th February 2010, the irradiance level was 134 μmol m−2 s−1 at 0 m (immediately below the ice), which corresponded to the 50% of the incident irradiance level
The samples were kept in the dark and cool for 24–36 h, while they were transported from Germany to the location of the laboratory measurements at the University of Pannonia, Veszprém, Hungary. Subsamples of 300 ml were preserved in Lugol solution for microscopic counting (Utermöhl, 1958). The chlorophyll a concentrations of the samples were measured according to Wetzel & Likens (2000).
P–I and P–T measurements
The measurements were carried out in a laboratory incubation system (Üveges et al., 2011). The photosynthetic activity of the natural population was measured by the 14C method (Steemann-Nielsen, 1952). The rate of photosynthesis was determined by adding NaH14CO3 with known activity (0.099181–0.114185 MBq) to 15 ml of samples in 20 ml scintillation vials (Econo Glass Vial, PerkinElmer, Waltham, MA, USA). The vials were pre-incubated at appropriate temperatures for 1 h before the addition of radioisotope to equilibrate the contents to the experimental condition. Three vials were incubated as replicates at each irradiance level. The photosynthesis of the natural population was measured for 2 h after the addition of radioisotope at nine irradiance levels (0; 7.5; 30; 75; 150; 260; 500; 920; 1,320 μmol m−2 s−1 PAR) at each applied temperature (2; 5; 10; 15; 20; 25; 30; 35°C). In all experiments, three vials were wrapped in aluminium foil to serve as dark control; they were incubated at 0 μmol m−2 s−1 irradiance. After the 2 h of incubation and removing of the externally attached radioisotope, the incorporation of 14C into algal protein was measured in each vial with liquid scintillation analyzer (Packard Tri-Carb 3180TR/SL, GMI Inc., Ramsey, MN, USA). Non-photosynthetic C uptake, which was determined in control vials kept in the dark, was subtracted from photosynthetic C uptake.
To fit curves for photosynthesis vs. irradiance data and to determine the P–I parameters (Table 1), the GraFit software was applied (GraFit by R. Leatherbarrow, 1989–1992 Erithacus Software Ltd.) with the equation of Platt et al. (1980). The Platt et al. (1980) equation fits most photoinhibition data fairly well:
where P is the photosynthetic rate at irradiance I, P s is the maximum photosynthesis obtained in the absence of photoinhibition, and α and β are parameters describing the initial slope and the photoinhibited section of the P–I curve, respectively. Photosynthetic parameters like maximal photosynthetic rate (P max) and photoadaptation parameter (I k = P max/α) were derived from the previous parameters (Table 1).
Q 10
The Q 10 model (Ahlgren, 1987) was employed to describe the relationship between photosynthesis and temperature:
where \( P_{{{\text{I}}(T_{2} )}} \) and \( P_{{{\text{I}}(T_{1} )}} \) are photosynthetic activity at given irradiance level (I) at two temperatures, T 2 and T 1, respectively. In fact, algal growth rates increase up to the optimal temperature beyond which they decrease due to stressful conditions, so Q 10 was calculated from the linear section of the photosynthesis–temperature (P–T) curves.
Results
Phytoplankton sample
On 26th February 2010, the chlorophyll a content of the integrated sample from 0 to 10 m upper layer of Lake Stechlin was 4.39 ± 0.40 μg chl a L−1. According to the microscopic analysis, A. flos-aquae dominated in the samples; the aggregated filaments of A. flos-aquae provided more than 99% of the total biomass. The rest 1% was provided by the diatom Stephanodiscus neoastraea. Therefore, from ecophysiological point of view the natural samples can be considered as a monoculture of A. flos-aquae.
P–I characteristics
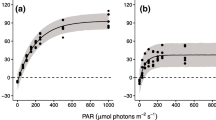
The maximum photosynthetic rate (P max) varied between 3.96 and 19.42 μg C L−1 h−1 (Fig. 2; Table 1). The population reached the highest P max at 30°C, and the lowest at 2°C. At 2 and 5°C the fixed carbon was 20 and 27% of that fixed at 30°C. A substantial decrease in the P max was measured between 10 and 5°C: A. flos-aquae photosynthesised at 50% of the maximum rate at 10°C. At 35°C, the P max declined to 54% of that measured at 30°C. In the temperature range of 2–30°C, the P max correlated positively with the temperature (r = 0.975; P < 0.001; n = 7).
The initial slope of the P–I curve (Fig. 2), α correlated negatively (r = −0.876; P < 0.001; n = 7) with the temperature; varied between 0.109 and 0.195 μg C L−1 h−1 (μmol m−2 s−1)−1. Photoinhibition was observed at different irradiance levels in the experiments carried out at different temperatures (Fig. 2). At 2°C, the community was inhibited at the irradiance of 500 μmol m−2 s−1 by 20% and at 1,320 μmol m−2 s−1 by 55% relative to photosynthesis at 75 μmol m−2 s−1. At 25°C, the photosynthesis was inhibited only at the highest irradiance levels, at 920 and 1,320 μmol m−2 s−1. The strongest photoinhibition was observed in the experiment carried out at 10°C, the community was inhibited at 1,320 μmol m−2 s−1 by 54% relative to photosynthesis at irradiance of 260 μmol m−2 s−1, in the region of P max.
The photoadaptation parameter, I k showed a strong positive correlation with the temperature in the range of 2–30°C (r = 0.985; P < 0.001; n = 7).
P–T characteristics
In the irradiance range of 260–1,320 μmol m−2 s−1, the carbon uptake increased with the increasing temperature until the temperature reached the 30°C (Fig. 3A), strong positive correlation was observed in all cases (P < 0.001; n = 21): r = 0.989 at 1,320 μmol m−2 s−1; r = 0.986 at 920 μmol m−2 s−1; r = 0.983 at 500 μmol m−2 s−1; and r = 0.954 at 260 μmol m−2 s−1. The photosynthesis at these irradiance levels was inhibited by 41–48% at 35°C relative to that at 30°C. Strong positive correlation between carbon uptake and temperature was found only at the upper temperature region (2–10°C) at the irradiances of 150 and 75 μmol m−2 s−1 (r = 0.984 and r = 0.971; P < 0.001; n = 9) (Fig. 3B). At low irradiance levels, the photosynthetic activity correlated negatively with the temperature (Fig. 3C). At irradiance level of 30 μmol m−2 s−1, the carbon uptake did not change remarkably with the increasing temperature, but above 15°C a negative correlation was observed (r = −0.851; P < 0.001; n = 15). At the lowest irradiance level (7.5 μmol m−2 s−1), the photosynthesis of A. flos-aquae showed a strong negative correlation with the temperature in the whole temperature range (r = −0.955; P < 0.001; n = 24) (Fig. 3C).
Q 10 of the photosynthetic activity
Q 10 values computed for the linear section of the P–T curve (Fig. 3) varied from 0.67 to 2.88 (Fig. 4). At the lowest applied irradiances the photosynthesis did not show positive correlation to the temperature, the Q 10 was less than 1. At the mid-irradiance levels (75–260 μmol m−2 s−1) the average Q 10 was 1.70 ± 0.03, which suggests lower variation of photosynthesis with temperature, than at the highest irradiance levels (500–1,320 μmol m−2 s−1), where Q 10 varied from 1.85 to 2.88.
Discussion
After the first appearance of A. flos-aquae in Lake Stechlin (Padisák et al., 2010), the life cycle of the species corresponded to the usual patterns described by Yamamoto & Nakahara (2009b) until 2009, when it overwintered with filaments near to the water surface. Overwintering filaments of the species were observed in previous studies (e.g. Simona, 2003; Yamamoto, 2009), however, physiological activity of the observed winter populations has not been studied. Yamamoto & Nakahara (2007) explained the winter appearance with the high biomass in summer which did not disappear completely in winter. It was certainly not the case for the Lake Stechlin A. flos-aquae population in winter of 2009, since morphology of summer and autumn filaments were clearly different: in contrast to the summer filaments, those found in winter did not include heretocytes. In a study from 2009, Yamamoto & Nakahara (2009a) concluded that the increase of population density of A. flos-aquae in winter might be a result of the adaptation of the species to low water temperatures.
The influence of temperature on the cellular metabolism of the species was addressed in several studies. Konopka & Brock (1978) isolated Aphanizomenon from Lake Mendota, and studied the influence of temperature on rate of carbon uptake under low-light intensities. They found, that the optimal temperature of A. flos-aquae for photosynthesis was 20°C, but at 10°C Aphanizomenon was still photosynthesising at 8% of the maximum rate. In other studies, the optimum temperature of Aphanizomenon’s growth varied between 20 and 28°C similar to our results at saturating and sub-saturating irradiances, but in these studies the lower limit was found between 4 and 10°C (Uehlinger, 1981; Tsujimura et al., 2001). We assume that the pre-experiment growth temperature could affect the temperature responses of A. flos-aquae, as it has been shown in case of Anabaena (Scherer et al., 1981). This was confirmed by the results of Yamamoto & Nakahara (2005, 2006). They found, that the growth of A. flos-aquae maintained at 20°C, ceased at water temperature 11–12°C, and the lowest temperature at which A. flos-aquae could grow was 14°C. Konopka & Brock (1978) observed significant photosynthesis of natural samples at 4°C, but those samples were collected or maintained at higher temperatures than the natural samples in this study. At the highest temperature (35°C) applied in our experiment, there was a drop in the photosynthesis of the natural population of A. flos-aquae; the species showed moderate photosynthetic activity, similar to the results of Butterwick et al. (2005). Previously the photosynthetic activity of overwintering natural populations of A. flos-aquae was not studied at such a low temperature (2°C) and irradiance (7.5 μmol m−2 s−1), therefore our findings in this temperature and irradiance levels cannot be compared to results of previous studies.
According to the P–T curves, the photosynthetic activity correlated negatively with the temperature under light-limited conditions. New and interesting result is that at the lowest irradiance level (7.5 μmol m−2 s−1) the temperature optimum was at 2°C, and it was between 2 and 10°C at the irradiance of 30 μmol m−2 s−1. For biological functions, the Q 10 value is ranges generally between 1 and 3 (e.g. Bissinger et al., 2008). According to our results, the Q 10 values at the applied lowest irradiances were <1 (0.67–0.87), and at irradiances >150 μmol m−2 s−1 it was between 1.67 and 2.88, similarly to other studies. These values suggest responsiveness to temperature changes only at mid and higher irradiance levels. These results can change our previous knowledge about the temperature dependence of the photosynthetic activity, and suggest that the responsiveness to temperature changes can be affected by other environmental parameters, like irradiance level. The interaction between temperature and the light availability affected the photosynthetic characteristics of A. flos-aquae, but the physiological distinctive features and survival strategies of the species contributed to the success at such extreme conditions.
The ability of A. flos-aquae to adapt to light-limited conditions was found higher than other cyanobacterial species (De Nobel et al., 1998). According to our study, the photoadaptation parameter was at each temperature low, the values were similar to those measured by De Nobel et al. (1998). The natural population of A. flos-aquae was more susceptible to photoinhibition due to the low-light conditions they grow in Lake Stechlin. At 2°C, the photoadaptation parameter was very low (20 μmol m−2 s−1), but the photosynthetic efficiency was at this temperature the highest in the light-limited region, which suggests a competitive advantage of A. flos-aquae in case of light-limited conditions. The increase in the photosynthetic efficiency could have been caused by the changes in the ratio of phycobilin/chlorophyll a (Vincent, 2007). Photoinhibition was observed at each temperature (except 35°C), the poor ability to utilize high irradiances indicates the adaptation to low irradiance levels. Photoinhibition by PAR usually becomes increasingly severe at low temperatures (e.g. Krause, 1994), but in this study we found low photoinhibition in the low-temperature range. The low photoinhibition at low temperatures can be attributed to the changed pigment composition and function of the photosystems (Lazarova et al., 2009); similar responses were observed in polar cyanobacteria to withstand the extremes of their environment (e.g. Vincent, 2007).
The deficiency of this study is that the pigment composition of the community was not examined; only the chlorophyll a was measured. According to previous studies cyanobacteria which contain phycoerythrin and phycoerythrocyanin, like A. flos-aquae, have competitive advantage in light-limited environments (Huisman et al., 1999). Phycoerythrin and phycoerytrocyanin synthesis did appear to be affected by light intensity (Gervais et al., 1997) and composition (Huisman et al., 1999). The light availability under the ice and snow cover was very low in Lake Stechlin in winter 2009/2010, the irradiance varied between 15 and 130 μmol m−2 s−1 in the upper 3 m layer during the day. Assuming that the spectral composition of light was similar in Lake Stechlin to that in Lake Pääjärvi (Lei et al., 2011), the green light dominated in the euphotic layer. At low-light conditions, A. flos-aquae could absorb green light (525–600 nm range) more efficiently because of its pigment composition (De Nobel et al., 1998; Huisman et al., 1999), therefore the spectral composition of the light under the ice and snow cover was favourable to its development. Despite our results show that the physiological plasticity of the species could enable them to develop deep chlorophyll maxima (DCM), in previous studies the appearance of the species in deeper water layers was not described nor in winter either in summer periods.
According to our results, the studied A. flos-aquae population had very similar ecophysiological characteristics to high-latitude cyanobacteria, which are dominant in cold ecosystems (Vincent, 2000): (i) A. flos-aquae could grow over a wide temperature range; (ii) at low temperatures it grows at slow rates; (iii) the low photoadaptation parameter enables the growth in dim light environment. Most of the previous studies about cyanobacteria supported the tenet that the optimum of photosynthesis and growth are above 20°C (e.g. Paerl & Huisman, 2008). This was also observed for cyanobacteria isolated from Antarctica: the species could grow over a wide temperature range (5–30°), but most of them were unable to grow at temperatures <5°C. Therefore, the polar cyanobacteria species are thought to be originated from warmer temperate regions (Seaburg et al., 1981). During long period of their evolution, they developed various adaptive mechanisms (e.g. Vincent, 2000), which contributed to their success and dominance in cold ecosystems. Microbial communities of cold environments are often unusual and intrinsically interesting because they have been subject to long periods of isolation with relatively low levels of disturbance (Vyverman et al., 2010). Not only the climate change (e.g. Vincent et al., 2009, 2011), but the species with high ecophysiological plasticity and success may have profound effects on the structure and efficiency of the food webs. A. flos-aquae, because of its ecophysiological plasticity and two temperature optima of its photosynthesis, can spread in habitats where its appearance was inconceivable according to our previous knowledge. The spread of different cyanobacterial species in temperate and polar regions at higher latitudes is often explained with the climate change, but an ongoing cyanobacterial adaptation cannot be ignored. The ecophysiological characteristics of species, like A. flos-aquae, may help us to understand the previous adaptation of cyanobacteria to cold environments, and their success in invasion and in extreme environments.
References
Ahlgren, G., 1987. Temperature functions in biology and their application to algal growth constants. Oikos 49: 177–190.
APHA – American Public Health Association, 1998. Standard Methods for the Examination of Water and Wastewater, 20th ed. United Book Press, Inc., Baltimore, MD.
Bissinger, J. E., D. J. S. Montagnes, J. Sharples & D. Atkinson, 2008. Predicting marine phytoplankton maximum growth rates from temperature: improving on the Eppley curve using quantile regression. Limnology and Oceanography 53: 487–493.
Butterwick, C., S. I. Heaney & J. F. Talling, 2005. Diversity in the influence of temperature on the growth rates of freshwater algae, and its ecological relevance. Freshwater Biology 50: 291–300.
De Nobel, W. T., C. P. H. Matthijs, E. von Elert & L. R. Mur, 1998. Comparison of the light-limited growth of the nitrogen-fixing cyanobacteria Anabaena and Aphanizomenon. New Phytologist 138: 579–587.
Dokulil, M. T. & K. Teubner, 2000. Cyanobacterial dominance in lakes. Hydrobiologia 438: 1–12.
Gervais, F., J. Padisák & R. Koschel, 1997. Do light quality and low nutrient concentration favour picocyanobacteria below the thermocline of the oligotrophic Lake Stechlin? Journal of Plankton Research 19: 771–781.
Huisman, J., R. R. Jonker, C. Zonneveld & F. J. Weissing, 1999. Competition for light between phytoplankton species: experimental tests of mechanistic theory. Ecology 80: 211–222.
Jones, R. I., 1979. Notes on the growth and sporulation of a natural population of Aphanizomenon flos-aquae. Hydrobiologia 62: 55–58.
Kirk, J. T. O., 1994. Light and Photosynthesis in Aquatic Ecosystems, 2nd ed. Cambridge University Press, Cambridge.
Konopka, A. & T. D. Brock, 1978. Effect of temperature on blue-green algae (cyanobacteria) in Lake Mendota. Applied and Environmental Microbiology 36: 572–576.
Krause, G. H., 1994. Photoinhibition induced by low temperatures. In Baker, N. R. & J. R. Bower (eds), Photoinhibition of Photosynthesis from Molecular Mechanisms to the Field. Bios Scientific Publishers, Oxford: 331–348.
Lazarova, D., A. Popova, D. Stanoeva & M. Velitchkova, 2009. Involvement of reactive oxygen radicals in photoinhibition of primary photosynthetic reactions—effect of temperature and oxygen radical scavengers. Biotechnology & Biotechnological Equipment 23: 511–515.
Lei, R., M. Leppäranta, A. Erm, E. Jaatinen & O. Pärn, 2011. Field investigations of apparent optical properties of ice cover in Finnish and Estonian lakes in winter 2009. Estonian Journal of Earth Sciences 60: 50–64.
Mehnert, G., F. Leunert, S. Cirés, K. D. Jöhnk, J. Rücker, B. Nixdorf & C. Wiedner, 2010. Competitiveness of invasive and native cyanobacteria from temperate freshwaters under various light and temperature conditions. Journal of Plankton Research 32: 1009–1021.
Padisák, J., 1997. Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju, an expanding highly adaptive bluegreen algal species: worldwide distribution and review of its ecology. Archiv für Hydrobiologie Supplement 107: 563–593.
Padisák, J., É. Hajnal, L. Krienitz, J. Lakner & V. Üveges, 2010. Rarity, ecological memory, rate of floral change in phytoplankton—and the mystery of the Red Cock. Hydrobiologia 653: 45–64.
Paerl, H. W. & J. Huisman, 2008. Blooms like it hot. Science 320: 57–58.
Platt, T., C. L. Gallegos & W. G. Harrison, 1980. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. Journal of Marine Research 38: 687–701.
Preußel, K., G. Wessel, J. Fastner & I. Chorus, 2009. Response of cylindrospermopsin production and release in Aphanizomenon flos-aquae (Cyanobacteria) to varying light and temperature conditions. Harmful Algae 8: 645–650.
Price, G. D., D. Sültemeyer, B. Klughammer, M. Ludwig & M. R. Badger, 1998. The functioning of the CO2 concentrating mechanism in several cyanobacterial strains: a review of general physiological characteristics, genes, proteins, and recent advances. Canadian Journal of Botany 76: 973–1002.
Reynolds, C. S., 1984. The Ecology of Freshwater Phytoplankton. Cambridge University Press, Cambridge.
Reynolds, C. S., V. Huszar, C. Kruk, L. Naselli-Flores & S. Melo, 2002. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research 24: 417–428.
Scherer, S., E. Stürlz & P. Boger, 1981. Arrhenius plots indicate localization of photosynthetic and respiratory electron transport in different membrane regions of Anabaena. Zeitschrift für Naturforschung Teil C 36: 1036–1040.
Seaburg, K. G., B. C. Parker, R. A. Wharton & G. M. Simmons, 1981. Temperature growth responses of algal isolates from Antarctic oases. Journal of Phycology 17: 353–360.
Simona, M., 2003. Winter and spring mixing depths affect the trophic status and composition of phytoplankton in the northern meromictic basin of Lake Lugano. Journal of Limnology 62: 190–206.
Steemann-Nielsen, E. J., 1952. The use of radioactive carbon (14C) for measuring organic production in the sea. Journal du Conseil Permanent International pour l’Exploration de la Mer 18: 117–140.
Tsujimura, S., K. Ishikawa & H. Tsukada, 2001. Effect of temperature on growth of the cyanobacterium Aphanizomenon flos-aquae in Lake Biwa and Lake Yogo. Phycological Research 49: 275–280.
Uehlinger, U., 1981. Experimental studies on the autecology of Aphanizomenon flos-aquae (Experimentelle Untersuchungen zur Autökologie von Aphanizomenon flos-aquae). Archiv für Hydrobiologie Supplement 60: 260–288.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitteilungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie 9: 1–38.
Üveges, V., L. Vörös, J. Padisák & A. W. Kovács, 2011. Primary production of epipsammic algal communities in Lake Balaton (Hungary). Hydrobiologia 660: 17–27.
Vincent, W. F., 2000. Cyanobacterial dominance on Polar regions. In Whitton, B. A. & M. Potts (eds), The Ecology of Cyanobacteria. Kluwer, Netherlands: 321–340.
Vincent, W. F., 2007. Cold tolerance in cyanobacteria and life in the cryosphere. In Seckbach, J. (ed.), Algae and Cyanobacteria in Extreme Environments. Springer, UK: 287–301.
Vincent, W. F., L. G. Whyte, C. Lovejoy, C. W. Greer, I. Laurion, C. A. Suttle, J. Corbeil & D. R. Mueller, 2009. Arctic microbial ecosystems and impacts of extreme warming during the International Polar Year. Polar Science 3: 171–180.
Vincent, W. F., T. V. Callaghan, D. Dahl-Jensen, M. Johansson, K. M. Kovacs, C. Michel, T. Prowse, J. D. Reist & M. Sharp, 2011. Ecological implications of changes in the arctic cryosphere. AMBIO 40: 87–99.
Vyverman, W., E. Verleyen, A. Wilmotte, D. A. Hodgson, A. Willems, K. Peeters, B. Van de Vijver, A. De Wever, F. Leliaert & K. Sabbe, 2010. Evidence for widespread endemism among Antarctic micro-organisms. Polar Science 4: 103–113.
Wagner, C. & R. Adrian, 2009. Cyanobacteria dominance: quantifying the effects of climate change. Limnology and Oceanography 54: 2460–2468.
Walsby, A. E., 1994. Gas vesicles. Microbiological Reviews 58: 94–144.
Wetzel, R. G. & G. E. Likens, 2000. Limnological Analyses. Springer, New York.
Wildman, R., J. H. Loescher & C. L. Winger, 1975. Development and germination of akinetes of Aphanizomenon flos-aquae. Journal of Phycology 11: 96–104.
Wilhelm, S. & R. Adrian, 2008. Impact of summer warming on the thermal characteristics of a polymictic lake and consequences for oxygen, nutrients and phytoplankton. Freshwater Biology 53: 226–237.
Yamamoto, Y., 2009. Environmental factors that determine the occurrence and seasonal dynamics of Aphanizomenon flos-aquae. Journal of Limnology 68: 122–132.
Yamamoto, Y. & H. Nakahara, 2005. The formation and degradation of cyanobacterium Aphanizomenon flos-aquae blooms: the importance of pH, water temperature, and day length. Limnology 6: 1–6.
Yamamoto, Y. & H. Nakahara, 2006. Importance of interspecific competition in the abundance of Aphanizomenon flos-aquae (Cyanophyceae). Limnology 7: 163–170.
Yamamoto, Y. & H. Nakahara, 2007. Factors affecting the fluctuation of akinete and heterocyst numbers of Aphanizomenon flos-aquae (Cyanobacteria) population. Algological Studies 125: 79–96.
Yamamoto, Y. & H. Nakahara, 2009a. Seasonal variations in the morphology of bloom-forming cyanobacteria in a eutrophic pond. Limnology 10: 185–193.
Yamamoto, Y. & H. Nakahara, 2009b. Life cycle of cyanobacterium Aphanizomenon flos-aquae. Taiwania 54: 113–117.
Acknowledgments
This research was implemented through the CENTRAL EUROPE Programme (European Lakes Under Environmental Stressors, 2CE243P3) and was supported by the Leibniz Gemeinschaft (Klimabetriebene Veränderung der Biodiversität von Mikrobiota—TemBi), and by the Hungarian National Science Foundation (OTKA Nr. 105827). We thank Dr. Lajos Vörös, Dr. Attila W. Kovács (Balaton Limnological Research Institute of the Hungarian Academy of Science), Dr. János Somlai and Borbála Máté (University of Pannonia, Institute of Radiochemistry and Radioecology) for their help in the radiocarbon measurements and the technical assistance by the staff of the Leibniz-Institute of Freshwater Ecology and Inland Fisheries for their contributions to the samplings and field measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: N. Salmaso, L. Naselli-Flores, L. Cerasino, G. Flaim, M. Tolotti & J. Padisák / Phytoplankton responses to human impacts at different scales: 16th workshop of the International Association of Phytoplankton Taxonomy and Ecology (IAP)
Rights and permissions
About this article
Cite this article
Üveges, V., Tapolczai, K., Krienitz, L. et al. Photosynthetic characteristics and physiological plasticity of an Aphanizomenon flos-aquae (Cyanobacteria, Nostocaceae) winter bloom in a deep oligo-mesotrophic lake (Lake Stechlin, Germany). Hydrobiologia 698, 263–272 (2012). https://doi.org/10.1007/s10750-012-1103-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1103-3