Abstract
High pCO2 environments, such as volcanic carbon dioxide (CO2) vents, which mimic predicted near-future scenarios of ocean acidification (OA), offer an opportunity to examine effects of low pH conditions on marine biodiversity and adaptation/acclimatization of marine organisms to such conditions. Based on previous field studies in these systems, it is predicted that the stress owing to increasing CO2 concentrations favours the colonization by invertebrate species with a brooding habit. The goal of this study was to investigate the relative occurrence of the two sibling species Platynereis dumerilii (Audouin & Milne-Edwards, 1834) (free spawner) and Platynereis massiliensis (Moquin-Tandon, 1869) (egg brooder) in two shallow CO2 vents off Ischia and Vulcano islands (Italy, Tyrrhenian Sea), and in various areas with ambient pH conditions, where they represent one of the dominant genera. Phylogeographic analyses were integrated with reproductive biology and life-history observations on some selected populations thriving in the vent areas. This approach revealed the presence of four distinct Platynereis clades. Whereas two clades primarily inhabit CO2 vents and are presumably all brooders, the other two clades dominate the non-acidified sites and appear to be epitokous free spawners. We postulate that one of the brooding, vent-inhabiting clades represents P. massiliensis and one of the free spawning, non-vent-inhabiting clades represents P. dumerilii, although confirmation of the species status with sequence data from the respective-type localities would be desirable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increase of atmospheric carbon dioxide (CO2) levels since the industrial revolution, has contributed to the phenomenon known as ‘ocean acidification’ (OA) (IPCC 2013). Increased pCO2 in seawater leads to the formation of carbonic acid, which then releases hydrogen ions, lowering the pH of the water (Gattuso and Hansson 2011). From the industrial revolution to the present time, oceanic pH has dropped from 8.2 to 8.07, and a further pH drop by 0.3–0.4 pH units is predicted by the year 2100 (Gattuso and Lavigne 2009; Caldeira and Wickett 2003). The focus of research on the potential impacts of OA has initially resided on potentially vulnerable stress-intolerant species (Fischlin et al. 2007), mainly calcifiers depending on calcium carbonate for the growth of their shells and tests. Much less information is available about non-calcifying organisms, which climate change could affect also at the physiological (acclimatization) or genetic level (adaptation) (Calosi et al. 2013; Harvey et al. 2014).
Volcanic CO2 vents represent useful model systems and natural laboratories to investigate short as well as long-term effects of OA on benthic biota and sea-floor ecosystems (Hall-Spencer et al. 2008; Fabricius et al. 2011). Polychaetes are a dominant group in the benthic communities in these systems, especially in temperate areas such as the island of Ischia (Gambi et al. 2016) (Castello Aragonese vent’s area; Kroeker et al. 2011) and Vulcano island (Levante Bay vent’s area; Vizzini et al. 2017) in Italy (Figs. 1, 2). Therefore, polychaetes are appropriate models to address various aspects of acclimatization/adaptation to OA, such as settlement pattern (Ricevuto et al. 2014), and assemblage responses along pH gradients (Gambi et al. 2016), functional traits analyses (Lucey et al. 2015, 2016; Gambi et al. 2016), trophic habit and acclimatization (Calosi et al. 2013; Ricevuto et al. 2015a), and biochemical responses to OA stress (Turner et al. 2015; Ricevuto et al. 2015b, 2016).
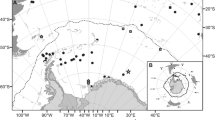
Map of Castello Aragonese study area (Ischia island, Italy) with location of the sampling stations on the north and south sides along a pH gradient (N1, N2, N3–S1, S2, and S3) (from Ricevuto et al. 2014). pH values measured with in situ sensors by Kroeker et al. (2011) are as follows: N1 = 8.0 ± 0.1; N2 = 7.8 ± 0.2; N3 = 7.2 ± 0.4; S1 = 8.1 ± 0.1; S2 = 7.8 ± 0.3; and S3 = 6.6 ± 0.5
Platynereis dumerilii (Audouin and Milne-Edwards, 1834) is a non-calcifying annelid worm of the family Nereididae (see Vieitez et al. 2004 for a morphological description). This meso-herbivore species (Gambi et al. 2000; Ricevuto et al. 2015a) has a semelparous reproduction, with a breeding period that occurs in the Mediterranean Sea between May and September (Giangrande et al. 2002). The breeding period begins with a “sexual metamorphosis” during which an immature, benthic atokous individual transforms in a sexually mature pelagic epitokous form called heteronereis (Fischer and Dorresteijn 2004). During metamorphosis, animals increase their eyes size, subdivide their trunk into two parts with different shapes of parapodia, and develop sexually dimorphic body coloration. Mature pelagic heteronereids swim rapidly and attract individuals of the opposite sex through the release of sex pheromones (Zeeck et al. 1988; Fischer and Dorresteijn 2004). Swarming culminates in the nuptial dance, with sexual partners rapidly swimming in a circle and delivering their gametes into the water column, leading to the fertilization of the eggs. After spawning, males and females die (Fischer and Dorresteijn 2004).
Platynereis dumerilii is a well-understood Evo-Devo model species especially for comparative studies, since its evolutionary lineage has been slow-evolving (Zantke et al. 2014). Highly conserved gene structure and cell types with protein sequences, as well as the position and the number of introns in its genome, show low rates of divergence from vertebrates as opposed to other faster evolving species (Raible et al. 2005; Zantke et al. 2014). P. dumerilii is also considered a bioindicator of organic pollution (Bellan 1980). It has been reported as one of the most abundant species from the vegetated rocky reefs of natural CO2 vents off Ischia Island (Italy) in the Mediterranean Sea (Kroeker et al. 2011; Ricevuto et al. 2014). Calosi et al. (2013) found that the population inhabiting the naturally acidified water of the CO2 vents at Ischia was genetically and physiologically distinct from nearby non-acidified control population. Genetic distances between the vent-inhabiting lineages and those from nearby non-acidified control locations suggested that they represent different sibling species (Calosi et al. 2013). The habitat preference of the two sibling species is not absolute; however, occasional individuals of the presumably vent-adapted lineage appeared in control environments and vice versa. Calosi et al. (2013) surmised that these individuals may not actually reproduce in the mismatched environments and that competitive exclusion insured that they only formed a small portion (1:10–1:15) of the respective population. Lucey et al. (2015), reared specimens in the lab and observed egg brooding in the vent-inhabiting population, indicating that it represents likely the only sibling species of P. dumerilii known to date, P. massiliensis (Moquin-Tandon, 1869). These two sibling species are morphologically indistinguishable as immature adults but are easily identified upon reproduction. In contrast to P. dumerilii, P. massiliensis shows no epitokous transformation and mature worms are protandric hermaphrodites, characterized by egg brooding inside the tube, lecithotrophic larval stages with a semi-direct development, and egg hatching into juveniles within the parental tube (Hauenschild 1951; Helm et al. 2014; Lucey et al. 2015).
Sibling species represent cryptic sister species that are the closest relative of each other and have not been morphologically, and therefore, taxonomically distinguished (Bickford et al. 2007).
Here, we analyze phylogeographic patterns among populations from two CO2 vent systems (Ischia and Vulcano in the Souther Tyrrhenian Sea) and various non-acidified control sites in the Mediterranean Sea and the Atlantic Ocean in conjunction with the reproductive modes of the vent-inhabiting lineages. This integrated approach aims to shed light on the presence and relative proportion of the sibling species in relation with OA and to check for the occurrence of possible selected genotypes and other cryptic species.
Materials and methods
Field collection of Platynereis spp. specimens
Morphologically identified P. dumerilii specimens were collected in two different CO2 vent systems located in Ischia island (Bay of Naples, Italy) and Vulcano island (Aeolian Archipelago, Tyrrhenian Sea, Italy) (Figs. 1, 2). The vent system of Ischia island includes both the South and North sides of the Castello Aragonese, a small islet of volcanic origin connected by an artificial bridge to the main Island of Ischia on the north-eastern side. The continued CO2 (90–95%) gas emissions from the shallow waters have created pH gradients on both sides of the Castello Aragonese based on which six areas with different pH, respectively, three on the north and three on the south, can be distinguished (N1, N2, N3–S1, S2, and S3) (Fig. 1). Sites numbered 1 are designated as control stations with ambient pH values (mean values N1 = 8.0 ± 0.1, S1 = 8.1 ± 0.1), sites numbered 2 are considered intermediate pH stations (N2 = 7.8 ± 0.2, S2 = 7.8 ± 0.3), while sites numbered 3 are considered as the most acidified ones (N3 = 7.2 ± 0.4, S3 = 6.6 ± 0.5; Kroeker et al. 2011; Ricevuto et al. 2014).
Specimens were collected in the two most acidified areas of the south side, S3 and S2, and in the most acidic one of the north side, N3 (e.g., Calosi et al. 2013; Ricevuto et al. 2014) (Fig. 1). For additional pH and carbonate chemistry data of these vents, see Ricevuto et al. (2014).
In the vent area, the dominant taxon is P. massiliensis (Calosi et al. 2013; Lucey et al. 2015); however, most specimens were not mature, so it was impossible to examine gametes. In addition, various swimming heteronereids (P. dumerilii epitokous specimens in reproduction) were collected over the south acidified areas S3 and S2 of the Castello at night (11.00 PM–1.00 AM, 24 May 2011; Larsson T., Gambi M.C. & Hardege J. personal observation) with long-handed nets from a rubber-boat with a strong light to attract the worms (Hardege et al. 1990). Since the depth at the south acidified areas is no more than 3 m, we assume that the pH on the surface is similar to the pH near the bottom. In this zone, the pH shows relatively high temporal and spatial variability, with mean values ranging according to the season from 7.75 to 7.69 in S2 and from 7.32 to 6.59 in S3 (see Ricevuto et al. 2014 for data pH overview). The collected specimens were immediately transferred into RNALater solution (Sigma-Aldrich Company Ltd., Gillingham, UK) on the boat and stored at −20 °C for genetic analyses.
The shallow Levante Bay, situated on the eastern side of Vulcano island, was used as an additional naturally acidified study area, since the main vent system (primary vents) of the island (lat 38°25′01″N and long 14°57′36″E) occurs there at less than 2 m depths (Boatta et al. 2013) (Fig. 2). Similar to Ischia, this vent system has also been partitioned into different sites (S1, S2, and S3) along a pH gradient, at different distances from the primary vents (Fig. 2) (Johnson et al. 2013). Platynereis specimens were collected in the most acidified station S3 (mean pH 7.49) in early May 2013. Further specimens were collected in other geographic areas away from the influence of the vents to check for the possible presence of the sibling species. Sampling sites out of the vents were located around Ischia, at various distances from the vent areas and in the Gulf of Naples (Italy): Sant’ Anna rocks (Ischia), San Pietro (Ischia), Forio (Ischia), Nisida (Gulf of Naples); in some areas of the Western Mediterranean: Palinuro (Tyrrhenian Sea), Ustica island (Tyrrhenian Sea), STARESO Belgian Marine Station at Calvi (Corsica, France), Blanes (Catalunia, Spain); in the Eastern Mediterranean: Santa Caterina (Ionian Sea, Eastern Mediterranean, Italy), and in the North Atlantic: Arcachon (Atlantic, France), and Bristol (Atlantic, UK; see Table 1).
Worms were collected by either snorkeling or SCUBA diving (at 1–3 m depth) by detaching thalli of macroalgae (in the Mediterranean sites mainly of the brown algae, Halopteris scoparia, Dictyota spp., Cladophora spp. at Ischia, and Cystoseira compressa and Dictyota dichotoma at Vulcano) and inserting them inside of fabric bags (20 × 20 cm). The bags were then inserted in cool boxes with seawater from the collection site, and transported to the laboratory until sorting of the algae. In the laboratory, the algae were placed into large plastic trays and the worms were visually identified as Platynereis, showing a very typical swimming behavior, and gently collected with a pipette and inserted in petri dishes. Worms from each sampling site were fixed in 95% ethanol in separate vials for phylogeographic analysis.
Some of the individuals from the Ischia (S3/S2) and Vulcano (S3) vent’s sites were maintained alive after collection and reared under controlled laboratory conditions to check for their reproductive features.
Phylogeographic analysis
The molecular analysis was conducted on specimens collected, as reported in Table 1, and also included the sequences previously published in Calosi et al. (2013) and Lucey et al. (2015). We sequenced a ~600 bp fragment of the mitochondrial cytochrome c oxidase subunit I gene (COI) for 3–15 individuals from each population. Total genomic DNA was extracted from each individual worm using the DNeasy Blood & Tissue Kit (Qiagen, Manchester, UK) following the manufacturer’s instructions. The COI region was amplified using the established primers described by Folmer et al. (1994). PCR products were sequenced directly (Macrogen Europe, Amsterdam, The Netherlands) and the sequence identities were verified using BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi). All sequences were submitted to Genbank under accession numbers KT124668 through KT124717. We further included in our analyses two sequences generated by Lucey et al. (2015), representing a brooding female from the Ischia vents and a free-spawning male from a nearby control site and a COI sequence from the mitochondrial genome of Platynereis dumerilii (Boore and Brown 2000) (see Supplementary Information for list of all Genbank accession numbers and haplotype assignment). The sequences were aligned using the ClustalW algorithm in MEGA 7.0.21 (Kumar et al. 2016). MEGA 7.0.21 was also used to calculate average genetic distances among clades using the Kimura-2-Parameter model. The final alignment length was 568 bp. A phylogenetic analysis was conducted using Bayesian Inference in MrBayes 3.2.1 (Ronquist et al. 2012) through the CIPRES Science Gateway v 3.3 (Miller et al. 2010), using two runs with four Metropolis Coupled Markov Chains Monte Carlo (MCMCMC) each for 10,000,000 generations under a General Time Reversible Model plus Gamma, with the first 2,500,000 generations discarded as burn-in. Trees were sampled every 1000 generations from the posterior distribution after the burn-in period and a 50% majority rule consensus tree was generated. Nereis pelagica (Genbank accession GU672554) was chosen as the outgroup and N. zonata (HQ024403) was included additionally. Minimum Spanning Networks (Bandelt et al. 1999) of the haplotypes were generated in PopART (http://popart.otago.ac.nz). Given the significant genetic divergence among the major clade, haplotype networks were generated for each clade separately. Genetic diversity indices were calculated in MEGA 7.0.21 (Kumar et al. 2016).
Laboratory rearing and reproductive biology of Platynereis vent populations
We kept specimens of Platynereis spp. collected both in the vents of Ischia and Vulcano islands, under similar laboratory conditions. The Ischia specimens (Castello Aragonese stations S2/S3) were kept in petri dishes (100 ml, approx. 5 specimens per bowl) using filtered sea water (0.22 µm), and kept in a summer regime of temperature, light and long day photoperiod (21 ± 1 °C and L:D = 16 h:8 h) inside a thermostatic chamber. Chopped fresh spinach was used to feed the worms and specimens checked for water change, food supply, and reproductive status approximately once a week. The specimens collected at the end of May 2015 in the Levante Bay vent’s area off Vulcano island were transported to Ischia laboratory alive and reared under the same controlled conditions of the Ischia specimens. For each brooding specimen observed, egg size was measured and larval development was followed and documented by photographs at the stereomicroscope (Leica MZ 125) and optical microscope (Leitz Dialux 20-EB).
Results
Phylogenetic and genetic diversity analysis
The 141 sequences of Platynereis spp. grouped into 48 haplotypes (Table 2, Supplementary Table). The phylogenetic tree (Fig. 3) shows that the Platynereis spp. sequences of the studied populations form four distinct clades. Clade 1 comprises most of the Ischia vent samples (North and South acidified sites), a few specimens form control areas (San Pietro, Santa Caterina, and Blanes) as well as a confirmed brooding female from the study of Lucey et al. (2015). Clade 1 is formed by three haplotypes, with the dominant haplotype (Hap_01) shared between the Ischia vent samples and one of the individuals from a nearby control site. Clade 2 consists of four haplotypes, mainly of the Vulcano vent samples, plus a single individual from the Ischia vents (S2/S3). Clade 3 includes specimens from three control locations and one heteronereid collected at Ischia swimming in the south vent area. Clade 3 has five haplotypes, with the heteronereid forming its own haplotype. Clade 4 comprises the largest number of sequences (103) and haplotypes (36), all except one (Ischia S3 11) from non-vent sites, and the P. dumerilii heteronereids collected at night on the south, acidified areas of the Castello. This last clade also includes the GenBank COI sequence for P. dumerilii. The two dominant haplotypes are Hap_15 and Hap_16. Hap_15 is widely distributed throughout the Mediterranean and the Atlantic and also includes one of the heteronereids and the single “outlier” vent sample from the Ischia vents. Hap_16 consists primarily of Mediterranean samples from non-acidified sites and three of the heteronereids. No clear separation between Mediterranean and Atlantic haplotypes is obvious.
50% Majority rule consensus tree based on Bayesian Inference. Inference and haplotype networks for each of the four clades. Asterisks at nodes indicate posterior probability (double asterisks 100%; asterisks >90%; branch support values <90% not shown). The size of the circles in the haplotype networks indicates the number of sequenced individuals with this haplotype. Hash marks on the connecting lines indicate the number of mutational steps between two haplotypes. The colours identify: Ischia vents specimens in pink, Vulcano vents specimens in blue, Heteronereis specimens in green, and Mediterranean and Atlantic specimens from non-acidified sites in grey and brown, respectively. Genbank accession numbers and haplotype assignments are listed in Supplementary Table
Our analyses show that clades 1 and 2 form sister groups, as well as clades 3 and 4. Average genetic distances (Kimura-2-parameter model) are 25.5% between clades 1 and 2 and 22% between clades 3 and 4.
Among the four clades, clade 1 has the highest nucleotide diversity and clade 4 the lowest (Table 1). The Tajima D index is negative in all four clades, with the most negative value in clade 4, indicating that all clades may have gone through recent selective sweeps or population expansions. The starburst shape of the haplotype network of clade 4, with one dominant haplotype and many descendant haplotypes, supports the notion of population expansion. If population genetic indices are calculated by habitat instead of clade, the vent group displays higher nucleotide diversity than the non-vent group. In contrast to all other groupings, the vent population has a positive Tajima D index which may indicate balancing selection and/or a population contraction.
Observation of reproductive features of Platynereis vent populations
Some of the specimens of Platynereis collected at the Ischia vent south side area and reared under controlled conditions (see “Materials and methods”) laid eggs inside their tubes after a few weeks. Individuals brooding eggs inside their tubes were observed in mid-May 2014 (n = 3), mid-June 2014 (n = 2), and end of October 2014 (n = 2). The eggs measured 250–350 µm in diameter (sample size = 21, sample mean = 308.95 µm; σ = 27.38) (Fig. 4), according to the embryo developmental stage, and were actively oxygenated through ventilation by regular movement of the parent inside the tube. The large, yolk rich eggs hatched approximately 2 weeks after being laid. Eggs hatched with three-segment juveniles that remained inside the parental tube until they had 5–6 segments (Fig. 4); at 9–10 segments, they started to build their own tubes (Fig. 4). Platynereis specimens collected in Vulcano and reared under the same controlled conditions of the Ischia specimens also showed a very similar brooding behavior with deposition of several eggs inside the tube. Specimens with eggs were observed in mid-June 2015 (n = 8), mid-July 2015 (n = 2), and end of October 2015 (n = 1). These parents also were ventilating the eggs, which showed a range size between 250 and 350 µm (sample size = 33, sample mean = 292.21 µm; σ = 26.63); eggs hatched with three-segment juveniles (Fig. 5), as observed for the Ischia specimens.
Platynereis massiliensis-like from the Castello Aragonese S3/S2 vent’s areas (Ischia island, Italy) with brooding behavior. The pictures depict: the female specimen inside the brooding tube with the laid eggs; the developing embryo inside the egg; a three-segment juvenile rich in yolk; and a six-segment juvenile with some yolk remnants
Platynereis massiliensis-like from the S3 vents site off the Levante Bay (Vulcano island, Italy). The pictures depicted: the parent specimen inside the brooding tube, while it is taking care of the laid eggs; a laid egg; three-, four-, and five-segmented juveniles in which the yolk content decreases with body growth
Discussion
Our phylogeographic study, in conjunction with reproductive observations on some of the studied populations, reveals several interesting insights. Platynereis clades 1 and 2 are primarily comprised of individuals from the vents at Ischia (clade 1) and the Vulcano vent site (clade 2). Both these populations showed brooding behavior, with parental care within the tube and hatching of juveniles form the eggs (Figs. 4, 5). Although most of the sequenced specimens in clades 1 and 2 were sexually immature at the time of preservation, we are confident that clade 1 represents a clade of brooders, because we included the confirmed brooding female from Lucey et al. (2015) in our analyses as a reference specimen. For clade 2, a brooding habit is presumed because of the results from our reproductive studies, which indicated that brooding is the dominant reproductive mode also at the Vulcano vent’s site. Additional reproductive studies with specimens from Vulcano would shed more light onto potential differences between clades 1 and 2.
We cannot conclude with certainty whether clades 1 or 2 represent Platynereis massiliensis, because we have not been able to obtain samples from the type locality for this species (off Marseille by Moquin-Tandon in 1869). However, the congruence of our developmental observations with those of Hauenschild (1951) and Schneider et al. (1992), and more recently by Helm et al. (2014), suggests that the Ischia population represents P. massiliensis. Hauenschild (1951) collected a population of brooding Platynereis, that he named P. massiliensis, in the Mergellina harbour (city of Naples) not far from Ischia (approx. 18 nm). Schneider et al. (1992) also recorded a brooding population of P. massiliensis in Banyuls sur Mer (France). Despite these studies, P. massiliensis was not included in polychaete checklists, including the Italian fauna (Castelli et al. 2008; Mikac 2015), because these previous records were not taken into account in taxonomic/ecological investigations, and ecological surveys, based on the analysis of preserved adult specimens, always reported only P. dumerilii (Valvassori et al. 2015). Therefore, we suspect that P. massiliensis has been largely overlooked and previous records of P. dumerilii should be reconsidered at the light of this “sibling problem”. Similarly, based on molecular studies, the occurrence of a complex of species has recently been hypothesized for P. dumerilii along the coast of Brazil (Santos C. and Halanych K., pers. comm.).
The significant mean genetic distance in COI between clades 1 and 2 (25.5%) suggests that clade 2 forms another brooding sister species. While genetic distances among sister species in polychaetes can vary greatly, 25.5% is on the high end of the spectrum (Nygren 2014), lending support to the existence of two separate brooding Platynereis species. The Ischia and the Vulcano vents populations of putative P. massiliensis are more than 600 km apart and such genetic complexity is well known from other nereidid worms that reproduce without epitokous spawning such as in Neanthes acuminata (Reish et al. 2014) and in Hediste diversicolor (Virgilio et al. 2009). Therefore, comparative sequence data for P. massiliensis from its type locality (Marseille, France) would be desirable to verify whether the Ischia or the Vulcano populations (or neither of them) truly represents the originally described P. massiliensis.
It was long speculated that the low dispersal rate in many marine species, that have no spawning and no planktonic larval stage, increases genetic diversity (Palumbi 1994) and it is not surprising that a brooding species with a direct or semi-direct development and potentially lower dispersal may show genetically isolated populations. Sato and Masuda (1997) observed two different forms of the nereidid polychaete Hediste japonica (Izuka 1908), which differs in life-history strategies (small-egg form and large-egg form). The results demonstrate that the free-swimming larvae of the smaller-eggs form easily migrate, resulting in frequent gene flow among populations; in contrast, the larger eggs form is characterized by direct development into benthic juveniles without a true pelagic phase resulting in limited gene flow between populations (Sato and Tsuchiya 1991; Sato and Masuda 1997). Therefore, the modes of larval development influence the scale of gene flow, consequently affecting genetic differentiation between populations (Sato and Masuda 1997). Low gene flow levels and regional phylogeographic fragmentation have also been observed by Teske et al. (2007) in two direct developer’s marine isopods species and by Reish et al. (2014) in the direct developer polychaete species Neanthes acuminata. The P. massiliensis genetic complexity (clades 1 and 2) could derive from the reproductive isolation caused by the low dispersal capacity of this semi-direct developer species.
The present data regarding the Platynereis spp. population here examined show lineage divergence and presence of putative cryptic species between both types of larval development and potential of larval dispersal. The “free spawner” clade (the P. dumerilii complex) has overall lower nucleotide diversity (despite significantly larger sample sizes). The positive Tajima D index of the vent samples (as opposed to negative values for all other groupings) may indicate balancing selection in which a high level of polymorphism within the vent populations may have adaptive advantages. This aspect deserves further study with larger samples sizes collected from multiple venting areas.
A few individuals from non-acidified control populations, including S. Caterina (Eastern Mediterranean), also fall into clade 1. Other individuals from S. Caterina fall into clade 4. This sampling site could represent the geographic area characterized by a species sympatry, in which both putative P. massiliensis (clade 1) and P. dumerilii (clade 4) coexist.
Clade 4 comprises individuals from non-acidified sites, with one exception (Ischia S2/S3 specimen #11). It further includes the sequence from a confirmed free-spawning male (from Lucey et al. 2015) and the COI sequence from the P. dumerilii mitochondrial genome, suggesting that clade 4 represents this species. In our phylogenetic tree (Fig. 3), we treated the P. dumerilii mitochondrial genome sequences as originating near the type locality, although the geographic origin of the specimen is actually unknown (Jeff Boore, pers. comm.), and considering the existence of multiple cryptic lineages in Platynereis, we cannot exclude the possibility that it was misidentified. However, we have also included sequences from specimens collected in Arcachon on the French Atlantic coast, less than 180 km south of the type locality of P. dumerilii in La Rochelle, France, providing that additional support that clade 4 is indeed P. dumerilii. Considering that the vents are open systems, it is conceivable that larvae of the epitokous P. dumerilii settle in these areas and survive to adulthood. Whether they successfully reproduce under the acidified conditions remains to be fully studied and demonstrated.
Clade 3 is comprised of individuals from several non-acidified sites (including S. Anna, only 600 m from the vent’s south side) and one heteronereid from the Ischia vents. The mode of reproduction of these populations has not been studied, but considering that a heteronereid falls into this clade, it appears that they exhibit a similar reproductive mode as clade 4. The significant genetic distance to clade 4 (22%), however, suggests that they represent another sibling species, corresponding in this case to the typical reproductive habit of P. dumerilii. From our data, it appears that P. dumerilii and P. massiliensis represent complexes of sibling species.
The phenomenon of sibling/cryptic speciation is particularly common within the polychaetes even among species used as bioindicators in environmental monitoring (Grassle and Grassle 1976; Durou et al. 2007), or in ecotoxicological and bioaccumulation studies (Virgilio et al. 2005; Burlinson and Lawrence 2007; Vázquez-Núñez et al. 2007; Dean 2008; Blake et al. 2009). There are several species in the cryptic Perinereis nuntia group and in the Marphysa sanguinea complex that are used in fishing bait trade and correct identification may be crucial for proper management (Glasby and Hsieh 2006; Lewis and Karageorgopoulos 2008). Due to the economic and ecological importance of these polychaetes, proper characterization of local populations is essential to detect potential ongoing cryptic speciation and to evaluate areas of endemism, and thus has fundamental implications for conservation and management (Nygren 2014). One such example of an endangered cryptic species is Hediste japonica, whose distribution has been found to diminish at a fast rate (Sato and Nakashima 2003).
The growing availability of DNA sequence data, when combined with more traditional taxonomy based on morphological, life-history and reproductive observations, is leading to an exponential increase in the perception of actual biodiversity (Bickford et al. 2007).
The predominance of the brooding P. massiliensis complex in the acidified areas of both Ischia and Vulcano vents might be not directly correlated with the effect of the OA on the reproductive isolation and cryptic speciation, but it seems to be much more related to the success of a brooding habit in stressful conditions. Further experiments to investigate if effectively, the low pH conditions favour a brooding reproductive strategy rather than a broadcasting one would be necessary. In the meantime, the evidence that vent populations only showed a brooding strategy seems to confirm that the extreme conditions in CO2 vents favour the survival and development of the parental-care taxa rather than free spawners (Lucey et al. 2015; Gambi et al. 2016). Larval stages are often more susceptible to stress than adults and the low pH could represent a sort of barrier for settlement of pelagic larvae coming often from habitat outside the acidified conditions. In contrast, the eggs laying inside a brood tube or egg mass and their successive ventilation and parental care may allow the embryos development until young worms (Schneider et al. 1992). The brooding habit, especially within a tube, might then perform a buffering effect that minimizes the effects of OA by providing a microclimate more favourable for hatching and juvenile development. A further buffer effect of low pH might derive by the photosynthetic process and oxygen production carried out by algae, where these brooding polychaetes live, thus further facilitating the embryos’ survival, as showed also by development of Spirorbis spirorbis (Polychaeta, Serpulidae) on the brown alga Fucus serratus (Saderne and Wahl, 2012).
Since the extreme conditions of different CO2 vents systems can be different and may generate different substantial selective pressures besides OA, they can favour cryptic speciation, especially when coupled with the brooding habit of some of the species involved in the selective process. The case of Platynereis spp. here discussed prove the occurrence of at least four different species (two complexes of siblings) of which two of them were suspected to belongs to P. dumerilii and P. massiliensis, respectively. Once the species identity will be resolved, Platynereis spp. could represent a good model to study evolutionary implications of climate change environmental stressors on the marine biota, and deserve further analysis in other vent’s zones or stressed habitat.
References
Bandelt H, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48
Bellan G (1980) Relationship of pollution to rocky substratum polychaetes on the French Mediterranean coast. Mar Pollut Bull 1:318–321
Bickford D, Lohman DJ, Sodhi NS, Ng PKL, Meier R, Winker K, Ingram KK, Das I (2007) Cryptic species as a window on diversity and conservation. Trends Ecol Evol 22:148–155
Blake JA, Grassle JP, Eckelbarger KJ (2009) Capitella teleta a new species designation for the opportunistic and experimental Capitella sp I with a review of the literature for confirmed records. Zoosymposia 2:25–53
Boatta F, D’Alessandro W, Gagliano AL, Liotta M, Milazzo M, Rodolfo-Metalpa R, Hall-Spencer JM, Parello F (2013) Geochemical survey of Levante Bay Vulcano Island (Italy) a natural laboratory for the study of ocean acidification. Mar Pollut Bull 73(2):485–494
Boore JL, Brown WM (2000) Mitochondrial genomes of Galathealinum, Helobdella and Platynereis: sequence and gene arrangement comparisons indicate that Pogonophora is not a phylum and Annelida and Arthropoda are not sister taxa. Mol Biol Evol 17(1):87–106
Burlinson FC, Lawrence AJ (2007) A comparison of acute and chronic toxicity tests used to examine the temporal stability of a gradient in copper tolerance of Hediste diversicolor from the Fal estuary, Cornwall, UK. Mar Pollut Bull 54:66–71
Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425:365
Calosi P, Rastrick SPS, Lombardi C, de Guzman HJ, Davidson L, Jahnke M, Giangrande A, Hardege JD, Schulze A, Spicer JI, Gambi MC (2013) Adaptation and acclimatization to ocean acidification in marine ectotherms: an in situ transplant experiment with polychaetes at a shallow CO2 vent system. Phil Trans R Soc Lond B Biol Sci 368:20120444. doi:10.1098/rstb20120444
Castelli A, Bianchi CN, Cantone G, Çinar ME, Gambi MC, Giangrande A, IraciSareri D, Lanera P, Licciano M, Musco L, Sanfilippo R, Simonini R (2008) Annelida Polychaeta. In: Relini G (ed) Checklist della flora e della fauna dei mari italiani. Parte I Biol Mar Mediterr 15(1):323–373
Dean HK (2008) The use of polychaetes (Annelida) as indicator species of marine pollution: a review. Rev Biol Trop 56:11–38
Durou C, Smith BD, Roméo M, Rainbow PS, Mouneyrac C, Mouloud M, Gnassia-Barelli M, Gillet P, Deutsch B, Amiard-Triquet C (2007) From biomarkers to population responses in Nereis diversicolor: assessment of stress in estuarine ecosystems. Ecotoxicol Environ Saf 66:402–411
Fabricius KE, Langdon C, Uthicke S, Humphrey C, Noonan S, De’ath G, Okazaki R, Muehllehner N, Glas MS, Lough JM (2011) Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat Clim Change 1:165–169
Fischer A, Dorresteijn A (2004) The polychaete Platynereis dumerilii (Annelida): a laboratory animal with spiralian cleavage lifelong segment proliferation and a mixed benthic/pelagic life cycle. BioEssays 26:314–325
Fischlin A, Midgley GF, Price J, Leemans R, Gopal B, Turley C, Rounsevell M, Dube P, Tarazona J, Velichko A (2007) Ecosystems their properties goods and services. In: Parry ML (ed) Climate Change 2007: Impacts Adaptation and Vulnerability Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Ecosystems their properties goods and services. Cambridge University Press, Cambridge, pp 211–272
Folmer O, Black M, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit 1 from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Gambi MC, Zupo V, Buia MC, Mazzella L (2000) Feeding ecology of Platynereis dumerilii (Audouin & Milne-Edwards) in the seagrass Posidonia oceanica system: the role of the epiphytic flora (Polychaeta, Nereididae). Ophelia 53(3):189–202
Gambi MC, Musco L, Giangrande A, Badalamenti F, Micheli F, Kroeker KJ (2016) Distribution and functional traits of polychaetes in a CO2 vent system: winners and losers among closely related species. Mar Ecol Prog Ser 550:121–134
Gattuso JP, Hansson L (eds) (2011) Ocean acidification. Oxford University Press, New York
Gattuso JP, Lavigne H (2009) Technical note: approaches and software tools to investigate the impact of ocean acidification. Biogeosciences 6:2121–2133
Giangrande A, Fraschetti S, Terlizzi A (2002) Local recruitment differences in Platynereis dumerilii (Polychaeta Nereididae) and their consequences for population structure. Ital J Zool 69:133–139
Glasby CJ, Hsieh HL (2006) New species and new records of the Perinereis nuntia species group (Nereididae: Polychaeta) from Taiwan and other Indo-West Pacific shores. Zool Stud 45:553–577
Grassle JP, Grassle JF (1976) Sibling species in the marine pollution indicator Capitella (Polychaeta). Science 192:567–569
Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, Rowley SJ, Tedesco D, Buia MC (2008) Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454:96–99. doi:10.1038/nature07051
Hardege JD, Bartels-Hardege HD, Zeeck E, Grimm FT (1990) Induction of swarming in Nereis succinea. Mar Biol 104:291–294
Harvey BP, Al-Janabi B, Broszeit S, Cioffi R, Kumar A, Aranguren-Gassis M, Bailey A, Green L, Gsottbauer CM, Hall EF, Lechler ML, Mancuso FP, Pereira CO, Ricevuto E, Schram JB, Stapp LS, Stenberg S, Santa Rosa LT (2014) Evolution of marine organisms under climate change at different levels of biological organisation. Water 6:3545–3574
Hauenschild C (1951) Nachweis de sogenannten atoken Geschlechtsform des Polychaeten Platynereis dumerilii Aud et M Edw als eigene Art auf Grund von Zuchtversuchen. Zoolo Jahr Abteil all Zool Physiol der Tiere 63:107–128
Helm C, Adamo H, Hourdez S, Bleidorn C (2014) An immunocytochemical window into the development of Platynereis massiliensis (Annelida Nereididae). Int J Dev Biol 58:613–622
IPCC Climate Change (2013) The Physical Science Basis Contribution of Working Group I to the Fifth Assessment Report of the Intergovernamental Panel on Climate Change. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Cambridge University Press, Cambridge, pp 1–33
Johnson VR, Brownlee C, Rickaby REM, Graziano M, Milazzo M, Hall-Spencer JM (2013) Responses of marine benthic microalgae to elevated CO2. Mar Biol 160:1813–1824
Kroeker KJ, Micheli F, Gambi MC, Martz TR (2011) Divergent ecosystem responses within a benthic marine community to ocean acidification. Proc Natl Acad Sci USA 108:14515–14520
Kumar A, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Lewis C, Karageorgopoulos P (2008) A new species of Marphysa (Eunicidae) from the western Cape of South Africa. J Mar Biol Assoc UK 88:277–287
Lucey NM, Lombardi C, De Marchi L, Schulze A, Gambi MC, Calosi P (2015) To brood or not to brood: are marine invertebrates that protect their offspring more resilient to ocean acidification? Nat Sci Rep 5:12009. doi:10.1038/srep12009
Lucey NL, Lombardi C, Florio M, De Marchi L, Nannini M, Rundle S, Gambi MC, Calosi P (2016) No evidence of local adaptation to low pH in a calcifying polychaete population from a shallow CO2 vent system. Evolut Appl. doi:10.1111/eva.12400
Mikac B (2015) A sea of worms: polychaete checklist of the Adriatic Sea. Zootaxa 3943(1):1–172
Miller JR, Koren S, Sutton G (2010) Assembly algorithms for next-generation sequencing data. Genomics 95(6):315–327
Moquin-Tandon A (1869) Note sur une nouvelle Annèlide hermaphrodite. Ann Sc Nat 11:34
Nygren A (2014) Cryptic polychaete diversity: a review. Zoolog Scr 43:172–183
Palumbi SR (1994) Genetic divergence reproductive isolation and marine speciation. Ann Rev Ecol Syst 25:547–572
Raible F, Tessmar-Raible K, Osoegawa K, Wincker P, Jubin C, Balavoine G, Ferrier D, Benes V, de Jong P, Weissenbach J, Bork P, Arendt D (2005) Vertebrate-type intron-rich genes in the marine annelid Platynereis dumerilii. Science 310:1325–1326
Reish DJ, Anderson FE, Horn KM, Hardege JD (2014) Molecular phylogenetics of the Neanthes acuminata (Annelida: Nereididae) species complex. Mem Mus Vic 71:271–278
Ricevuto E, Kroeker KJ, Ferrigno F, Micheli F, Gambi MC (2014) Spatio-temporal variability of polychaete colonization at volcanic CO2 vents indicates high tolerance to ocean acidification. Mar Biol 161:2909–2919
Ricevuto E, Benedetti M, Regoli F, Spicer JI, Gambi MC (2015a) Antioxidant capacity of polychaetes occurring along a natural pCO2 gradient: results of an in situ reciprocal transplant experiment. Mar Environ Res 112(Part A):44–51
Ricevuto E, Vizzini S, Gambi MC (2015b) Ocean acidification effects on stable isotope signatures and trophic interactions of polychaete consumers and organic matter sources at a CO2 shallow vent system. J Exp Mar Biol Ecol 468:105–117
Ricevuto E, Lanzoni I, Fattorini D, Regoli F, Gambi MC (2016) Arsenic speciation and susceptibility to oxidative stress in the fanworm Sabella spallanzanii (Gmelin) (Annelida Sabellidae) under naturally acidified conditions: an in situ transplant experiment in a Mediterranean CO2 vent system. Sci Total Environ 544:765–773
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 32: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61(3):539–542
Saderne V, Wahl M (2012) Effect of Ocean acidification on growth, calcification and recruitment of calcifying and non-calcifying epibionts of brown algae. Biogeosci Discuss 9:3739–3766
Sato M, Masuda Y (1997) Genetic differentiation in two sibling species of the brackish-water polychaete Hediste japonica complex (Nereididae). Mar Biol 130:163–170
Sato M, Nakashima A (2003) A review of Asian Hediste species complex (Nereididae Polychaeta) with descriptions of two new species and a redescription of Hediste japonica (Izuka 1908). Zool J Linn Soc 137:403–445
Sato M, Tsuchiya M (1991) Two patterns of early development in nereidid polychaetes keying out to Neanthes japonica (Izuka). Ophelia 5(Suppl):371–382
Schneider S, Fischer A, Dorresteijn AW (1992) A morphometric comparison of dissimilar early development in sibling species of Platynereis (Annelida Polychaeta). Roux’s Arch Dev Biol 201:243–256
Teske PR, Papadopoulos I, Zardi GI, McQuaid CD, Edkins MT, Griffiths CL, Barker NP (2007) Implications of life history for genetic structure and migration rates of southern African coastal invertebrates: planktonic, abbreviated and direct development. Mar Biol 152:697–711
Turner LM, Ricevuto E, Massa-Gallucci A, Gambi MC, Calosi P (2015) Energy metabolism and cellular homeostasis trade-offs provide the basis for a new type of sensitivity to ocean acidification in a marine polychaete at a high CO2 vent: adenylate and phosphagen energy pools versus carbonic anhydrase. J Exp Biol 163:211. doi:10.1242/jeb117705
Valvassori G, Massa-Gallucci A, Gambi MC (2015) Reappraisal of Platynereis massiliensis (Moquin-Tandon) (Annelida Nereididae) a neglected sibling species of Platynereis dumerilii (Audouin & Milne Edwards). Biol Mar Mediterr 22(1):113–116
Vázquez-Núñez R, Méndez N, Green-Ruíz C (2007) Bioaccumulation and elimination of Hg in the fireworm Eurythoe complanata (Annelida: Polychaeta) from Mazatlan, Mexico. Arch Environ Contam Toxicol 52(4):541
Vieitez J, Alos C, Parapar J et al (2004) Fauna Iberica, Vol 25. Annelida Polychaeta I. Consejo Superior de Investigaciones Cientificas, Madrid, p 536
Virgilio M, Maci S, Abbiati M (2005) Comparisons of genotype-tolerance responses in populations of Hediste diversicolor (Polychaeta: Nereididae) exposed to copper stress. Mar Biol 147:1305–1312
Virgilio M, Fauvelot C, Costantini F, Abbiati M, Backeljau T (2009) Phylogeography of the common ragworm Hediste diversicolor (Polychaeta: Nereididae) reveals cryptic diversity and multiple colonization events across its distribution. Mol Ecol 18(9):1980–1994
Vizzini S, Martínez-Crego B, Andolina C, Massa-Gallucci A, Connell SD, Gambi MC (2017) Ocean acidification as a driver of community simplification via the collapse of higher-order and rise of lower-order consumers. Nat Sci Rep 7:4018. doi:10.1038/s41598-017-03802-w
Zantke J, Bannister S, Rajan VBV, Raible F, Tessmar-Raible K (2014) Genetic and genomic tools for the marine annelid Platynereis dumerilii. Genet Soc Am 197(1):19–31. doi:10.1534/genetics.112.148254
Zeeck E, Hardege JD, Bartels-Hardege H, Wesselmann G (1988) Sex pheromone in a marine polychaete: determination of the chemical structure. J Exp Zool part A 246(3):285–292
Acknowledgements
This project was supported by an EU-ASSEMBLE Grant to JDH, JW, and MCG and partially by National Science Foundation Grant DEB-1036186 to AS. GV was supported by a Stazione Zoologica Anton Dohrn PhD fellowship. We thank A. Massa-Gallucci for some early observation on P. massiliensis brooding in the laboratory on the Ischia vent’s population, and Thomas Larsson for collaboration in the collection of heteronerids at the Castello vents. MCG is indebted to Prof. Vizzini S. (University of Palermo) and the ESF COST Action (ES 0906) “Seagrass Productivity: From Genes to Ecosystem Management” for the invitation at the Workshop in Vulcano island in early May 2013, and the opportunity to sample Platynereis specimens at the vents.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was supported by an EU-ASSEMBLE Grant to JDH, JW and MCG and partially by National Science Foundation Grant DEB-1036186 to AS. GV was supported by a Stazione Zoologica Anton Dohrn PhD fellowship.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: A-E. Todgham.
Reviewed by Undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wäge, J., Valvassori, G., Hardege, J.D. et al. The sibling polychaetes Platynereis dumerilii and Platynereis massiliensis in the Mediterranean Sea: are phylogeographic patterns related to exposure to ocean acidification?. Mar Biol 164, 199 (2017). https://doi.org/10.1007/s00227-017-3222-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3222-x