Abstract
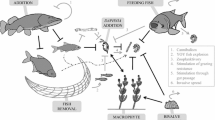
Biomanipulation through fish removal is a tool commonly used to restore a clear-water state in lakes. Biomanipulation of ponds is, however, less well documented, although their importance for biodiversity conservation and public amenities is undisputed. In ponds, a more complete fish removal can be carried out as compared to lakes and therefore a stronger response is expected. Fish recolonization can, however, potentially compromise the longer term success of biomanipulation. Therefore, we investigated the impact of fish recolonization on zooplankton, phytoplankton, and nutrients for several years after complete drawdown and fish removal in function of submerged vegetation cover in 12 peri-urban eutrophic ponds situated in Brussels (Belgium). Fish recolonization after biomanipulation had a considerable impact on zooplankton grazers, reducing their size and density substantially, independent of the extent of submerged vegetation cover. Only ponds with <30% cover of submerged vegetation shifted back to a turbid state after fish recolonization, coinciding with an increase in density of small cladocerans, rotifers, and cyclopoid copepods. In ponds with >30% submerged vegetation cover, macrophytes prevented an increase in phytoplankton growth despite the disappearance of large zooplankton grazers. Our results suggest that macrophytes, rather than by providing a refuge for zooplankton grazers, control phytoplankton through other associated mechanisms and confirm that the recovery of submerged macrophytes is essential for biomanipulation success. Although the longer term effect of biomanipulation is disputable, increased ecological quality could be maintained for several years, which is particularly interesting in an urban area where nutrient loading reduction is often not feasible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the past decades, eutrophication due to human activities has become a serious threat for many European lakes and ponds. An increase in nutrient loading can possibly lead to an increased phytoplankton biomass, often dominated by potentially toxic algae (Graham & Wilcox, 2000; Peretyatko et al., 2007b) and has caused a decline of macrophytes throughout Europe (de Nie, 1987). This decline was not only associated with a decrease of biological diversity in general, but also associated with a reduction of the amenity and conservation values of many lakes and ponds (Moss et al., 1996).
In recent years, many efforts have been made to restore shallow lakes to a vegetated clear-water state (van Donk et al., 1990b; Meijer et al., 1999; Skov et al., 2003), which is preferred to a non-vegetated state because of the higher biodiversity that is associated with plant communities (Brönmark, 1985; Scheffer et al., 1993; Gee et al., 1997; Van Onsem et al., 2010) and the reduced risk of toxic algal blooms (Moss et al., 1996). In addition to nutrient loading reduction (Jeppesen et al., 2007a), which is often used to restore lakes, biomanipulation, through complete or partial fish removal, is a commonly used technique to increase ecological quality in shallow lakes (Hosper & Jagtman, 1990; Jeppesen et al., 1990; Shapiro, 1990; Meijer & Hosper, 1997). Fish are known to have an important structuring role in eutrophic lakes (Jeppesen et al., 2000; De Backer et al., 2010) and their total or partial removal can have marked effects on the ecology of lakes and ponds (Lammens, 1999). By changing fish community structure through plankti-benthivorous fish removal (Brönmark & Weisner, 1992), predation pressure on large zooplankton grazers (mainly Daphnia spp.) is reduced, resulting in an increased grazing on phytoplankton in spring (Meijer et al., 1999). The increased transparency caused by increased zooplankton grazing on phytoplankton is often followed by the recovery of submerged vegetation (Ozimek et al., 1990; van Donk et al., 1990a; Hutorowicz & Dziedzic, 2008) that, in turn, can stabilize the clear-water state through a number of associated mechanisms (Jeppesen et al., 1997; Madsen et al., 2001; van Donk & van de Bund, 2002).
Although initially the results of such restoration measures were promising (Hutorowicz & Dziedzic, 2008), a shift back to a turbid state is often observed after a few years, generally accompanied by a decline of submerged macrophytes (Meijer et al., 1994; Søndergaard et al., 2007). Increased predation pressure on zooplankton grazers (Shapiro, 1990; Romare & Bergman, 1999) is often stated as an important reason for deterioration during the years after biomanipulation, shifting the zooplankton community toward smaller cladocerans, rotifers, and cyclopoid copepods (Meijer et al., 1990; Moss et al., 1996; Vakkilainen et al., 2004) which are typical for more turbid waters (Cottenie et al., 2001). Provision of a suitable refuge for large zooplankton, for instance by submerged vegetation, could potentially reduce predation pressure on zooplankters and as such prevent a shift back to the turbid state (Shapiro, 1990). Use of macrophytes as a refuge for zooplankton can be especially important in shallower ponds and lakes, as compared to deeper lakes where light limitation often prevents colonization of macrophytes (Jeppesen et al., 2007b). Many studies have been carried out on the refuge capacity of submerged vegetation for large zooplankton in shallow lakes, often with contrasting results. Schriver et al. (1995) observed that the impact of fish predation on the zooplankton community was lower inside dense macrophyte beds and that the refuge capacity decreased when fish density increased. Conversely, in three lakes in England, large populations of grazing Cladocera were maintained even under high predation pressure from a high density of zooplanktivorous 0+ fish (Stansfield et al., 1997). This is in contradiction with the findings of Perrow et al. (1999), where any refuge effect was nullified if fish density exceeded 1 m−2. Similar results were reported by Nicolle et al. (2010), who found that large cladocerans were unable to use macrophytes as a refuge from 0+ fish predation. Iglesias et al. (2007) also suggested that the refuge for zooplankton was lost under very high densities of fish and invertebrates in a study on subtropical lakes.
Due to the small size of ponds, a complete drawdown and an (almost) complete fish removal is generally possible as compared to larger lakes where often only partial removal of fish can be carried out (Lammens, 1999). Except for the lake Zwemlust example, few other cases of total fish removal are known (van Donk et al., 1990a). However, despite the complete removal of fish, in some cases, fish (mostly 0+) recolonize the ponds which can potentially compromise the success of biomanipulation (Peretyatko et al., 2009). The impact of planktivorous fish on lower trophic levels is known to increase with decreasing depth, as indicated by the higher density of planktivorous fish per unit of volume that is generally found in shallower lakes as compared to deeper lakes (Jeppesen et al., 1990). This suggests that, should fish return, the potential predation pressure on large zooplankton can become very high in eutrophic ponds.
The main objective of this study was to investigate different post-biomanipulation situations in ponds in which a clear-water state was obtained after complete fish removal and water drawdown. We studied the interaction between different factors that are known to potentially influence the stability of the ponds on the longer term, such as the recovery of submerged vegetation, zooplankton community structure, and influence of recolonizing fish to address the following questions in this article:
-
Is zooplankton grazing alone sufficient to restore a clear-water state in eutrophic ponds?
-
What is the impact of fish recolonization on zooplankton community structure?
-
Do macrophytes act as a refuge for large Cladocera?
-
Does fish recolonization lead to a shift back to the turbid state and does this depend on the extent of the submerged vegetation cover?
To answer these questions, we investigated zooplankton community structure, phytoplankton biomass, nutrients, submerged vegetation cover, and fish presence in 12 biomanipulated peri-urban eutrophic ponds.
Materials and methods
Study area
Twelve ponds located in the Brussels Capital Region were selected for this study, most of them situated within the catchment of the Woluwe river, a typical lowland river, and one of its tributaries. All of the ponds were manmade and created more than a century ago by the damming of small low order streams (Marlier, 1971). They are shallow (maximum depth 3 m), flat-bottomed and range in surface area from 0.2–6 ha. All the ponds are mainly fed by small rivulets and ground water seepage. Before biomanipulation, all ponds were overstocked with fish typical for European freshwaters (>500 kg ha−1). For more details on fish species and community structure in Brussels ponds before biomanipulation, we refer to De Backer et al. (2010). Twelve ponds were biomanipulated during 2005–2009 by means of water drawdown and complete fish removal in winter. They were slowly refilled again in early spring (a few weeks to several months later) through overflow of neighboring ponds, groundwater seepage, or small rivulets entering the ponds.
Sampling and sample processing
Samples were taken one year before and 1–4 years after biomanipulation for each studied pond during the warm season (May–September; Table 1). Secchi depth was measured using a 30-cm diameter disk. To determine the actual Secchi depth more accurately in case the disk was still visible at the pond bottom, we added 1 m to the Secchi depth when the disk was still clearly visible, and 0.1 m when it was only partially visible, similar to Cottenie et al. (2001). Quantitative phytoplankton, Chlorophyll a (Chl a), main nutrient (total phosphorus—TP, soluble reactive phosphorus—SRP, and DIN—dissolved inorganic nitrogen, i.e., NH4 and NOx (NO2 and NO3)), and zooplankton samples were collected on each occasion. Mixed water samples based on ten random subsamples were taken from each pond (including vegetated parts) with a plastic tube sampler of 4.5 cm diameter and 70 cm length that closes in the lower part. An extension was fixed to the sampler to reach the deeper parts of the ponds when appropriate. After stirring the collected water, 500 ml was taken for phytoplankton identification and enumeration, 1l for chemical analyses and 1l for Chl a determination. Samples for Chl a analysis were filtered onto Whatman GF/C filters and stored at −18°C for a maximum of 7 days before analysis. Pigments were extracted in 90% acetone in the dark for 8 h. Pigment concentrations were measured spectrophotometrically. Nutrient concentrations were measured according to standard methods (APHA-AWWA-WEF, 1995). Phytoplankton samples were fixed in the field with alkaline lugol, sodium thiosulfate and buffered formalin (Sherr & Sherr, 1993) and stored in the dark before identification and enumeration to genus level using inverted microscopy. Biovolumes were calculated using the approximations of cell shapes to simple geometrical forms (Wetzel & Likens, 2000). Total phytoplankton biovolume was mainly used as a proxy for turbidity of the water column.
In order to assess the zooplankton community structure, ten subsamples of 1l were collected with the same sampler used for phytoplankton and nutrients. The samples from a given pond were mixed and filtered through a 64 μm-mesh net and preserved in 5% formaldehyde (final concentration) at 4°C before being identified and enumerated using inverted microscopy. Different levels of identification were used: cladocerans were identified to genus level, copepods were divided into cyclopoids, calanoids, and nauplii. Rotifers were not discriminated. For the analysis, cladocerans were divided into large (Daphnia spp., Diaphanosoma spp., Eurycercus spp., Sida spp., and Simocephalus spp.) and small (Acroperus spp., Alona/Biapertura spp., Alonella spp., Bosmina spp., Camptocercus spp., Ceriodaphnia spp., Chydorus spp., Disparalona spp., Graptoleberis spp., Pleuroxus spp., and Scapholeberis spp.) taxa, as proposed by Moss et al. (2003). Predatory cladocerans such as Polyphemus spp. were not included in the analysis, as our main interest here was to study phytoplankton grazing zooplankters.
Submerged vegetation cover was estimated from a boat during each field visit, visually and by the use of a rake, along transects throughout the whole pond. Macrophytes were identified to species level, except for Characeae that were identified to genus level, as were filamentous algae. For statistical analyses, total submerged vegetation cover (SV; based on submerged macrophyte and filamentous algae cover) was classified as low (<30%), intermediate (30–60%), or high (>60%) cover for each sampling occasion.
Many of the biomanipulated ponds were recolonized at a certain time by large numbers of small planktivorous fish. As most ponds are fed by groundwater or small rivulets, fish recolonizing the ponds were generally small (less than 50 mm). Since they always occurred in high abundances, and because of their small size, estimating fish abundance quantitatively was difficult. Therefore, we differentiated between fish presence (large schools of small fish observed) or absence (no fish observed), visually and by the use of a landing net (4 mm mesh size).
Statistical analyses
Phytoplankton biovolume and Chl a showed similar spatial–temporal patterns and were significantly positively correlated (Spearman rank-order correlation: P < 0.001; R s = 0.82), which suggests that the former gives a reasonable estimation of the latter. As phytoplankton was identified to genus level, it has a greater discriminative power than Chl a. Therefore, biovolume was used as a proxy for phytoplankton biomass instead of Chl a.
Due to high intra-annual variation, for all analyses, data of each individual sampling occasion were used instead of annual means. Samples from ponds in which fish recolonization took place for more than three years were removed from all analyses, as the goal of this study was to investigate the short-term effect of fish recolonization and the long-term situation is essentially different.
A redundancy analysis (RDA) was performed using Canoco (ter Braak & Smilauer, 1998) to explore the phytoplankton data (expressed as total biovolume per division) in different post-biomanipulation situations. Data were transformed to achieve normality when possible. Environmental data were centered and standardized. The automatic forward selection procedure was used to select the variables (nutrients (TP, NOx, NH4, SRP), total submerged vegetation cover, fish presence) that contributed the most to the explanation of the phytoplankton data. This analysis allowed us to identify the factors that differentiated phytoplankton biovolume the most after biomanipulation. Different post-biomanipulation situations where then studied based on these factors.
A one-sample Kolmogorov–Smirnov test was used to investigate the data for normality. A Levene’s test was used to check for homogeneity of variance. When appropriate, a two-way ANOVA was used to study the individual effect of fish presence and submerged vegetation cover and their interaction on selected variables (i.e., total phytoplankton biovolume and large cladocera size).
Next, One-way ANOVA was used to compare the before and post-biomanipulation situations to investigate the effect of fish presence and submerged vegetation cover. A Tukey HSD test, modified for unequal sample size, was used as a post-hoc comparison test to compare mean values between individual groups. In case the assumption of homoscedasticity was not met, a Kruskal–Wallis (K–W) ANOVA was used instead of a one-way ANOVA. Additionally, Spearman rank-order correlation tests were performed to further investigate the relationships between zooplankton community structure and phytoplankton biovolume.
All statistics and graphs (except for the RDA) were done using Statistica version 8 (Statsoft Inc., Tulsa, OK, USA) and Sigmaplot version 11 (Systat Software GmbH, Erkrath, Germany).
Results
Before biomanipulation
Before biomanipulation, all ponds were eutrophic to hypereutrophic when considering total phosphorus concentrations (TP > 0.3 mg l−1 on average; Table 2). They were all overstocked with fish (>500 kg ha−1) typical for European freshwaters, mainly benthivorous species such as carp (Cyprinus carpio L.) and bream (Abramis brama L.). All ponds resided in a turbid state before biomanipulation with an average phytoplankton biovolume of >25 mm3 l−1 and no submerged vegetation cover, (except for two ponds (VKn2 and WtMl) with <20% cover of Ceratophyllum demersum). Zooplankton was dominated by rotifers, cyclopoid copepods and small cladocerans. Density of large cladocerans varied considerably between ponds. Their size was low in all cases (0.5 mm on average), probably as a result of high fish predation pressure (Table 3).
After biomanipulation
During the period after biomanipulation, 9 out of 12 ponds were recolonized by small juvenile fish (<50 mm), mainly dominated by cyprinids. Large fish remained absent, as most ponds are fed by groundwater or small rivulets and only small fish could recolonize the ponds. After biomanipulation, TP concentrations remained high, corresponding to (hyper)eutrophic conditions (Table 2; Fig. 1). TP concentrations were not significantly lower after biomanipulation, except in ponds with a high cover of submerged vegetation in absence of fish. SRP concentrations on the contrary, increased after biomanipulation, and were significantly higher in all post-biomanipulation cases, except for the ponds with high vegetation cover and no fish. The concentration of DIN was only significantly higher after biomanipulation in case of low submerged vegetation cover in absence of fish. Although not statistically significantly different, median and maximum DIN concentrations were considerably lower in ponds with high cover of submerged vegetation (Table 2).
Nutrient concentrations before and after biomanipulation. Different characters indicate significant differences (P < 0.05) using Kruskal–Wallis ANOVA (TP: H = 14.36, P = 0.026, n = 154; SRP: H = 47.11, P < 0.001, n = 150; DIN: H = 35.70, P < 0.001, n = 154). A dashed line separates the before and after situations. FFF overstocked with fish, F fish observed after biomanipulation, NF no fish observed after biomanipulation, SV submerged vegetation cover, bm biomanipulation
Despite the generally high nutrient concentrations, average phytoplankton biovolume was considerably lower after biomanipulation in ponds where no fish were observed (Table 2). Before fish recolonization, phytoplankton biovolume remained low: <2 mm3 l−1 on average, independent of the extent of the submerged vegetation cover that had developed after biomanipulation (Table 2). Average large Cladocera size generally exceeded 1 mm. Large Cladocera density was high, as compared to the situation before biomanipulation, and varied from 81 to 41 and 39 individuals l−1 on average in ponds with low, intermediate and high submerged vegetation cover, respectively (Table 3). Submerged vegetation was restored in 11 out of 12 ponds, ranging from very sparse growth to dense macrophyte beds covering the entire pond surface, generally consisting of several Potamogeton species, C. demersum and/or Characeae (Nitella or Chara spp.).
An RDA based on phytoplankton and environmental data was performed to explore different post-biomanipulation situations. The first two axes explained 89% of the variation in the phytoplankton-environment relationship, of which 81% was explained by the first and 8% by the second axis (Table 4). As suggested by the arrows of the phytoplankton groups that are all directed toward the right side of the diagram, a gradient of increasing total phytoplankton biovolume is shown from left to right (Fig. 2). The RDA results show that the factor having the strongest relationship with phytoplankton after biomanipulation is fish presence, emphasizing the importance of fish return after biomanipulation (Fig. 2). TP, NH4, SV, and LCD also showed a significant relationship with phytoplankton biovolume (Table 5). Together, these variables explained 34% of the variation in the phytoplankton data out of 36% explained by all the variables used in the model. As is often the case with ecological data, a large part of the variation remains unexplained. This could be caused by stochasticity, inter-pond variability, or by other sources of variability.
Redundancy analysis based on phytoplankton and environmental data (per pond per sampling) after biomanipulation. FISH fish observed/not observed, LCD large Cladocera density, LCL large Cladocera length, SV submerged vegetation cover, TP total phosphorus, SRP soluble reactive phosphorus, NOx NO2 + NO3
The two-way ANOVA showed a significant fish presence × submerged vegetation cover interaction for phytoplankton biovolume (Table 6; Fig. 3). Considering large Cladocera length, the effect of fish presence alone was significant. No significant interaction was found between fish presence and submerged vegetation cover, suggesting that the effect of fish on large Cladocera length is independent of the extent of submerged vegetation cover (Table 6).
Phytoplankton biovolume and large Cladocera density and length before and after biomanipulation. Different characters indicate significant differences (P < 0.05) using One-Way or Kruskal–Wallis ANOVA (LCD (K–W): H = 40.22, P < 0.001, n = 154; LCL (One-way): F = 9.274, df = 6, P < 0.001, n = 122; Phytoplankton biovolume (One-way): F = 30.16, df = 6, P < 0.001, n = 166). A dashed line separates the before and after situations. FFF overstocked with fish, F fish observed after biomanipulation, NF no fish observed after biomanipulation, SV submerged vegetation cover, bm biomanipulation
Phytoplankton biovolume was significantly lower in all post-biomanipulation situations compared to before biomanipulation (Fig. 3). Phytoplankton biovolume increased significantly after fish recolonization in ponds with a low cover of submerged vegetation, not in ponds with an intermediate or high cover. However, in high cover ponds (both with and without fish), phytoplankton biovolume was significantly lower compared to the situation with low vegetation cover after fish recolonization.
Large Cladocera length was significantly higher after biomanipulation for all situations (Fig. 3). In ponds with a low cover of submerged vegetation, length decreased significantly after fish recolonization, however, not to such an extent as it was before biomanipulation. Although not significantly, a decrease in length was also observed in ponds with an intermediate and high cover of vegetation after fish recolonization.
Average large Cladocera density was significantly higher after biomanipulation in ponds with a low cover of submerged vegetation (Fig. 3). Although not statistically significant, considerably higher densities of large Cladocera were reached in all other post-biomanipulation situations, especially after fish recolonization, except for ponds with a high submerged vegetation cover where densities remained low both in absence and presence of fish (Fig. 3).
Coinciding with the significant increase in phytoplankton biovolume after fish recolonization in ponds with a low cover of submerged vegetation, the zooplankton community structure changed toward more but smaller zooplankters. Densities of small cladocerans, rotifers, and cyclopoid copepods tended to increase with increasing phytoplankton biomass. Small cladocerans were only present in high numbers in cases where phytoplankton biomass was high. This was not the case after fish recolonization in ponds with an intermediate or high cover of submerged vegetation, when phytoplankton biovolume remained low (Fig. 4). The association of elevated phytoplankton biovolume with an increase in densities of smaller zooplankton was confirmed by Spearman rank correlations on data before and after biomanipulation. A significant positive correlation was found between phytoplankton biovolume and small Cladocera (R s = 0.24; n = 154; P = 0.003), rotifer (R s = 0.72; n = 154; P < 0.001), cyclopoid copepod (R s = 0.34; n = 154; P < 0.001), and nauplius density (R s = 0.21; n = 154; P = 0.009). Large Cladocera density (R s = −0.41; n = 154; P < 0.001) was negatively correlated to phytoplankton biovolume, as was calanoid density (R s = −0.24; n = 154; P = 0.003).
Mean densities (and standard deviations) of cladocerans, copepods (a) and rotifers (b). Rotifer densities are shown on a log scale. Situations before and after biomanipulation are separated by a dashed line. FFF overstocked with fish before biomanipulation, F fish observed after biomanipulation, NF no fish observed after biomanipulation, SV submerged vegetation cover, bm biomanipulation
Discussion
Is zooplankton grazing alone sufficient to restore a clear-water state in eutrophic ponds?
After fish removal, large zooplankton grazers increased considerably in density and size in all ponds that were not recolonized by fish. Associated with the increase in density and size of large cladocerans, phytoplankton biomass decreased and submerged vegetation was restored in several ponds. Despite high nutrient concentrations that did not change significantly after fish removal, a shift to the clear-water state was achieved. This is consistent with the alternative stable states, suggesting that, within a certain nutrient range, two alternative states can exist at the same nutrient level (Scheffer et al., 1993). The low phytoplankton biomass in absence of fish suggests that zooplankton grazing is an important factor for phytoplankton control and that recovery of submerged vegetation is not indispensable to obtain a clear-water state, even in (hyper-)eutrophic conditions. Despite the removal of fish, control of large Cladocera by macroinvertebrates (Benndorf et al., 2000) was not observed during the study period. Fluctuations in large Cladocera densities seemed to be related to food availability rather than predation (Peretyatko et al., 2011). Although macroinvertebrates could have influenced large Cladocera densities positively by feeding selectively on smaller cladocerans (Pinel-Alloul, 1995), the same effect could also be attributed by the absence of predation by fish, resulting in a competitive advantage for larger filter feeding individuals (Brooks & Dodson, 1965; Gliwicz, 1990).
What is the impact of fish recolonization on zooplankton community structure?
When predation is absent, it is likely that large-bodied individuals outcompete the smaller individuals by reducing food level concentration below the minimum food concentration necessary for smaller cladocerans to grow. Gliwicz (1990) found that for large-bodied daphniids, the threshold food concentration at which assimilation equals respiration was lower than for small-bodied individuals. The competitive advantage of larger bodies species thus could eliminate coexistence of small and large zooplankton species in absence of fish (Gliwicz et al., 2010). However, when planktivorous fish are present, they will selectively feed more on the larger individuals (Brooks & Dodson, 1965), allowing the smaller zooplankters to increase their abundance, but only if the disappearance of large cladocerans coincides with an increase in food availability. The size-efficiency hypothesis suggests that co-existence of large and small-bodied zooplankton can be explained by a balance between predation, that generally forces the community toward smaller individuals, and competition that pushes the balance toward larger individuals (Brooks & Dodson, 1965; DeMott & Kerfoot, 1982). This is in agreement with our results, where, in absence of fish predation, generally only large-bodied Cladocera were found (mainly Daphnia spp.). In the presence of fish, predation removed the larger cladocerans, resulting in an overall decrease in size of large Cladocera (but not their density) and an increase in density of small Cladocera species, as a result of phytoplankton biomass increase. This was, however, only the case in ponds with a low submerged vegetation cover. In ponds with an intermediate or high cover of submerged vegetation, phytoplankton biomass did not increase after the decline in large Cladocera densities and as a likely consequence of low food resources (i.e., low phytoplankton biomass) due to macrophyte presence, the density of small Cladocera did not increase either.
Do macrophytes act as a refuge for large Cladocera?
In this study, the reappearance of small planktivorous fish in some of the ponds after biomanipulation had a significant negative impact on large Cladocera. Although their density did not decrease in low or intermediately vegetated ponds, large cladocerans almost disappeared in highly vegetated ponds where fish were present. The overall reduced size in presence of fish suggests a high predation pressure, independent of the extent of submerged vegetation cover. It might seem contradictory that in some ponds with a low or intermediate submerged vegetation cover, higher densities of large cladocerans were found after fish recolonization compared to before, as in densely vegetated ponds all large cladocerans generally disappear. This phenomenon could be explained by the lack of food availability in the densely vegetated ponds, preventing cladocerans to recover from predation. Another factor could be an increased predation efficiency of fish in clear-water situations. The results of Castro et al. (2007) who observed diel horizontal migration of zooplankton toward macrophytes only when the water was clear but not in turbid water, suggests that predation efficiency of fish could be more efficient in clear-water conditions. The decline in densities of large cladocerans in densely vegetated ponds, in combination with the overall decrease of their size after fish recolonization, suggests that no refuge against fish predation was provided by macrophytes. Perrow et al. (1999) already suggested that any refuge effect was nullified if fish density exceeded 1 m−2. Other papers indicate that the refuge for zooplankton was lost under very high densities of fish and invertebrates (Iglesias et al., 2007; Nicolle et al., 2010). The high densities and size of large cladocerans that were observed in absence of fish, probably provided a high food stock for juvenile fish population that quickly grew to very high densities. Especially in eutrophic conditions, food availability for fish in ponds can become very high, as already suggested by the high densities of large cladocerans found in this study (see also Peretyatko et al. (2009)). In absence of any predator or competition from larger fish, juvenile 0+ fish can occur in very high densities (Romare & Bergman, 1999). The impact of planktivorous fish on lower trophic levels is known to increase with decreasing depth, as indicated by the higher density of planktivorous fish per unit of volume that is generally found in shallower lakes as compared to deeper (Jeppesen et al., 1990). Our results, showing that the effect of fish on large Cladocera size was independent of submerged vegetation cover, suggest that, if fish return, predation pressure on large zooplankton can potentially become very high and it seems unlikely that any vegetation would still be able to provide shelter for zooplankton against predation.
Does fish recolonization lead to a shift back to the turbid state and does this depend on the extent of the submerged vegetation cover?
Despite the considerable impact of fish on zooplankton, even in highly vegetated ponds, phytoplankton biomass only significantly increased after fish recolonization in ponds when cover of submerged vegetation was <30%. Thus, as long as macrophytes persist, phytoplankton biomass generally remains low. In these ponds, the increase of phytoplankton biomass coincided with a shift in zooplankton community structure toward smaller bodied individuals. Tessier et al. (2001) found that smaller daphniids were less effective in suppressing phytoplankton, as a diverse assemblage of green algae persisted in the presence of smaller bodied daphniids, which was not the case for the larger bodies daphniids. This could explain the increase of phytoplankton biomass after the decline in large Cladocera size in ponds with a low vegetation cover. Although small cladocerans increased in density, phytoplankton biomass also increased because of the lower ability of small cladocerans to suppress phytoplankton (Brooks & Dodson, 1965). Another factor influencing phytoplankton biomass and composition could be the difference in feeding habits of smaller cladocerans. Bosmina sp. for example, are known to have a more selective feeding strategy by moving actively toward their preferred food and feeding only on highly edible algae (DeMott & Kerfoot, 1982), in contrast to the larger Daphnia spp. that are more generalist filter feeders and have a different impact on phytoplankton biomass and composition.
Ponds with an intermediate or a high cover of submerged vegetation were not associated with an increased phytoplankton biomass upon fish recolonization. This suggests that in these ponds, as long as macrophytes persist, submerged macrophytes are able to maintain the clear-water state, despite the lack of refuge for large zooplankton grazers. Submerged macrophytes are known to inhibit phytoplankton growth through several associated mechanisms such as reduction of nutrient availability, increased sedimentation, allelopathy, and shading (Søndergaard & Moss, 1998; van Donk & van de Bund, 2002; Peretyatko et al., 2007a). N-limitation of phytoplankton by submerged vegetation is often suggested as a potential mechanism for stabilization of the clear-water state after biomanipulation (Ozimek et al., 1990; Meijer et al., 1994). This is in agreement with our results showing that in absence of fish, DIN concentrations are lower inside highly vegetated ponds as compared to ponds where no or only a low cover of submerged vegetation was restored. The slightly lower densities and length of large Cladocera that were observed in intermediate and highly vegetated ponds, reinforce the idea of a negative impact of submerged vegetation on phytoplankton (Jeppesen et al., 1997; Madsen et al., 2001; van Donk & van de Bund, 2002), resulting in a lower food availability for zooplankters inside vegetated ponds. This could also explain why, despite the decline in large Cladocera densities after fish recolonization in highly vegetated ponds, small Cladocera density did not increase, on the contrary to the situation in ponds with a low vegetation cover where phytoplankton biomass increased sufficiently for small cladocerans to be able to survive.
Long-term effects
Although a high cover of submerged macrophytes seems to be able to stabilize the clear-water state to a certain extent, a long-term effect is disputable. As no nutrient loading reduction measures were taken, the combination of high nutrient concentrations and growth of the recolonized fish population could, on a longer term, result in a return to the initial situation before biomanipulation (i.e., overstocked with fish). Especially when nutrient concentrations are too high, submerged vegetation might not be capable of controlling phytoplankton or epiphyton growth efficiently and may no longer be able to maintain a clear-water state throughout the whole summer resulting in a reduced light availability, eventually leading to a total disappearance of macrophytes and a shift back to a turbid state during summer, often coinciding with cyanobacterial blooms (Scheffer, 1998). This was for example the case in Leyb-b in 2008 (Table 1). The following year, as large Cladocera might not be present in spring due to predation by fish, reduced light conditions due to increased phytoplankton biomass could inhibit macrophyte growth. This was the case in Leyb-b in 2010 (data not shown). In our study, the RDA results already showed that TP could play an important role in determining biomanipulation outcome. In addition, an increased phytoplankton biovolume was observed in a few cases despite the presence of a submerged vegetation cover of >60% (see extreme outliers on Fig. 3). It should be noted that TP concentrations in these cases were rather high (>0.85 mg P l−1), suggesting that when nutrients are too high, submerged macrophytes might no longer be able to control phytoplankton sufficiently. In such case, additional measures to further reduce nutrient loading are unavoidable to improve the situation. Nevertheless, as suggested by our results, biomanipulation without nutrient reduction can potentially increase ecological quality considerably for several years and is worth considering in an urban area such as Brussels, in which a strong nutrient reduction is often not feasible.
Conclusions
In conclusion, our results show that fish recolonization after biomanipulation can have a considerable impact on zooplankton community structure and consequently phytoplankton biomass in eutrophic ponds. Submerged macrophytes did not provide sufficient shelter for large Cladocera grazers, irrespective of the extent of the submerged vegetation cover. Although large cladocerans were not protected from a high predation pressure, even in ponds with a high vegetation cover, elevated phytoplankton biomass was only found when submerged vegetation cover was low. Therefore, we believe that submerged macrophytes, as long as they can persist, stabilize the clear-water state after biomanipulation by other associated mechanisms rather than by providing a refuge for zooplankton grazers. As recolonization by fish at some point is difficult to avoid, recovery of submerged macrophytes is essential for a long-term stabilization of the clear-water state after biomanipulation in eutrophic ponds. On the longer term, it is uncertain how the situation might evolve. A return to conditions similar to the situation before biomanipulation is likely to occur in ponds where fish recolonized. Although the longer term effect of biomanipulation is disputable in ponds recolonized by fish, our results show that biomanipulation can increase ecological quality of eutrophic ponds for several years, which is particularly interesting in case nutrient loading reduction is not feasible.
References
APHA-AWWA-WEF, 1995. Standard Methods for the Examination of Water and Wastewater. American Public Health Association, Washington, DC.
Benndorf, J., B. Wissel, A. F. Sell, U. Hornig, P. Ritter & W. Boing, 2000. Food web manipulation by extreme enhancement of piscivory: an invertebrate predator compensates for the effects of planktivorous fish on a plankton community. Limnologica 30: 235–245.
Brönmark, C., 1985. Freshwater snail diversity: effects of pond area, habitat heterogeneity and isolation. Oecologia 67: 127–131.
Brönmark, C. & S. E. B. Weisner, 1992. Indirect effects of fish community structure on submerged vegetation in shallow, eutrophic lakes: an alternative mechanism. Hydrobiologia 243(244): 293–301.
Brooks, J. L. & S. I. Dodson, 1965. Predation, body size, and composition of plankton. Science 150: 28–35.
Castro, B. B., S. M. Marques & F. Gonçalves, 2007. Habitat selection and diel distribution of the crustacean zooplankton from a shallow Mediterranean lake during the turbid and clear water phases. Freshwater Biology 52: 421–433.
Cottenie, K., N. Nuytten, E. Michels & L. De Meester, 2001. Zooplankton community structure and environmental conditions in a set of interconnected ponds. Hydrobiologia 442: 339–350.
De Backer, S., S. Van Onsem & L. Triest, 2010. Influence of submerged vegetation and fish abundance on water clarity in peri-urban eutrophic ponds. Hydrobiologia 656: 255–267.
de Nie, H. W., 1987. The decrease in aquatic vegetation in Europe and its consequences for fish populations. EIFAC/CECPI Occasional paper No. 19, Rome: 52 pp.
DeMott, W. R. & W. C. Kerfoot, 1982. Competition among Cladocerans – nature of the interaction between Bosmina and Daphnia. Ecology 63: 1949–1966.
Gee, J. H. R., B. D. Smith, K. M. Lee & S. W. Griffiths, 1997. The ecological basis of freshwater pond management for biodiversity. Aquatic Conservation-Marine and Freshwater Ecosystems 7: 91–104.
Gliwicz, Z. M., 1990. Food thresholds and body size in cladocerans. Nature 343: 638–640.
Gliwicz, Z. M., E. Szymanska & D. Wrzosek, 2010. Body size distribution in Daphnia populations as an effect of prey selectivity by planktivorous fish. Hydrobiologia 643: 5–19.
Graham, L. E. & L. W. Wilcox, 2000. Algae. Prentice-Hall, Upper Saddle River.
Hosper, S. H. & E. Jagtman, 1990. Biomanipulation additional to nutrient control for restoration of shallow lakes in The Netherlands. Hydrobiologia 200(201): 523–534.
Hutorowicz, A. & J. Dziedzic, 2008. Long-term changes in macrophyte vegetation after reduction of fish stock in a shallow lake. Aquatic Botany 88: 265–272.
Iglesias, C., G. Goyenola, N. Mazzeo, M. Meerhoff, E. Rodó & E. Jeppesen, 2007. Horizontal dynamics of zooplankton in subtropical Lake Blanca (Uruguay) hosting multiple zooplankton predators and aquatic plant refuges. Hydrobiologia 584: 179–189.
Jeppesen, E., M. Søndergaard, E. Mortensen, P. Kristensen, B. Riemann, H. J. Jensen, J. P. Müller, O. Sortkjær, J. P. Jensen, K. Christoffersen, S. Bosselmann & E. Dall, 1990. Fish manipulation as a lake restoration tool in shallow, eutrophic temperate lakes 1: cross-analysis of 3 Danish case-studies. Hydrobiologia 200(201): 205–218.
Jeppesen, E., J. P. Jensen, M. Søndergaard, T. Lauridsen, L. J. Pedersen & L. Jensen, 1997. Top-down control in freshwater lakes: the role of nutrient state, submerged macrophytes and water depth. Hydrobiologia 342(343): 51–164.
Jeppesen, E., J. P. Jensen, M. Søndergaard, T. Lauridsen & F. Landkildehus, 2000. Trophic structure, species richness and biodiversity in Danish lakes: changes along a phosphorus gradient. Freshwater Biology 45: 201–218.
Jeppesen, E., M. Søndergaard, M. Meerhoff, T. L. Lauridsen & J. P. Jensen, 2007a. Shallow lake restoration by nutrient loading reduction – some recent findings and challenges ahead. Hydrobiologia 584: 239–252.
Jeppesen, E., M. Meerhoff, B. A. Jacobsen, R. S. Hansen, M. Søndergaard, J. P. Jensen, T. L. Lauridsen, N. Mazzeo & C. W. C. Branco, 2007b. Restoration of shallow lakes by nutrient control and biomanipulation – the successful strategy varies with lake size and climate. Hydrobiologia 581: 269–285.
Lammens, E., 1999. The central role of fish in lake restoration and management. Hydrobiologia 395: 191–198.
Madsen, J. D., P. A. Chambers, W. F. James, E. W. Koch & D. F. Westlake, 2001. The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia 444: 71–84.
Marlier, G., 1971. Les étangs de la Fôret de Soignes. Les Naturalistes Belges 52: 177–192.
Meijer, M.-L. & H. Hosper, 1997. Effects of biomanipulation in the large and shallow Lake Wolderwijd, The Netherlands. Hydrobiologia 342(343): 335–349.
Meijer, M.-L., E. H. R. R. Lammens, A. J. P. Raat, M. P. Grimm & S. H. Hosper, 1990. Impact of cyprinids on zooplankton and algae in ten drainable ponds. Hydrobiologia 191: 275–284.
Meijer, M.-L., E. Jeppesen, E. van Donk, B. Moss, M. Scheffer, E. Lammens, E. van Nes, J. A. van Berkum, G. J. de Jong & J. P. Jensen, 1994. Long-term responses to fish-stock reduction in small shallow lakes: interpretation of five-year results of four biomanipulation cases in The Netherlands and Denmark. Hydrobiologia 275(276): 457–466.
Meijer, M.-L., I. de Boois, M. Scheffer, R. Portielje & H. Hosper, 1999. Biomanipulation in shallow lakes in The Netherlands: an evaluation of 18 case studies. Hydrobiologia 408(409): 13–30.
Moss, B., J. Madgwick & G. Phillips, 1996. A Guide To The Restoration Of Nutrient-enriched Shallow Lakes. Environment Agency Broads Authority & European Union Life Programme, Norwich: 180 pp.
Moss, B., D. Stephen, C. Alvarez, E. Becares, W. Van, S. E. de Bund, E. Collings, E. Van Donk, T. De Eyto, C. Feldmann, M. Fernandez-Alaez, R. J. M. Fernandez-Alaez, F. Franken, E. M. Garcia-Criado, M. Gross, L. A. Gyllstrom, K. Hansson, A. Irvine, J. P. Jarvalt, E. Jensen, T. Jeppesen, R. Kairesalo, T. Kornijow, H. Krause, A. Kunnap, E. Laas, B. Lille, H. Lorens, M. R. Luup, P. Miracle, T. Noges, M. Noges, I. Nykanen, W. Ott, E. Peczula, G. Peeters, S. Phillips, V. Romo, J. Russell, M. Salujoe, K. Scheffer, H. Siewertsen, C. Smal, H. Tesch, L. Timm, I. Tuvikene, T. Tonno, E. Virro, D. Vicente & Wilson, 2003. The determination of ecological status in shallow lakes – a tested system (ECOFRAME) for implementation of the European Water Framework Directive. Aquatic Conservation-Marine and Freshwater Ecosystems 13: 507–549.
Nicolle, A., L.-A. Hansson & C. Brönmark, 2010. Habitat structure and juvenile fish ontogeny shape zooplankton spring dynamics. Hydrobiologia 652: 119–125.
Ozimek, T., R. D. Gulati & E. Van Donk, 1990. Can macrophytes be useful in biomanipulation of lakes? The Lake Zwemlust example. Hydrobiologia 200(201): 399–407.
Peretyatko, A., J. J. Symoens & L. Triest, 2007a. Impact of macrophytes on phytoplankton in eutrophic peri-urban ponds, implications for pond management and restoration. Belgian Journal of Botany 140: 83–99.
Peretyatko, A., S. Teissier, J. J. Symoens & L. Triest, 2007b. Phytoplankton biomass and environmental factors over a gradient of clear to turbid peri-urban ponds. Aquatic Conservation: Marine and Freshwater Ecosystems 17: 584–601.
Peretyatko, A., S. Teissier, S. De Backer & L. Triest, 2009. Restoration potential of biomanipulation for eutrophic peri-urban ponds: the role of zooplankton size and submerged macrophyte cover. Hydrobiologia 634: 125–135.
Peretyatko, A., S. Teissier, S. De Backer & L. Triest, 2011. Biomanipulation of hypereutrophic ponds: when it works and why it fails. Environmental Monitoring Assessment. doi: 10.1007/s10661-011-2057-z.
Perrow, M. R., A. J. D. Jowitt, J. H. Stansfield & G. L. Phillips, 1999. The practical importance of the interactions between fish, zooplankton and macrophytes in shallow lake restoration. Hydrobiologia 395(396): 199–210.
Pinel-Alloul, B., 1995. Impacts des prédateurs invertébrés sur les communautés aquatiques. In Pourriot, R. & M. Meybeck (eds), Limnologie Générale. Masson, Paris: 628–686.
Romare, P. & E. Bergman, 1999. Juvenile fish expansion following biomanipulation and its effect on zooplankton. Hydrobiologia 404: 89–97.
Scheffer, M., 1998. Ecology of shallow lakes. Kluwer Academic Publishers, Dordrecht.
Scheffer, M., S. H. Hosper, M. L. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology Evolution 8: 275–279.
Schriver, P., J. Bøgestrand, E. Jeppesen & M. Søndergaard, 1995. Impact of submerged macrophytes on fish-zooplankton-phytoplankton interactions: Large-scale enclosure experiments in a shallow eutrophic lake. Freshwater Biology 33: 255–270.
Shapiro, J., 1990. Biomanipulation: the next phase – making it stable. Hydrobiologia 200(201): 13–27.
Sherr, E. B. & B. F. Sherr, 1993. Preservation and storage of samples for enumeration of heterotrophic protists. In Kemp, P. F., B. F. Sherr, E. B. Sherr & J. J. Cole (eds), Handbook of Methods in Aquatic Microbial Ecology. Lewis Publishers, Boca Raton: 207–212.
Skov, C., O. Lousdal, P. H. Johansen & S. Berg, 2003. Piscivory of 0+ pike (Esox lucius L.) in a small eutrophic lake and its implication for biomanipulation. Hydrobiologia 506–509: 481–487.
Søndergaard, M. & B. Moss, 1998. Impact of submerged macrophytes on phytoplankton in shallow freshwater lakes. In Jeppesen, E., M. Søndergaard, M. Søndergaard & K. Christoffersen (eds), The structuring role of submerged macrophytes in lakes. Springer, New York: 115–133.
Søndergaard, M., E. Jeppesen, T. L. Lauridsen, C. Skov, E. H. Van Nes, R. Roijackers, E. Lammens & R. Portielje, 2007. Lake restoration: successes, failures and long-term effects. Journal of Applied Ecology 44: 1095–1105.
Stansfield, J. H., M. R. Perrow, L. D. Tench, A. J. D. Jowitt & A. A. L. Taylor, 1997. Submerged macrophytes as refuges for grazing Cladocera against fish predation: Observations on seasonal changes in relation to macrophyte cover and predation pressure. Hydrobiologia 342/343: 229–240.
ter Braak, C. J. F. & P. Smilauer, 1998. CANOCO reference manual and user’s guide to Canoco for Windows: software for canonical community ordination (version 4). Microcomputer Power, Ithaca.
Tessier, A. J., E. V. Bizina & C. K. Geedey, 2001. Grazer-resource interactions in the plankton: Are all daphniids alike? Limnology and Oceanography 46: 1585–1595.
Vakkilainen, K., T. Kairesalo, J. Hietala, D. M. Balayla, E. Becares, W. J. Van, E. de Bund, M. Van Donk, M. Fernandez-Alaez, L. A. Gyllstrom, M. R. Hansson, B. Miracle, S. Moss, J. Romo, D. Rueda & Stephen, 2004. Response of zooplankton to nutrient enrichment and fish in shallow lakes: a pan-European mesocosm experiment. Freshwater Biology 49: 1619–1632.
van Donk, E. & W. J. van de Bund, 2002. Impact of submerged macrophytes including charophytes on phyto- and zooplankton communities: allelopathy versus other mechanisms. Aquatic Botany 72: 261–274.
van Donk, E., M. P. Grimm, R. D. Gulati & K. B. J. P. G, 1990a. Whole-lake food-web manipulation as a means to study community interactions in a small ecosystem. Hydrobiologia 200(201): 275–289.
van Donk, E., M. P. Grimm, R. D. Gulati, P. G. M. Heuts, d. K. W. A & L. van Liere, 1990b. First attempt to apply whole-lake food-web manipulation on a large scale in The Netherlands. Hydrobiologia 200(201): 291–301.
Van Onsem, S., S. De Backer & L. Triest, 2010. Microhabitat–zooplankton relationship in extensive macrophyte vegetations of eutrophic clear-water ponds. Hydrobiologia 656: 67–81.
Wetzel, R. G. & G. E. Likens, 2000. Composition and biomass of phytoplankton. In Wetzel, R. G. & G. E. Likens (eds), Limnological Analyses. Springer-Verlag, New York: 147–174.
Acknowledgments
This study was supported by the Brussels Institute of Environment (BIM/IBGE), the Research in Brussels Action 2003–2006 and the Belgian Science Policy (BELSPO). We thank Anatoly Peretyatko for sampling and processing of the phytoplankton data. We would also like to thank the several anonymous reviewers that provided helpful suggestions to improve this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: D. Boix, B. Oertli, R. Céréghino, T. Kalettka, J. Biggs & A. P. Hull / Pond Research and Management in Europe – Proceedings of the 4th conference of the European Pond Conservation Network (Berlin 2010)
Rights and permissions
About this article
Cite this article
De Backer, S., Teissier, S. & Triest, L. Stabilizing the clear-water state in eutrophic ponds after biomanipulation: submerged vegetation versus fish recolonization. Hydrobiologia 689, 161–176 (2012). https://doi.org/10.1007/s10750-011-0902-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0902-2