Abstract
Lakes and reservoirs present contrasting differences regarding origin, age and trophic state that may influence their biological communities. In the face of the inevitably rising number of reservoirs worldwide, our objective was to investigate the differences in zooplankton community structure and diversity patterns from 98 tropical shallow lakes and reservoirs (northeast Brazil). We tested the hypothesis that reservoirs have less diverse communities, which could be associated with ecosystem age or high productivity (a typical local pattern). The results show that most reservoirs are eutrophic ecosystems that hold distinct zooplankton communities in comparison with lakes. Despite their higher productivity, reservoirs played an essential role in subsidizing zooplankton diversity as they had higher gamma diversity because of the number of exclusive species, especially for the Rotifera group. The zooplankton density and biomass were also higher in the reservoirs, but this pattern was not associated with higher species dominance. Lakes also played a central role in zooplankton diversity, having a distinct species composition. Jointly, lakes and reservoirs help to maintain the zooplankton species pool at a regional level, suggesting the importance of complementarity in community composition between artificial and natural aquatic ecosystems on large-scale patterns of zooplankton biodiversity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural lakes (hereafter lakes) and reservoirs are inland water ecosystems recognized for their ecological, economic and social importance (Tundisi and Matsumura-Tundisi 2003; Irz et al. 2006; Manatunge et al. 2008; Downing 2010; Fang et al. 2016). They constitute an essential source of water resources for ecosystem services and biodiversity development, besides acting as fundamental components for hydrological and biogeochemical cycles (Tranvik et al. 2009; Cooke et al. 2016). Lakes are usually ancient ecosystems formed by geomorphological processes (Havel et al. 2005; Dodson et al. 2007), while reservoirs are human-made environments, which have been recently implemented (≈ 50–100 years) to fulfill a variety of human needs, such as recreation, hydroelectricity production, waste disposal, drinking water and irrigation (Lehner and Döll 2004; Lehner et al. 2011). In this sense, lakes and reservoirs differ in origin, age, basin morphology, hydrological processes and human use (Timms 2009; Branstrator 2010; Doubek and Carey 2017). Such differences may influence community diversity through differences in patterns of species richness, composition, and population abundance (Pinto-Coelho et al. 2005; Simões et al. 2015).
Studies about biological diversity in lentic ecosystems are primarily conducted in lakes, with many gaps about how biological communities respond to the differences presented by lakes and reservoirs (Merrix-Jones et al. 2013). Understanding how biological diversity differs between lakes and reservoirs is central to community conservation of inland water biodiversity, owing to the increasing fraction of global inland surface waters turning into reservoirs and the concomitant reduction in the number of lakes worldwide (Nilsson 2009; Pekel et al. 2016). This scenario is even more prominent in drylands (i.e., arid and semiarid regions), where extreme droughts are becoming more frequent due to climate changes, leading to the disappearance of small lakes and ponds and the need to construct artificial inland water bodies (Pekel et al. 2016).
In low-latitude regions of Brazil, reservoirs are usually built to supply electricity and for general anthropogenic water demands (Rosenberg et al. 2000; Lazzaro et al. 2003; Havel et al. 2005; Moss 2009). In northeast Brazil, where the semiarid climate determines water shortages, reservoirs and lakes are subjected to detrimental effects of irregular rainfall and high evaporation (Sousa et al. 2008; Da Costa et al. 2016). Reservoirs in this region are generally small (< 1 km2) and constitute the primary water source for the local populations (Molle 1991; Bouvy et al. 2001). Under this scenario, they have suffered with the impacts of increasing nutrient concentrations causing eutrophication (Chellappa and Costa 2003; Costa et al. 2006), which may act as an environmental filter selecting species that are adapted to eutrophic and hypereutrophic conditions and resulting in the decrease in local species diversity (Crossetti et al. 2008; Arthaud et al. 2012). Despite this, the reservoirs may contain exclusive species that contribute to the regional species pool in lentic ecosystems, since they constitute hybrid systems (i.e., lotic and lentic characteristics) and harbor species adapted to lotic conditions.
Contrasting with these patterns, the geomorphological and hydrodynamic changes that occurred throughout the history of the lakes have subjected the biological community to a long process of adaptive selection to such conditions (Wellborn et al. 1996; Albrecht and Wilke 2008). According to the structural heterogeneity hypothesis, habitats that have existed for more extended periods are structurally more complex. Such complexity may act as an environmental filter for the regional species pool, selecting different combinations of functional traits and increasing the community diversity (McKindsey and Bourget 2001). Similarly, the ecological time hypothesis states that old habitats (such as lakes when compared to reservoirs) had more time to be colonized and therefore should have higher diversity (Pianka 1988).
In the face of the inevitably rising number of reservoirs worldwide, our objective was to verify the contribution of reservoirs and lakes on the patterns of local and regional diversity of zooplankton communities. Zooplankton constitute a diverse and abundant group, with species that vary greatly in size, ontogenetic development, growth, reproductive and feeding strategies, and trophic levels (Brown et al. 1996; Figuerola and Green 2002; Beisner et al. 2006). These species play an essential role in the aquatic food webs, linking primary production to upper trophic levels (Soranno et al. 1993; Harris et al. 2000; Suthers and Rissik 2009). The high diversity of trait variability and dispersal capacity across zooplankton species makes them very sensitive to changes in aquatic ecosystems across space and time (Leibold 1999; Dodson et al. 2000) and consequently to the processes governing community assembly at local and regional scales (Beisner et al. 2006; Allen 2007).
Following these conjectures, we expect that: (1) lakes should have higher alpha diversity (local scale) than reservoirs when expressed by indices such as species richness, Simpson diversity and evenness, and higher gamma diversity, when expressed by total species richness at the regional scale. This scenario will dictate higher species dominance and regional homogenization in reservoirs due to environmental conditions that favor the emergence of few widespread tolerant species (Ghidini et al. 2009; Wojciechowski et al. 2017). In addition, (2) total local zooplankton community biomass and density are expected to be, on average, higher in reservoirs due to higher nutrient concentrations. Finally, (3) although reservoirs are expected to harbor more homogeneous communities than natural lakes, they may play a significant role in the regional biodiversity conservation as they host a fraction of exclusive species from the regional pool.
Materials and methods
Study area
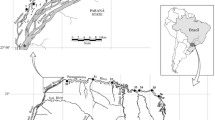
This study was performed using data from 98 low-latitude lentic freshwater environments (30 lakes and 68 reservoirs) sampled during the dry season (September 2012) (Fig. 1). Ecosystems were predominantly shallow (91% < 4 m deep) with small surface areas (87% < 1 km2) (Table 1). Only two lakes and five reservoirs presented depths varying between 4 and 9 m. Their distribution spans about 36,000 km2 from the coastal region (tropical humid and tropical semi-humid climatic subdomains; annual precipitation ≈ 800–1200 mm) to the semiarid region (semiarid climatic subdomain; annual precipitation ≈ 400–800 mm) (Diniz and Pereira 2015; INMET 2015). Despite the large geographic distribution of the sampled ecosystems, all of them were perennials (SUDENE 2010). The sampled reservoirs consisted of systems formed by stream damming, constructed in 1915 by the National Department of Works for Drought Control (DNOCS 2015). They were built to store water, so that there are no water outflows unless the water level rises above the dam. These reservoirs present low outflow and high water residence time associated with a negative hydric balance and high temperatures during most of the hydrological cycle (Barbosa et al. 2012). They are generally surrounded by shallow and low-permeable soils, which prevent groundwater accumulation. The sampled lakes are mostly coastal systems originating from the tertiary period with subsequent quaternary sediment deposition, are mainly surrounded by sandy soils, and primarily supplied by groundwater (Esteves et al. 2008).
Sampling procedures of zooplankton and environmental variables
The sampled lakes and reservoirs were not stratified. Therefore, water samples for zooplankton and physical/chemical analysis were collected from the subsurface (approximately 0.3 m deep) portion of the water column with a Van Dorn bottle. Samplings were taken from six different spots in each ecosystem. Three sampling spots were randomly positioned near the shores of the littoral region, and the other three were taken from limnetic habitats, near the central portion of the water body. The six water samples from each lake were mixed and integrated into a single sample in a graduated bucket from which aliquots to analyze abiotic water parameters and zooplankton were taken. Dissolved oxygen (DO) concentration (% of saturation) was measured in the field with a portable digital multiparameter system (YSI), and values were averaged across the six sampling spots. Aliquots of water (500 ml) were stored in pre-washed plastic vials and frozen until laboratory analysis of chlorophyll-a (Chl-a) determination, according to Jespersen and Christoffersen (1987), total phosphorus (TP), according to Murphy and Riley (1962), and total nitrogen (TN) (nitrogen analyzer TOC-V Shimadzu). The ecosystem area was calculated for each lake and reservoir using the software ArcGIS 10.5.1 (ESRI 2011). To describe the parameters related to the landscape properties and land cover across the lakes and reservoirs, we measured a set of variables related to the morphometry of the aquatic ecosystems and their catchments, as well as the land use on these catchments. To delineate catchments for each of the 98 aquatic ecosystems, a 30-m digital elevation model (DEM) mosaic comprising the states of Rio Grande do Norte and Paraíba was gathered from the Brazilian Geomorphometric database (TOPODATA), available at http://www.dsr.inpe.br/topodata/acesso.php. The “fill sinks” function was used as a DEM manipulation procedure to fill any existing depressions in the DEM file that could obstruct water flow. Delineation of catchments was based on polygon shapefiles for the lakes and reservoirs and was performed using the Arc Hydro 2.0 Toolbox (ESRI 2011) in ArcGIS 10.5.1. Morphometric variables that can potentially affect the degree of connectivity between aquatic and terrestrial systems, including catchment area (CA), lake area (LA) and catchment area/lake area ratio (CA/LA), were calculated for each lake and reservoir and catchment in ArcGIS 10.5.1 (ESRI 2011).
Land use was characterized for individual catchments, using 2012 data for land-use classification for the Caatinga and Atlantic Forest biomes, available through the MapBiomas Initiative—v.2.3 (http://mapbiomas.org/). The MapBiomas classification of land use provides a suite of precise classifications of diverse natural vegetation physiognomies and human use, including forests, fields, natural non-forest areas, agriculture, pasture, urban, developed, water, among others. The area of each land-use type was calculated using the “tabulate area” tool in ArcGIS 10.5.1. Subsequently, categories of land use provided by MapBiomas were grouped in three main groups: forest cover, agriculture/pasture cover and urban cover (Table S1).
Zooplankton samplings were performed by filtering a known volume of water (30 to 70 L depending on the number of suspended particles in the water) from the bucket through plankton net (50-µm mesh). The samples were rapidly fixed with a sugar formaldehyde solution (4% final concentration) and stored in 150-ml glass flasks. Zooplankton individuals (Rotifera, Copepoda, and Cladocera) were identified to the species level using a Sedgewick-Rafter camera and microscope (for Rotifera), a Bogorov camera and stereo microscope (for Copepoda and Cladocera), and taxonomic guides (e.g., Elmoor-Loureiro 1997; Koste 1978; Santos-Silva 2000). Identification and counting were performed on three replicates per sample with at least 100 individuals of the most abundant organisms in each. The samples were thoroughly evaluated to search for rare species. Total zooplankton density (ind m−3) was calculated by multiplying the number of individuals by the total volume of water filtered.
To estimate Cladocera, Calanoida and Cyclopoida biomass as dry weight (µm DW m−3) per sample, we first determined the individual species weight by measuring 30 random individuals of each species and used their body sizes in allometric equations available in the literature (e.g., Hall et al. 1970; Bottrell et al. 1976; Wetzel and Likens 1991). Then, we multiplied the species biovolume by its density and summed this over the species within each group. To determine rotifers biomass (µm DW m−3), we used literature data on average individual weight for each species (e.g., Bottrell et al. 1976; Pauli 1989) and multiplied them by each species density followed by summing of the species. Total zooplankton biomass was then estimated by summing the biomass of rotifers, cladocerans and copepods in a sample.
Data analysis
To test the average differences in abiotic parameters that characterize the trophic status of the aquatic ecosystems of natural lakes and reservoirs, we used the Mann–Whitney test to compare the Chl-a, TP, TN, DO and ecosystem area (these parameters were not normally distributed) and the Student’s t test to compare the water temperature (normally distributed among samples).
We used individual-based rarefaction curves to assess whether regional species richness (gamma diversity) would be higher in lakes than in reservoirs. This method allows us to compare regional zooplankton species richness between lakes and reservoirs while removing the effect of different sampling efforts (i.e., differences in the number of sampled individuals) (Gotelli and Colwell 2001). The rarefaction curves were constructed by the bootstrap method, randomly selecting the individuals according to a growing sampling effort. The selection was repeated 1000 times to obtain an average pattern of species richness for lakes and reservoirs. The 95% confidence interval (CIŜ) was estimated by IC = Ŝ ± s × 1.96 (Manly 2006), where s is the standard deviation of the 1000 species richness values. We also performed a permutational multivariate analysis of variance (PERMANOVA; Anderson 2001) based on the Jaccard index with 999 permutations to test whether lakes and reservoirs differed in species composition, and a principal coordinate analysis (PCoA) to assist visualizing the results of the PERMANOVA.
To test whether the total zooplankton community biomass and density were, on average, higher in reservoirs than in lakes, we first transformed data (log10) to meet homogeneity of variances and then we used the Student’s t test, considering individual lakes and reservoirs as independent sample units. To measure local zooplankton species diversity, we calculated Simpson’s reciprocal index of diversity (D): \(D = \frac{1}{{\mathop \sum \nolimits_{i = 1}^{S} p_{i}^{2} }}\) (where pi is the proportion of species i, and S is the number of species). Zooplankton species evenness (E) was based on Simpson’s reciprocal index of diversity: \(E = \frac{{\left( {1 - D} \right)}}{S}\). We used the Mann–Whitney test and the Student’s t test to compare the Simpson’s diversity (not normally distributed) and the average local zooplankton species evenness between lakes and reservoirs.
All analyses were carried out in R 3.1.3 (R Core Team 2015). We wrote the routine for individual-based rarefaction curves. PERMANOVA, Simpson’s reciprocal index of diversity and evenness were calculated using the vegan package (Oksanen et al. 2015). The PCoA was performed using vegan and ape packages (Paradis 2012). The Student’s t test and Mann–Whitney test (Wilcoxon rank-sum test) were calculated using the stats package (R Core Team 2015).
Results
Abiotic parameters describing the trophic status of the aquatic ecosystems were statistically distinct between lakes and reservoirs. Reservoirs had higher values of Chl-a, TP, TN and lower values of DO than lakes. The average values of water temperature and area were not statistically different between the two types of ecosystems (Table 1). Regarding the landscape properties and the patterns of land use, we observed that the catchment area and the catchment area/ecosystem area ratio were significantly higher for reservoirs compared to lakes (Table S1). Only values referent to urban land cover were significantly different between the two kind of ecosystems, with lakes showing a slightly higher proportion of catchment converted to urban areas compared to reservoirs (Table S1).
For the zooplankton community, we recorded a total of 179 species across all sampled ecosystems. In lakes, 135 species were registered (16 copepods, 36 cladocerans, and 83 rotifers species), while in reservoirs a total of 148 species were found (19 copepods, 35 cladocerans, and 94 rotifers species) (Table S1). Thirty-one species were found exclusively in lakes, while 44 were exclusively from reservoirs (Table S2). Zooplankton community composition between lakes and reservoirs was statistically different (PERMANOVA; F = 4.30; P = 0.001), with plot groups for lakes and reservoirs separated in the ordination space (Fig. 2).
The rarefaction curves showed that reservoirs had proportionally more zooplankton species (gamma diversity) than lakes (Fig. 3). Although at low sampling effort the regional species richness accumulated faster across lakes than in reservoirs, at the maximum sampling effort for lakes (≈ 2,500,000 ind m−3) reservoirs had a significantly higher number of sampled zooplankton species (Fig. 3). Also, on average, reservoirs had higher total zooplankton abundance and biomass than lakes (Fig. 3; P < 0.001; unpaired Student’s t test). Finally, we did not find any statistical differences between lakes and reservoirs for alpha diversity, concerning the average local species richness (Fig. 4a; P = 0.241; unpaired Student’s t test), Simpson’s diversity (Fig. 4b; P = 0.285; Mann–Whitney test) and species evenness (Fig. 4c; P = 0.882; unpaired Student’s t test).
Zooplankton gamma diversity (regional species richness) for lakes (n = 30) and reservoirs (n = 68). The curves are individual-based rarefactions showing the average (solid lines) accumulated zooplankton species richness and their ± 95% confidence intervals (shaded areas). The scale number of individuals is shown until that difference in species richness for lakes and reservoirs is stabilized. The dashed vertical line indicates the species richness at the comparison point. (Inset) The bar graph shows average values and standard deviation (log10-transformed) of total zooplankton density and biomass for lakes and reservoirs
Comparisons of local average zooplankton: a species richness, b species diversity (measured as Simpson’s index) and c species evenness (measured as the Simpson Evenness) between lakes (n = 30) and reservoirs (n = 68). Differences in local average zooplankton species richness and species diversity were tested through a nonparametric Mann–Whitney test. Boxes, horizontal bars and vertical solid lines represent the interquartile range, median and the data range, respectively. Difference in local average zooplankton species evenness between lakes and reservoirs was tested through a t test. Vertical bars depict the ± 95% confidence intervals
Discussion
We analyzed the zooplankton diversity patterns between lakes and reservoirs in northeast Brazil by performing the first comprehensive comparative analysis of a large number of lentic aquatic ecosystems with different origins and age from tropical regions. As we expected, reservoirs had higher nutrient levels, which could indicate eutrophication processes. However, these characteristics were not found to be associated with zooplankton gamma or alpha diversities, contradicting our first hypothesis. This result was a consequence of the high percentage of exclusive species in the reservoirs, especially the Rotifera group. We also observed that zooplankton biomass and density were higher in reservoirs, confirming our second hypothesis. However, this pattern was not associated with higher species dominance as predicted. Finally, we observed that both reservoirs and lakes harbored significant zooplankton diversity in tropical and shallow lentic ecosystems.
Although the reservoirs are more recent ecosystems and our results showed that they are more productive than the lakes (Table 1), the reservoirs had higher gamma diversity than lakes but similar levels of alpha diversity. We attributed the higher gamma diversity in reservoirs to their high number of exclusive species, especially rotifers that contributed to 61% of the species registered solely in the reservoirs. The unique species effect influenced the regional species pool by increasing the gamma diversity observed in the reservoirs; however, it did not generate differences in the alpha diversity between reservoirs and lakes. Also, among newer ecosystems as the studied reservoirs, age-mediated dispersal limitation may be a limiting factor for zooplankton colonization and distribution across the regional community, since organisms had less time to transpose the terrestrial matrix (i.e., overland dispersion) and colonize new lentic habitats (Shurin 2000; Figuerola and Green 2002; Bohonak and Jenkins 2003). However, although the connection between the tributaries and the reservoirs is sporadic in the Brazilian semiarid (They et al. 2017), this process can reduce or even compensate the age-mediated dispersal limitation on the structure of reservoirs’ communities and facilitate the watercourse dispersion (Havel et al. 2005), contributing to their gamma diversity.
Regarding the influence of trophic state of the reservoirs, areas with higher productivity are associated with larger temporal variation in species richness and are more prone to have alternative stable states (Chase and Leibold 2002). This factor might influence species selection, the community assembly sequence and species permanence, thus contributing to higher regional richness in the reservoirs. Besides, among the zooplankton groups, Rotifers had the highest number of species in the two sets of ecosystems and contributed considerably to the higher zooplankton gamma diversity in reservoirs. The high dispersion capacity (Koste 1978; Fernández-Rosado and Lucena 2001) and adaptability of rotifer species to productive ecosystems (Araújo 1982; Sampaio et al. 2002; Malveira et al. 2011) may have counterbalanced the predicted adverse effects, not only for gamma diversity, but also for alpha diversity in reservoirs. Rotifers also produce resting eggs, which may be an adaptation against environmental harshness (Gilbert 1974; Ricci 2001), such as the frequent drought periods that occur in the Brazilian northeast region (Finan and Nelson 2001; Bouvy et al. 2003). Although resting eggs is also a reproductive strategy adopted by Cladocera to overcome environmental harshness, we believe that this strategy may have been negatively counterbalanced in reservoirs because most of cladocerans are not adapted to eutrophic and hypereutrophic ecosystems such as the sampled reservoirs (Nogueira et al. 2008; Sendacz et al. 2006; Parra et al. 2009), and because cladocerans are more affected by size-selective predation of planktivorous fish which are widespread among the sampled ecosystems (Menezes et al. 2012; Eskinazi-Sant’Anna et al. 2013).
Diverging from what we observed, Simões et al. (2015) reported higher zooplankton gamma diversity across lakes than reservoirs in the Paraná river basin in southern Brazil. The authors argue that the reservoirs are oligotrophic compared to natural lakes and attributed the highest zooplankton gamma diversity in lakes to the increased productivity. The predominance of oligotrophic reservoirs in southeast Brazil was evidenced from the 31 reservoirs studied by Pagioro et al. (2005). This scenario contrasts with our results, where the reservoirs had higher levels of nutrient concentrations related to productivity (e.g., Chl-a, TP, and TN), indicating that most of them are eutrophic or hypereutrophic. In another study, Dodson et al. (2007) did not record differences in zooplankton species richness for lakes and reservoirs considering the ecosystems age. However, they observed that for lakes (9500 years) and reservoirs (3–37 years) located in Wisconsin (USA), the watershed land use was more important than the lake age for generating differences in zooplankton richness. In our study, the higher trophic state of reservoirs compared to lakes was not apparently associated with the extent of land use of their catchments as advocated by others (De Araujo et al. 2006; Chellappa et al. 2009). Rather, patterns of nutrient concentration in reservoirs mirrored the patterns of catchment area and catchment area/ecosystem area ratio of these ecosystems. Therefore, compared to lakes, this result may indicate that reservoirs are, on average, more eutrophic because they have larger catchments which contribute with greater entrance of allochthonous material per unit area of the reservoirs.
Regarding ecosystem age, we hypothesized that since lakes are older ecosystems, they would have greater gamma and alpha diversity than zooplankton (i.e., ecological time hypothesis—Pianka 1988). However, it is possible that the higher water residence time in reservoirs is a more significant time-related variable for the aquatic ecosystem dynamics studied than their age in affecting the zooplankton diversities. Aquatic communities of the reservoirs in northeast Brazil are reset with lower frequency than those of lakes giving the reservoirs high water residence times face the frequent droughts (They et al. 2017). Meanwhile in lakes, the more frequent inputs of water and allochthonous organic matter may contribute to shifting the communities to an “early point” in evolutionary time (Wilkinson et al. 2013; They et al. 2017).
Due to the high resource availability in reservoirs, we predicted that the average local zooplankton abundance and biomass would be higher in reservoirs than in lakes. Similar to many studies that investigated the importance of resource availability for population and community abundance of zooplankton (Canfield Jr and Watkins 1984; Corgosinho and Pinto-Coelho 2006; Havens and Beaver 2011), we also found support for our predictions. However, the higher local zooplankton abundance was not associated with the success of a few dominant species adapted to the high productivity of reservoirs. Consequently, no differences were observed in Simpson’s diversity and evenness when comparing reservoirs and lakes. We conducted our sampling procedure in the limnetic and littoral regions, with the very majority of ecosystems (≈ 83%) being colonized by aquatic macrophytes at the littoral zone. The environmental heterogeneity created by the presence of macrophytes is recognized as harboring higher zooplankton species richness in the littoral region than in the limnetic ones (Kuczyńska-Kippen 2005; Maia-Barbosa et al. 2008). The macrophytes structural framework also favors the appearance of different niches colonized by specific species (Masclaux et al. 2014; Rennie and Jackson 2005; Thomaz et al. 2008), allowing the specialist species to coexist (i.e., narrow niches) and reducing species dominance.
In conclusion, we observed that reservoirs play an essential role in subsidizing zooplankton diversity, despite their high productivity. Therefore, the likely increase in the number of reservoirs in the landscape is expected to be crucial for the maintenance of a significant portion of aquatic diversity. Moreover, the lakes also play a central role in zooplankton diversity because they contain distinct species compositions. About a quarter of the species found were exclusive of either lake or reservoir systems. Hence, both ecosystem types contribute to zooplankton species pool, suggesting that the complementarity in species composition among artificial and natural aquatic ecosystems is important for large-scale patterns of zooplankton biodiversity in the studied region. In addition, as zooplankton is a connector element in trophic webs linking primary producers and higher trophic levels (Soranno et al. 1993; Harris et al. 2000), it is possible that the results found for zooplankton diversity extend to other groups of aquatic organisms. Therefore, we recommend that conservation and management policies that intend to act in the preservation of freshwater biodiversity on a regional scale must consider a scenario that includes a combination of reservoirs and lakes in the landscape.
References
Albrecht C, Wilke T (2008) Ancient Lake Ohrid: biodiversity and evolution. Hydrobiologia 615(1):103
Allen MR (2007) Measuring and modeling dispersal of adult zooplankton. Oecologia 153(1):135–143
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26(1):32–46
Araújo JAA (1982) Dams in the Northeast of Brazil, 2nd edn. DNOCS—Brazilian National Department of Droughts, Fortaleza
Arthaud F, Vallod D, Robin J, Bornette G (2012) Eutrophication and drought disturbance shape functional diversity and life-history traits of aquatic plants in shallow lakes. Aquat Sci 74(3):471–481
Barbosa JEL, Medeiros ESF, Brasil J, Cordeiro RS, Crispim MCB, Silva GHG (2012) Aquatic systems in semi-arid Brazil: limnology and management. Acta Limnol Braz 24(1):103–118
Beisner BE, Peres-Neto PR, Lindström ES, Barnett A, Longhi ML (2006) The role of environmental and spatial processes in structuring lake communities from bacteria to fish. Ecology 87(12):2985–2991
Bohonak AJ, Jenkins DG (2003) Ecological and evolutionary significance of dispersal by freshwater invertebrates. Ecol Lett 6(8):783–796
Bottrell H, Duncan A, Gliwicz Z et al (1976) A review of some problems in zooplankton production studies. Nor J Zool 24(4):419–456
Bouvy M, Pagano M, Troussellier M (2001) Effects of a cyanobacterial bloom (Cylindrospermopsis raciborskii) on bacteria and zooplankton communities in Ingazeira reservoir (northeast Brazil). Aquat Microb Ecol 25(3):215–227
Bouvy M, Nascimento SM, Molica RJR, Ferreira A, Huszar V, Azevedo SMFO (2003) Limnological features in Tapacurá reservoir (northeast Brazil) during a severe drought. Hydrobiologia 493(1):115–130
Branstrator DK (2010) Origins of types of lake basins. In: Likens GE (ed) Lake ecosystem ecology: a global perspective. Academic Press, Oxford, pp 191–202
Brown J, Stevens G, Kaufman D (1996) The geographic range: size, shape, boundaries, and internal structure. Annu Rev Ecol Syst 27(1):597–623
Canfield DE Jr, Watkins CE (1984) Relationships between zooplankton abundance and chlorophyll a concentrations in Florida lakes. J Freshw Ecol 2(4):335–344
Chase J, Leibold M (2002) Spatial scale dictates the productivity–biodiversity relationship. Nature 416(6879):427
Chellappa NT, Costa MAM (2003) Dominant and co-existing species of Cyanobacteria from a Eutrophicated reservoir of Rio Grande do Norte State, Brazil. Acta Oecol 24:S3–S10
Chellappa NT, Câmara FRA, Rocha O (2009) Phytoplankton community: indicator of water quality in the Armando Ribeiro Gonçalves reservoir and Pataxó channel, Rio Grande do Norte, Brazil. Braz J Biol 69(2):241–251
Cooke GD, Welch EB, Peterson SA, Nichols SA (2016) Restoration and management of lakes and reservoirs, 3rd edn. CRC Press, Boca Raton
Corgosinho PHC, Pinto-Coelho RM (2006) Zooplankton biomass, abundance and allometric patterns along an eutrophic gradient at Furnas Reservoir (Minas Gerais, Brazil). Acta Limnol Bras 182:213–224
Costa IAS, Azevedo SMF, Senna PAC, Bernardo RR, Costa SM, Chellappa NT (2006) Occurrence of toxin-producing cyanobacteria blooms in a Brazilian semiarid reservoir. Braz J Biol 66(1B):211–219
Crossetti LO, de Bicudo D, de Bicudo CEM, Bini LM (2008) Phytoplankton biodiversity changes in a shallow tropical reservoir during the hypertrophication process. Braz J Biol 68(4):1061–1067
da Costa MRA, Attayde JL, Becker V (2016) Effects of water level reduction on the dynamics of phytoplankton functional groups in tropical semi-arid shallow lakes. Hydrobiologia 778(1):75–89
De Araujo JC, Güntner A, Bronstert A (2006) Loss of reservoir volume by sediment deposition and its impact on water availability in semiarid Brazil. Hydrol Sci J 51(1):157–170
Diniz MTM, Pereira VHC (2015) Climatologia do estado do Rio Grande do Norte, Brasil: Sistemas atmosféricos atuantes e mapeamento de tipos de clima. Bol Goiano Geogr 35(3):488–506
DNOCS (2015) Departamento Nacional de Obras Contra às Secas. História do DNOCS. http://www.dnocs.gov.br/. Accessed 1 Nov 2015
Dodson SI, Arnott SE, Cottingham KL (2000) The relationship in lake communities between primary productivity and species richness. Ecology 81(10):2662–2679
Dodson SI, Everhart WR, Jandl AK, Krauskopf SJ (2007) Effect of watershed land use and lake age on zooplankton species richness. Hydrobiologia 579(1):393–399
Doubek JP, Carey CC (2017) Catchment, morphometric, and water quality characteristics differ between reservoirs and naturally formed lakes on a latitudinal gradient in the conterminous United States. Inland Waters 7(2):171–180
Downing JA (2010) Emerging global role of small lakes and ponds: little things mean a lot. Limnetica 29(1):9–24
Elmoor-Loureiro L (1997) Manual de Identificação de Cladóceros Límnicos Do Brasil. Ed. Universitária, Distrito Federal
Eskinazi-Sant’Anna EM, Menezes R, Costa IM, Araújo M, Panosso R, Attayde JL (2013) Zooplankton assemblages in eutrophic reservoirs of the Brazilian semi-arid. Braz J Biol 73(1):37–52
ESRI (2011) Arc hydro tools - tutorial, version 2.0. http://downloads.esri.com/archydro/ArcHydro/Tutorial/Doc/Arc%20Hydro%20Tools%202.0%20-%20Tutorial.pdf. Visited on 1 Oct 2015
Esteves FA, Caliman A, Santangelo JM, Guariento RD, Farjalla VF, Bozelli RL (2008) Neotropical coastal lagoons: an appraisal of their biodiversity, functioning, threats and conservation management. Braz J Biol 68(4):967–981
Fang Y, Cheng W, Zhang Y et al (2016) Changes in inland lakes on the Tibetan Plateau over the past 40 years. J Geogr Sci 26(4):415–438
Fernández-Rosado MJ, Lucena J (2001) Space-time heterogeneities of the zooplankton distribution in La Concepción reservoir (Istán, Málaga; Spain). Hydrobiologia 455(1–3):157–170
Figuerola J, Green A (2002) Dispersal of aquatic organisms by waterbirds: a review of past research and priorities for future studies. Freshw Biol 47(3):483–494
Finan TJ, Nelson DR (2001) Making rain, making roads, making do: public and private adaptations to drought in Ceará, Northeast Brazil. Clim Res 9(2):97–108
Ghidini AR, Serafim-Júnior M, Perbiche-Neves G, Brito L (2009) Distribution of planktonic cladocerans (Crustacea: Branchiopoda) of a shallow eutrophic reservoir (Paraná State, Brazil). Panam J Aquat Sci 4(3):294–305
Gilbert JJ (1974) Dormancy in rotifers. Trans Am Microsc Soc 43(4):490–513
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4(4):379–391
Hall DJ, Cooper WE, Werner EE (1970) An experimental approach to the production dynamics and structure of freshwater animal communities. Limnol Oceanogr 15(6):839–928
Harris R, Wiebe P, Lenz J, Skjoldal HR, Huntley M (eds) (2000) ICES zooplankton methodology manual. Academic Press, London
Havel JE, Lee CE, Vander Zanden MJ (2005) Do reservoirs facilitate invasions into landscapes? Bioscience 55(6):518–525
Havens KE, Beaver JR (2011) Composition, size, and biomass of zooplankton in large productive Florida lakes. Hydrobiologia 668(1):49–60
INMET (2015) Instituto Nacional de Meteorologia. Banco de Dados Meteorológicos para Ensino e Pesquisa. http://www.inmet.gov.br/portal/%0Aindex.php?r=bdmep/bdmep. Accessed 1 July 2015
Irz P, Odion M, Argillier C, Pont D (2006) Comparison between the fish communities of lakes, reservoirs and rivers: can natural systems help define the ecological potential of reservoirs? Aquat Sci 68(1):109–116
Jespersen A, Christoffersen K (1987) Measurements of chlorophyll-a from phytoplankton using ethanol as extraction solvent. Arch für Hydrobiol 109:445–454
Koste W (1978) Rotatoria. Die Rädertiere Mitteleuropas Ein Bestimmungswerk, Begründet von Max Voigt Überordnung Monogononta. (Gebrüder Borntraeger, ed.). Stuttgart
Kuczyńska-Kippen N (2005) On body size and habitat selection in rotifers in a macrophye-dominated lake Budzyńskie, Poland. Aquat Ecol. 239(4):447–454
Lazzaro X, Bouvy M, Ribeiro-Filho RA et al (2003) Do fish regulate phytoplankton in shallow eutrophic Northeast Brazilian reservoirs? Freshw Biol 48(4):649–668
Lehner B, Döll P (2004) Development and validation of a global database of lakes, reservoirs and wetlands. J Hydrol 296(1):1–22
Lehner B, Liermann CR, Revenga C et al (2011) High-resolution mapping of the world’s reservoirs and dams for sustainable river-flow management. Front Ecol Environ 9(9):494–502
Leibold M (1999) Biodiversity and nutrient enrichment in pond plankton communities. Evol Ecol Res 1(1):73–95
Maia-Barbosa PM, Peixoto RS, Guimarães S (2008) Zooplankton in littoral waters of a tropical lake: a revisited biodiversity. Braz J Biol 68:1069–1078
Malveira VTC, de Araújo JC, Güntner A (2011) Hydrological impact of a high-density reservoir network in semiarid northeastern Brazil. J Hydrol Eng 17(1):109–117
Manatunge J, Nakayama M, Priyadarshana T (2008) Environmental and social impacts of reservoirs: issues and mitigation. Ocean Aquat Ecosyst 1:212–255
Manly BFJ (2006) Randomization, bootstrap and monte carlo methods in biology. Champman and Hall/CRC, Boca Raton, FL
Masclaux H, Bourdier G, Jouve L, Duffaud E, Bec A (2014) Temporal changes in essential fatty acid availability in different food sources in the littoral macrophyte zone. Hydrobiologia 736(1):127–137
McKindsey CW, Bourget E (2001) Diversity of a northern rocky intertidal community: the influence of body size and succession. Ecology 82(12):3462–3478
Menezes RF, Attayde JL, Lacerot G, Kosten S, Souza LC, Costa LS, Van Nes EH, Jeppesen E (2012) Lower biodiversity of native fish but only marginally altered plankton biomass in tropical lakes hosting introduced piscivorous Cichla cf. ocellaris. Biol Invasions 14(7):1353–1363
Merrix-Jones FL, Thackeray SJ, Ormerod SJ (2013) A global analysis of zooplankton in natural and artificial fresh waters. J Limnol 72(1):12
Molle F (1991) Caractéristiques et potentialités des “açudes” du nordeste brésilien. Thesis, USTL Montpelier
Moss BR (2009) Ecology of Fresh waters: man and medium, past to future. Blackwell Science, Oxford
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nilsson C (2009) Reservoirs. In: Likens GE (ed) Encyclopedia of inland waters. Elsevier, Academic Press, Oxford, pp 211–219
Nogueira MG, Oliveira PCR, Britto YT (2008) Zooplankton assemblages (Copepoda and Cladocera) in a cascade of reservoirs of a large tropical river (SE Brazil). Limnetica 27(1):151–170
Oksanen J, Kindt R, Legendre P et al (2015) Vegan: community ecology package
Pagioro TA, Thomaz SM, Roberto MC (2005) Caracterização limnológica abiótica dos reservatórios. In: Rodrigues L, Thomaz SM, Agostinho AA, Gomes LC (eds) Biocenose de reservatórios: padrões espaciais e temporais. Rima, São Carlos, pp 17–38
Paradis E (2012) Analysis of phylogenetics and evolution with R. Springer, New York
Parra G, Matias NG, Guerrero F, Boavida MJ (2009) Short term fluctuations of zooplankton abundance during autumn circulation in two reservoirs with contrasting trophic state. Limnetica 28:175–184
Pauli HR (1989) A new method to estimate individual dry weights of rotifers. Hydrobiologia 186(1):355–361
Pekel J-F, Cottam A, Gorelick N, Belward AS (2016) High-resolution mapping of global surface water and its long-term changes. Nature 540(7633):418
Pianka E (1988) Evolutionary ecology, 4th edn. Harper & Rows, New York
Pinto-Coelho R, Pinel-Alloul B, Méthot G, Havens KE (2005) Crustacean zooplankton in lakes and reservoirs of temperate and tropical regions: variation with trophic status. Can J Fish Aquat Sci 62(2):348–361
R Core Team (2015) R: a language and environment for statistical computing. http://www.r-project.org/. Accessed 1 Jul 2015
Rennie MD, Jackson LJ (2005) The influence of habitat complexity on littoral invertebrate distributions: patterns differ in shallow prairie lakes with and without fish. Can J Fish Aquat Sci 69(2):2088–2099
Ricci C (2001) Dormancy patterns in rotifers. Hydrobiologia 446(1):1–11
Rosenberg DM, McCully P, Pringle CM (2000) Global-scale environmental effects of hydrological alterations: introduction. Bioscience 50(9):746–751
Sampaio EV, Rocha O, Matsumura-Tundisi T, Tundisi JG (2002) Composition and abundance of zooplankton in the limnetic zone of seven reservoirs of the Paranapanema River, Brazil. Braz J Biol 62(3):525–545
Santos-Silva E (2000) Revisão das espécies do “complexo nordestinus”(Wright, 1935) de Notodiaptomus Kiefer, 1936 (Copepoda: Calanoida: Diaptomidae). Thesis, Universidade de São Paulo
Sendacz S, Caleffi S, Santos-Soares J (2006) Zooplankton biomass of reservoirs in different trophic conditions in the state of São Paulo, Brazil. Brazil J Biol 66(1B):337–350
Shurin JB (2000) Dispersal limitation, invasion resistance, and the structure of pond zooplankton communities. Ecology 81(11):3074–3086
Simões NR, Nunes AH, Dias JD, Lansac-Tôha FA, Velho LFM, Bonecker CC (2015) Impact of reservoirs on zooplankton diversity and implications for the conservation of natural aquatic environments. Hydrobiologia 758(1):3–17
Soranno P, Carpenter S, He X (1993) Zooplankton biomass and body size. In: Carpenter S, Kitchell J (eds) The trophic cascade in lakes. Cambridge University Press, Cambridge, pp 116–152
Sousa W, Attayde JL, Rocha EDS, Eskinazi-Sant’Anna EM (2008) The response of zooplankton assemblages to variations in the water quality of four man-made lakes in semi-arid northeastern Brazil. J Plankton Res 30(6):699–708
SUDENE (2010) Superintendência do Desenvolvimento do Nordeste. MAPAS. http://www.sudene.gov.br/. Accessed 1 Oct 2011
Suthers IM, Rissik D (eds) (2009) Plankton: a guide to their ecology and monitoring for water quality. Csiro Publishing, Colinwood
They NH, Amado AM, Cotner JB (2017) Redfield ratios in inland waters: higher biological control of C: N: P ratios in tropical semi-arid high water residence time lakes. Front Microbiol 8:1505
Thomaz SM, Dibble ED, Evangelista LR, Higuti J, Bini LM (2008) Influence of aquatic macrophyte habitat complexity on invertebrate abundance and richness in tropical lagoons. Freshw Biol 53(2):358–367
Timms B (2009) Geomorphology of lake basins. In: Likens GE (ed) Encyclopedia of inland waters. Elsevier, Academic Press, Oxford, pp 203–210
Tranvik LJ, Downing JA, Cotner JB et al (2009) Lakes and reservoirs as regulators of carbon cycling and climate. Limnol Oceanogr 54(6 part 2):2298–2314
Tundisi JG, Matsumura-Tundisi T (2003) Integration of research and management in optimizing multiple uses of reservoirs: the experience in South America and Brazilian case studies. Aquat Biodivers 171:231–242
Wellborn GA, Skelly DK, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annu Rev Ecol Syst 27(1):337–363
Wetzel RG, Likens GE (1991) Limnological analysis, 2nd edn. Springer, New York
Wilkinson GM, Pace ML, Cole JJ (2013) Terrestrial dominance of organic matter in north temperate lakes. Global Biogeochem Cycles 27(1):43–51
Wojciechowski J, Heino J, Bini LM, Padial AA (2017) Temporal variation in phytoplankton beta diversity patterns and metacommunity structures across subtropical reservoirs. Freshw Biol 62(4):751–766
Acknowledgements
This study was supported by grants provided by the Brazilian National Council for Scientific and Technological Development (CNPq—www.cnpq.br) through the Universal Grant (Process 477637/2011-6) to LSC. CRC is thankful to Coordination for the Improvement of Higher Education (CAPES—www.capes.gov.br) for the concession of a PhD scholarship. AC and AMA gratefully acknowledge continuous funding through Research Productivity Grants provided by CNPq (Processes 304621/2015-3 and 310033/2017-9). AMA is thankful for the support from the National Council for Scientific and Technological Development—CNPq through the Universal Grant (Proc. # 475537/2012-2). We are thankful to all staff members at the Limnology Laboratory at UFRN for helping us with fieldwork and laboratory analysis. We are also indebted to Gustavo Fonseca for reviewing an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Télesphore Sime-Ngando.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10452_2019_9693_MOESM1_ESM.docx
Supplementary material includes two additional tables showing (i) data for landscape properties and land-use cover for the sampled lakes and reservoirs and (ii) the zooplankton species list, species mean abundance and the proportion of exclusive species from natural lakes and reservoirs. (DOCX 32 kb)
Rights and permissions
About this article
Cite this article
Cabral, C.R., Guariento, R.D., Ferreira, F.C. et al. Are the patterns of zooplankton community structure different between lakes and reservoirs? A local and regional assessment across tropical ecosystems. Aquat Ecol 53, 335–346 (2019). https://doi.org/10.1007/s10452-019-09693-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-019-09693-5