Abstract
Sparse monitoring of New Brunswick (Canada) lakes creates challenges for understanding mechanisms of deteriorating water quality, such as recent instances of increased cyanobacterial biomass in low-nutrient systems. To assess long-term environmental change experienced by low-nutrient, dimictic New Brunswick lakes we use sedimentary remains of algal pigments and Cladocera in dated cores from impact (prone to late-summer algal blooms, Lac Unique) and reference (no observed algal blooms, First and States) lakes. Overall, all three lakes now exhibit greater bosminid abundance and fewer daphniids, lower Cladocera richness, and smaller average cladoceran body size, although some lake-specific differences in assemblage response exist that cannot be related solely to top-down or bottom-up forces. Zooplankton trends are most pronounced at Lac Unique and First Lake, where algal production is generally greater. Across all three lakes, the only significant (IndVal: p < 0.05) cladoceran bioindicator of the post-1990 period is the pelagic bosminid group, whereas prior to ~ 1990, Daphnia longispina-complex and several littoral taxa were significant (IndVal: p < 0.05) bioindicators. These temporal shifts suggest that the smaller-bodied bosminid group may now be favored over its larger-bodied pelagic competitor Daphnia sp. in these lakes, irrespective of heterogeneous long-term algal patterns inferred from stable sedimentary pigments. We suggest that the indirect effects of climate change, principally during the spring and summer quarters in New Brunswick, may be associated with marked shifts in limnological structure, as evidenced by changes in dominant zooplankton of the pelagic zone. However, further research is needed to rule out whether or not size-selective predation, and its potential interactions with climate change and other stressors, are key mechanisms favoring increased bosminids in low-nutrient, dimictic lakes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ecological integrity of freshwaters is of concern globally. Deteriorating water quality has ecological and societal implications (Dodds et al. 2013). Managing aquatic ecosystems requires effective monitoring to understand the mechanisms driving change in a given system. In the maritime province of New Brunswick, the Department of Environment and Local Government estimates that surface waters support ~ 40% of the population’s municipal water needs. However, some NB lakes are exhibiting obvious signals of environmental change, as indicated by increased frequency of late-summer algal blooms. Cyanobacterial dominance has long been associated with nutrient loading and is often a consequence of cultural eutrophication. The causal mechanism is complex and may be the result of several factors including nutrient availability and cycling, hydrology, and alterations to lake food webs (Cottingham et al. 2015; Pick 2016). Other important drivers of cyanobacterial dominance include indirect climate change effects, such as higher water temperatures, a stronger thermal stratification, and a deepening of the mixed layer (Reynolds 2006; Wagner and Adrian 2009; Paerl et al. 2011; Pick 2016). Progressively shorter ice-cover periods in the region (Patterson and Swindles 2014; Lavery et al. 2014) and pelagic diatom shifts consistent with other paleolimnological inferences of warming trends across the Northern Hemisphere (Harris et al. 2006) suggest that regional climate warming is already influencing lake environments. A regional scale stressor such as climate warming would affect limnological variables across multiple systems. Therefore, increased instances of late-summer algal blooms in New Brunswick may be symptomatic of regional limnological change coincident with recent climate warming or other environmental changes related to human activities.
Threats to aquatic ecosystems, as evidenced by recent cyanobacterial blooms, necessitate investigation into the mechanisms influencing these systems. Of New Brunswick’s ~ 2500 lakes, extremely few are monitored regularly to assess water quality trends. The lack of systematic monitoring therefore calls for an indirect approach to assess environmental changes. Natural archives such as lake sediments have been instrumental in advancing knowledge of ecosystem changes over the past few centuries when monitoring data are scarce (Smol 2010). Biological indicators preserved in lake sediments are a powerful, integrative tool often used to assess regional shifts in community structure and document past lake conditions (Smol 2010; Taranu et al. 2015).
Cladoceran subfossils preserved in lake sediments are now widely-used bioindicators. Cladocera represent an important intermediate trophic level in lakes due to their response to key ecological drivers, including, bottom-up and top-down factors. Size-selective predation by planktivorous fish and macroinvertebrates on pelagic cladocerans is often recognized as important to structuring cladoceran assemblages (Davidson et al. 2010; Korosi et al. 2013a; Labaj et al. 2013). Additionally, habitat structure (Berström et al. 2000; Adamczuk 2015), water chemistry (Kurek et al. 2011; Labaj et al. 2015), ice cover and growing season length (Luoto and Nevalainen 2012; Nevalainen et al. 2014) are recognized as key drivers of Cladocera. Pelagic taxa such as bosminids and Daphnia sp. have been shown to be particularly sensitive to changes in lake thermal regimes driven by climate. For example, Bosmina sp. have replaced daphniids as the dominant pelagic cladoceran in some alpine lakes as the result of deeper epilimnetic mixing associated with increased air temperatures (Luoto and Nevalainen 2012; Nevalainen et al. 2014). Studies from a variety of lakes report cladoceran assemblage shifts associated with climate changes, including fewer Daphnia sp. and the increase of small-bodied pelagic grazers (Jiang et al. 2014; Nevalainen et al. 2014; Adamczuk 2015; Armstrong and Kurek 2018).
We use dated lake sediment cores and paleolimnological analyses of primary producers (algal pigments) and primary consumers (Cladocera) to assess long-term environmental changes in lakes from northwestern New Brunswick, Canada. We focus on the early 1900s to present, as this period captures a reasonable baseline for comparison with contemporary lake conditions. One of our sites is prone to late-summer algal blooms and has considerable land-use modifications in the watershed (in terms of residential and agricultural areas), whereas the other two lakes are in remote locations and thus serve as reference ecosystems. Recent studies from lakes around the world are showing that planktonic assemblages are often sensitive to changes in lake thermal stratification (Thompson et al. 2005; Harris et al. 2006; Adrian et al. 2009; Luoto and Nevalainen 2012; Nevalainen et al. 2014). Some studies suggest that these physical changes of the pelagic zone may elicit shifts towards smaller body sizes as a response to warming (Daufresne et al. 2009; Yvon-Durocher et al. 2011; Sheridan and Bickford 2011; Armstrong and Kurek 2018). This study enhances our understanding of the stressors affecting lake ecosystems, and provides insight into low-nutrient lake responses in remote regions of the maritime provinces of Canada. Additionally, it provides a much-needed long-term ecological context to ongoing monitoring efforts and potential restoration goals in New Brunswick lakes. Overall, the goals of this study are: (1) to determine whether low-nutrient New Brunswick lakes have changed significantly from baseline conditions, therefore signalling increased environmental stress and (2) to identify the main driver(s) of shifting ecological conditions.
Study Lakes
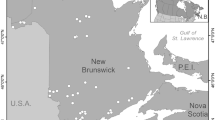
Lakes in northwestern New Brunswick, Canada, within ~ 100 km of one another, were selected for paleolimnological sampling (Fig. 1, Table 1). One “impact” lake experiencing late-summer cyanobacterial blooms since at least the late-2000s and two “reference” lakes with no observed blooms were studied. Reference sites were also selected because of their classification as potential long-term monitoring sites within an enhanced lake-monitoring program the province of NB is considering (Curry 2013, 2014). Surficial geology of the study region is typically composed of calcareous and non-calcareous metasedimentary rocks. Topography consists of rolling hills between ~ 150 and 300 m in elevation. The regional landscape is predominately forest cover by conifers and marked by moderate-sized rivers, including tributaries of the Saint John and Restigouche rivers. Forestry operations are important to the economy and logging is the dominant disturbance of the Canadian maritime landscape since at least the mid-1800s (Korosi et al. 2013b). The closest climate station to our most distant study site (~ 80 km) is in Edmunston (ID: 810AL00). Climate normals between 1980 and 2010 show that average annual temperature is 3.6 °C, average summer temperature is 16.8 °C, average winter temperature is − 9.7 °C, and annual precipitation is 1010 mm (http://climate.weather.gc.ca/).
Lac Unique served as the impact site (Table 1). Late-summer algal blooms were formally reported to the province of New Brunswick in July 2012 and 2015, but anecdotally observed earlier by residents (Maaref 2014). The catchment was permanently settled in ~ 1930 and Lac Unique is now surrounded by 116 full-time homes or seasonal cottages, which comprise 60% of the lake’s catchment. Forestry operations and some small-scale agriculture comprise the remaining catchment area (Maaref 2014). Lac Unique is a 121 ha dimictic system with a maximum water depth of ~ 6.4 m. Lac Unique is slightly alkaline and shows high epilimnetic chl-a of 16 µg L−1, and low total phosphorus (TP) (Table 1). Lac Unique has a calcium concentration of 9.7 mg L−1. Recent monitoring performed by New Brunswick’s Department of Environment and Local Government notes a slight increase in TP and decrease in dissolved oxygen (Maaref 2014). Brook Trout is the only recognized sportfish at Lac Unique.
First Lake and States Lake served as reference sites and are within remote watersheds compared to Lac Unique (Table 1). These lakes have completely forested catchments with no permanent dwellings or industrial activities, except for limited forestry operations. Both sites are accessible from unpaved roads with restricted access. First Lake is 500 ha and dimictic, with a maximum depth of ~ 20 m. States Lake is a 75 ha headwater lake with a maximum depth of ~ 28 m. There are no reports of late-summer algal blooms in either reference lake (Curry 2014). Both lakes are slightly alkaline, oligotrophic, with low epilimnetic chl-a (3 and 1 µg L−1, respectively) and low TP (Table 1). First and States lakes also show high calcium concentrations (24 and 11 mg L−1, respectively). Water colour of the reference lakes are clearer than Lac Unique. Compared to Lac Unique, the two reference lakes have more complex sportfish communities typical of deeper, cold-water fisheries. States Lake supports populations of Brook Trout and Lake Trout, whereas First Lake supports populations of Brook Trout, Landlocked Salmon, Yellow Perch, and Lake Whitefish. To our knowledge, no detailed, systematic assessments of fish communities or stocking efforts across recent decades exist at the study lakes.
Materials and methods
Sediment collection and dating
Sediment cores were retrieved from the central, deep basin of each lake during June 19–22, 2015, using a gravity corer (Glew 1991). Coring location was established with bathymetric maps and sonar depth soundings. Cores were immediately sectioned on shore in the shade at 0.5-cm intervals using a vertical extruder (Glew 1988). Sediments were then placed in labelled Whirl-Pak® bags, stored with ice packs in a cooler, and transported to a ~ 4 °C refrigerator in the Environmental Change and Aquatic Biomonitoring (ECAB) laboratory at Mount Allison University.
Gamma spectroscopy was used to estimate sediment age following standard methods (Schelske et al. 1994). 210Pb and 137Cs were measured from 15 (Lac Unique and First Lake) and 13 (States Lake) sediment intervals at the University of Ottawa (Fig. 2). Radiometric dates were estimated from natural fallout of 210Pb radionuclides and the constant rate of supply (CRS) model, which were corroborated with artificial fallout of 137Cs radionuclides (Appleby 2001). A second-order polynomial based on the established dates from each core sequence was then used to model ages as a function of sediment midpoint depth.
Sedimentary pigment processing and analysis
Sedimentary pigments were extracted, analyzed, and expressed following standard paleolimnological techniques. Twenty samples from Lac Unique, 21 samples from First Lake, and 18 samples from States Lake were processed. Sediment subsamples from selected intervals were placed into cryogenic vials and frozen until analyzed. Sample processing then took place in the dark or under green light whereby each subsample was first freeze-dried and then suspended in acetone for 24 h at − 20 °C to extract pigments (Zapata et al. 2000). Extracts were then centrifuged in a refrigerated unit and the supernatant was decanted, filtered (0.2 µm) and placed in vials for analysis using a Waters High Performance Liquid Chromatography system (HPLC; Mississauga, Ontario, Canada) fitted with a photodiode array (PDA) detector (model 2996). Sedimentary pigments were then separated using a reverse phase C18 column following methods adapted from Zapata et al. (2000). Isolated sedimentary pigments were identified and quantified from the chromatograms produced by matching the peak spectrum, retention time, and area to authentic standards (purchased from DHI Group, Water and Environment, Hørsholm, Denmark). Chromatograms of zeaxanthin were inseparable from lutein using HPLC and thus we reported zeaxanthin + lutein as a combined pigment (Patoine and Leavitt 2006). Sedimentary pigment concentrations were expressed as pigment mass normalized to sample organic matter (ng µg−1 OM). Only “stable” sedimentary pigments were considered for further examination and analysis, as chlorophyll a is highly susceptible to diagenesis (Leavitt and Hodgson 2001; Patoine and Leavitt 2006). Mann–Kendall trend tests were then used to recognize trends in the stable sedimentary pigments at each lake. This nonparametric, rank correlation test was used to determine significant (p < 0.05) monotonic patterns in pigment indicators within sediment records from impact and reference sites. Positive summed rank scores suggest an increasing pigment trend (i.e. more pairs in increasing order compared to pairs of observations in decreasing order) and negative summed rank scores support a decreasing pigment trend. This analysis was completed using the “Kendall” package in R version 3.2.3 (R Core Team 2015).
Cladoceran processing and analysis
Cladoceran remains were processed using standard methods (Korhola and Rautio 2001). Twenty-two samples from both Lac Unique and First Lake, and 21 samples from States Lake were examined. This includes every other 0.5-cm interval from 0 to 30 cm at Lac Unique and First Lake, and every other 0.5-cm interval from 0 to 22 cm at States Lake. Most of the cladoceran samples were adjacent to a sedimentary pigment sample and thus not from the same sediment interval. Wet sediments (1–2 g) were deflocculated in 10% KOH at ~ 70 °C for 30 min then rinsed over a 38-μm mesh sieve with deionized water. Retained sediments were then transferred to a glass vial with deionized water and a few drops of ethanol. Between one and three 50-μl aliquots of the mixed sample were applied to each coverslip, evaporated at room temperature, then permanently mounted on slides using Entellan®. Cladoceran remains were examined with bright-field illumination at 200× or 400× magnification. Taxonomy followed standard subfossil guides for eastern North America (Korosi and Smol 2012a, b). Entire coverslips were scanned and counting effort for all intervals at each lake exceeded minimum count guidelines of 70 individuals (Kurek et al. 2010). All identifiable cladoceran remains, such as carapaces, headshields, postabdomens, postabdominal claws and exopodite segments, were counted individually. The most abundant remain of each taxon was used to calculate the total number of individuals present in each sediment interval.
Daphniids were grouped as complexes based on the presence of stout spines on the middle pectin of the postabdominal claws: the Daphnia longispina-complex (potentially comprised of D. ambigua, D. mendotae, D. dentifera, D. dubia, and D. longiremis) and the Daphnia pulex-complex (potentially comprised of D. pulex, D. pulicaria, D. catawba and D. minehaha). Our bosminid group refers to the sum of all Bosmina sp. and Eubosmina sp., as the pore location on the headshield to separate genera was not always discernible, and often fragmented carapaces were the most abundant bosminid remains.
As sampling efforts of bioindicators often differ among sediment intervals, rare taxa may be more or less likely to be observed from high or low count intervals, respectively. Because of this inherent feature of count data, the total number of cladoceran remains counted for each sediment interval from each lake were rarefied based on the minimum total count from all intervals (Lac Unique = 75, First Lake = 76, States Lake = 70) using the “vegan” package (Oksanen et al. 2013). Rarefaction estimates the expected number of taxa in a standardized sampling count. Rarefied counts were then used only to estimate species richness trends at each lake. Prior to undertaking additional analyses, taxa that occurred in three or less intervals in a given core were deemed rare and excluded from further analyses. To facilitate the description of cladoceran assemblages, sediment intervals were then divided into biostratigraphic zones. Zones were identified by cluster analysis using constrained incremental sum of squares (CONISS) (Grimm 1987) with the number of zones determined with the broken-stick model (Bennett 1996) via the “rioja” package (Juggins 2009). Indicator value analyses (IndVal) were then used to recognize taxa that characterized each zone (Dufrene and Legendre 1997). Relative frequency and abundance data were used to determine significant (p < 0.05) indicator taxa of each zone using the “labdsv” package (Roberts 2007). The indicator metric is at a maximum (IndVal = 1) when all individuals of a taxon are observed in all samples of only one a priori defined zone. All numerical analyses were completed in R version 3.2.3 (R Core Team 2015).
To test the findings from meta-analyses that demonstrate decreased body size of aquatic invertebrates with increased temperatures associated with climate warming (Daufresne et al. 2009; Yvon-Durocher et al. 2011; Sheridan and Bickford 2011), we estimated average cladoceran body size for each interval. Specifically, estimates were made by using the average body sizes of cladoceran taxa from the Great Lakes Environmental Research Laboratory (GLERL) database (Sturtevant 2006) and the Norwegian Institute for Nature Research (NINA) Field Guide—Sizes database (Walseng and Halvorsen 2016). The number of a given cladoceran taxon in each interval was multiplied by the average body size as recorded by GLERL or NINA for all taxa in said interval. The products were summed together and divided by the total number of individuals counted in the interval to give an average body size of the assemblage at each sediment interval. A potential limitation with this approach may be differing average sizes of cladoceran taxa in Atlantic Canada compared to the database regions. In addition, our approach does not recognize that the size structure of an individual taxon may shift with time as lake conditions change. Thus, our average body size estimate is strongly influenced by the overall composition of the dominant taxa in an assemblage.
Results
Sediment ages based on CRS modelling
Lac Unique did not exhibit a clear decay of 210Pb between 0 and ~ 6.5 cm (Fig. 2). There was also no well-defined 137Cs peak that could be associated with the 1963 atmospheric maximum (Appleby 2001). The lack of a clear decay trend in 210Pb in the upper sediments could indicate sediment mixing or an increase in autochthonous productivity diluting the 210Pb activity. The 210Pb-based chronology indicated that Lac Unique sediments between 0 and 22.5 cm represent a time period of 2014 to ~ 1935 ± 4 years (Fig. 2). First Lake exhibited a well-defined exponential 210Pb decay pattern between 0 and ~ 13 cm. A 137Cs peak at ~ 15 cm is observed and corresponds to ~ 1950 according to the CRS model applied (Fig. 2). 210Pb chronology at First Lake indicated that the intervals between 0 and 18.5 cm represent a time period of 2014 to ~ 1925 ± 16 years. States Lake exhibited a typical pattern of exponential 210Pb decay between 0 and ~ 16.5 cm. 137Cs decay was variable and no peak exists. 210Pb chronology at States Lake indicated that the intervals between 0 and 12.5 cm represent a time period of 2013 to ~ 1925 ± 3 years (Fig. 2). All dates derived from intervals below the unsupported 210Pb activity should be viewed with caution as they are extrapolated with second-order polynomials and thus remain unanchored.
Sedimentary pigments
Stable sedimentary pigments indicated steady, low-nutrient conditions at both impact and reference lakes prior to environmental changes experienced during the 20th century (Fig. 3). Collectively, pigments indicated that all sites have experienced significant (p < 0.05) shifts in several algal groups after the mid-20th century (Fig. 4), although Lac Unique has generally exhibited greater algal shifts, especially with respect to cyanobacteria abundance, than the reference lakes. At Lac Unique, pigments began to shift following the ~ 1960s, although diatoxanthin showed a consistent decrease throughout the record. Echinenone, a proxy of cyanobacteria, increased at ~ 1960, whereas zeaxanthin + lutein (combined) decreased at ~ 1960 to present. During the 1980s–1990s, the stable pigments pheophytin a and ß-carotene, proxies for overall primary production, increased significantly (Figs. 3, 4). Of the reference lakes, First Lake showed a pigment signal that differed from that of States Lake (Figs. 3, 4). First Lake showed greater pigment increases than States Lake in recent decades. For example, echinenone was not detectable in States Lake, whereas in First Lake, this pigment increased slightly although to a lesser degree than Lac Unique (Fig. 4). States Lake also showed no change in the pigments ß-carotene and pheophytin a, whereas at First Lake both pigments have increased significantly. At States Lake, diatoxanthin decreased during ~ 1900 to present, whereas at First Lake it shows greater variability. Both reference lakes showed no significant increasing trend in zeaxanthin + lutein (combined).
Fossil pigment summary diagram of Mann–Kendall trend coefficients from lake sediment cores. Primary x-axis shows stable sedimentary pigments measured and secondary x-axis identifies their likely plant/algal contributors (Leavitt and Hodgson 2001). Horizontal grey lines denote significance (p < 0.05) in either a monotonic increase or decrease throughout the sediment record. At States Lake, echinenone was below detection (BD) throughout the core. (Color figure online)
Cladoceran assemblages from the impact site: Lac Unique
Overall, the bosminid group increased in relative abundance by three-fold during ~ 1900 to modern times in the Lac Unique record (Fig. 5a). Consequently, D. longispina-type and multiple littoral taxa showed decreased abundance and occurrence with the notable bosminid rise after the mid-20th century (Fig. 5a, Table 2). The primary zone delineation occurred at ~ 1950. IndVal scores indicated that prior to ~ 1950, 14 littoral species were indicative of zone 1 (Table 2). After ~ 1950, there was a pronounced decrease in the D. longispina-complex and the richness of littoral taxa declined substantially from ~ 17 taxa observed in zone 1 to ~ 6 taxa in zone 2 (Fig. 6). Additionally, average cladoceran body size decreased from stable baseline values of ~ 0.7 mm in zone 1 to ~ 0.5 mm in zone 2 (Fig. 6). A secondary zone designation at ~ 1992 showed further distinction of the modern cladoceran assemblage from that of zones 1 and 2. After ~ 1992, zone 3 showed that the bosminid group, the only significant indicator of this zone (Table 2), increased to its highest values of the 120-year record. Decreased relative abundances of daphniids, lower average body sizes, and the lowest richness values of the record were also apparent in zone 3 (Figs. 5a, 6).
Average body size and rarefied species richness of cladoceran assemblages from lake sediment cores. Red horizontal lines denote zone delineations based on cladoceran assemblage data (as shown in Fig. 5). Historical observations of air temperatures (white squares) from Edmundston climate station (ID: 810AL00) averaged across decades beginning in the 1920s. Mann–Kendall tests determined if significant monotonic trends in the quarterly climate observations exist. Only quarters (spring and summer) with significant trends (each τ = 0.60, p = 0.02) are presented. (Color figure online)
Cladoceran assemblages from the reference sites: First and States Lakes
Overall, the assemblage at First Lake showed dominance of the bosminid group and D. longispina-complex, which tend to be inversely correlated in their relative abundances (Fig. 5b). The bosminid group decreased gradually in relative abundance from ~ 1830 until ~ 1930, then increased from ~ 35 to ~ 90%, achieving its highest relative abundance over the ~ 140 year record. Consequently, D. longispina-complex exhibited a gradual increase in relative abundance reaching a record high of ~ 50% in ~ 1930, then decreased to a record low of < 5% in ~ 2014. Zone delineations were recognized at ~ 1890 and a primary zone at ~ 1990 (Fig. 5b). The IndVal metric identified Acroperus harpae and Chydorus brevilabris, species existing at low relative abundances, as significant indicators of zone 1 (Table 2). Zone 2 was marked by shifts in relative abundances of the bosminid group and D. longispina-complex. Bosminids decreased from stable relative abundances of ~ 70% in zone 1 to a record low of 35% in ~ 1930, then continued to increase to ~ 65% towards the end of zone 2. Daphnia longispina-complex, the only indicator taxon of zone 2 (Table 2) increased from stable relative abundances of ~ 15% in zone 1 to a record high of ~ 50% in ~ 1930, then continued to decrease to ~ 30% towards the end of zone 2. The bosminid group, the most abundant taxon in the core, is the only indicator taxon of zone 3 (Table 2). Species richness declined from stable values of ~ 8 species early in the record and reached < 5 species in zone 3 (Fig. 6). Average cladoceran assemblage body size reached a peak of ~ 1.4 mm in ~ 1930, coinciding with the highest relative abundance of D. longispina-complex, and then continued to decrease toward modern times, reaching a record low of ~ 0.5 mm in zone 3 (Fig. 6).
From the 1800s to modern times, States Lake exhibited minimal loss of species richness, increased dominance of bosminids, and decreased D. longispina-complex (Figs. 5c, 6). Only one zone delineation was identified and occurred at ~ 1998 (Fig. 5c). In zone 1, the bosminid group showed relative abundances of ~ 40% with a slight increasing trend. The IndVal-designated indicator D. longispina-complex (Table 2) showed relative abundances of 25% with a decreasing trend towards modern times. The most abundant littoral taxon, Alonella nana, also exhibited a decreasing trend following the mid-20th century (Fig. 5c). After ~ 1998, the bosminid group, the only indicator taxon of zone 2 (Table 2), increased and reached a record high of ~ 70% in ~ 2013. Daphnia longispina-complex exhibited a decreasing trend after ~ 1998 and reached a record low of ~ 6% in ~ 2013 (Fig. 5c). Average cladoceran assemblage body size decreased from ~ 0.8 mm in zone 1 to ~ 0.6 mm in zone 2 (Fig. 6).
Discussion
Sedimentary algal pigments and zooplankton bioindicators at both impact and reference systems in northwestern New Brunswick have shifted, in some respects markedly, from baseline conditions of the early-20th century. Similar to Harris et al. (2006), these paleolimnological trends are not consistent with acidification or lake recovery from this stressor. Lac Unique (impact site) and one reference site (First Lake) demonstrated significant increases in stable algal pigments, such as pheophytin a and echinenone, which are considered as proxies for chl-a and cyanobacterial abundance, respectively (Leavitt and Hodgson 2001; Patoine and Leavitt 2006). Additionally, all three lakes now exhibit generally greater bosminid abundance and fewer daphniids, lower Cladocera richness, and reduced average cladoceran body size relative to the mid-20th century. Based on our multi-proxy measures at the primary producer and consumer levels it is clear that cladoceran assemblage shifts have occurred in a consistent manner regardless of whether or not a lake has experienced recent late-summer algal blooms.
The overall timing and magnitude of the shifts at the producer and consumer levels differs among study lakes. Lac Unique and First Lake showed the earliest directional changes starting in the mid-20th century, followed by States Lake beginning at ~ 2000. States Lake may be less responsive to some environmental changes given its smaller surface area to depth ratio (Magnuson et al. 1990). Sensitivity to stressors and the degree of impact mediated through lake and catchment processes likely drives this offset among sites (Magnuson et al. 1990; Adrian et al. 2009; Labaj et al. 2015). For example, while ~ 1990 marks the primary zone delineation of cladocerans at First Lake, this reference site clearly experienced notable shifts in its pelagic zooplankton beginning at ~ 1900, whereas sedimentary algal pigments do not change at this time. While the main driver(s) of this early Cladocera shift at remote First Lake are unknown, high abundances of Daphnia sp. at ~ 30–40% lasted only a few decades as bosminids increased markedly beginning in ~ 1950. States Lake also shows some minor change and/or variability in cladoceran compositions prior to the primary zone delineation at ~ 2000. We suggest that these earlier periods at States Lake reflect relative stability in both producers and consumers prior to recent decades when zooplankton change is more substantial. Nonetheless, at both impact and reference lakes the overall trajectory of bioindicator shifts at the consumer level, especially in recent decades, shows surprising consistency.
At Lac Unique, the mid-20th century marks an important shift in the ecological structure of the system as evidenced by multiple trophic levels. This period signifies a clear shift from baseline conditions and the timing reflects the lake’s altered ecological trajectory over the last half century. Land-use alterations in the Lac Unique catchment beginning in ~ 1930 (Maaref 2014) likely contributed to the deterioration of littoral habitat (loss of Cladocera richness) and facilitated enhanced primary production in the pelagic zone (greater concentrations of pheophytin a, echinenone) likely through enhanced inputs of nutrients from the catchment. In turn, recently observed cyanobacterial blooms at Lac Unique may have been stimulated by a synergy between enhanced nutrient inputs and other recent environmental changes, namely the indirect effects of climate warming (Paerl et al. 2011; Taranu et al. 2015).
Stressors now affecting low-nutrient lakes in northwestern New Brunswick are likely to be exacerbated by further climatic changes, especially at lakes experiencing late-summer cyanobacterial blooms (Paerl and Huisman 2008; Jiang et al. 2014; Taranu et al. 2015) or those showing alterations in spring algal blooms (Patoine and Leavitt 2006). However, the overall response of regional lakes to shifting environmental conditions will be determined through processes regulated by factors such as nearby land use, basin morphometry, limnological conditions, and their complex interactions. Two of the three study sites diverged in a surprisingly consistent manner from baseline conditions of the early 1900s when the large-bodied grazer Daphnia sp. were more abundant. Similar shifts across sites suggest that these relatively low-nutrient systems are responding to a regional stressor, whether or not cyanobacterial blooms have occurred recently (Jiang et al. 2014). As such, climate change is a potential driver for the observed community changes.
Growing season air temperatures averaged across decades in both the spring and summer quarters have increased significantly (p = 0.02) since the 1920s in northwestern New Brunswick, whereas winter and fall show no monotonic trend (Fig. 6). In nearby Gaspé, Quebec, annual air temperatures have increased by ~ 3.4 °C from ~ 1890 to 2013 and earlier river ice-off dates of ~ 9 days were observed during the 20th century (Lavery et al. 2014). Patterson and Swindles (2014) also linked earlier lake ice-off dates by ~ 15 days to recent climatic changes experienced in northeastern North America, including New Brunswick and the adjacent state of Maine, USA. Likewise, Woolway et al. (2017) reported that mid-latitude lakes in central Europe are most susceptible to climatic warming during spring. Collectively, these all suggest that a lengthened open-water period and warmer spring and summer seasons associated with recent climate changes are likely now playing a greater role in driving producer and consumer dynamics in regional lakes. Although climate change is considered a regional stressor, lakes are expected to show responses that are dependent on lake-specific factors such as morphometry, water chemistry, and ecological structure. For example, Magnuson et al. (1990) showed that exposure to climatic factors as estimated by morphometric features, such as surface area to mean depth ratio, may explain how a region’s lakes collectively respond to environmental changes. Thus, we suggest that important lake-specific factors could ultimately determine the timing and/or magnitude of environmental changes among lakes experiencing a regional stressor such as climatic change.
The increased abundance of bosminids following the mid-20th century is a trend that has been observed in several oligotrophic lakes in remote regions of northern Canada (Thienpont et al. 2015; Hargan et al. 2016), high elevations of northern Nova Scotia, Canada (Korosi et al. 2013b), the Austrian Alps (Nevalainen et al. 2014), and central Italy (Manca et al. 1996). Similarly, declining body size in aquatic communities, observed at all our study sites as bosminids increase, is an expected response of aquatic communities to indirect effects of climate warming based on surveys and experimental work (Daufresne et al. 2009; Yvon-Durocher et al. 2011; Sheridan and Bickford 2011). Given increased abundance of bosminids, decreased average cladoceran body size, and higher spring and summer air temperatures (Fig. 6), it is likely that the structure and function of all three study lakes are responding to a regional stressor. We interpret these cladoceran responses as outcomes of climate-mediated shifts in key limnological processes affecting mostly the pelagic zone. The rise of bosminids and coincident declines of a taxon with higher Ca demands, such as Daphnia sp., cannot be related to dissolved Ca declines through time given that adequate dissolved Ca (> 10 mg L−1) is readily available to crustacean zooplankton at our study lakes (Jeziorski and Yan 2006). Additionally, evidence suggests that bosminids may hold some competitive advantages over daphniids when food resources change as primary producers respond to nutrient shifts, especially when you consider predation pressure (DeMott and Kerfoot 1982; Jiang et al. 2014). However, further studies are needed to better appreciate important drivers such as fish and macroinvertebrate predation, as well as nutrient cycling, in structuring the composition and average body size of pelagic Cladocera (Davidson et al. 2010; Korosi et al. 2013a; Labaj et al. 2013).
The success of bosminids and the associated decline of D. longispina-complex suggests that regional stressors affecting low-nutrient, hard-water lakes are principally altering zooplankton structure. This may result in poorer quality food for secondary consumers. Bosminids have much lower phosphorus and calcium content than Daphnia sp. (Schulz and Sterner 1999; Jeziorski and Yan 2006). Thus, a large bosminid increase across decades could negatively alter lake food webs, affecting energy and nutrient transfers to higher trophic levels. However, the lack of fish population measures through time makes it impossible to reconcile our findings in light of potential predation pressure on zooplankton by planktivorous fish. Given similar zooplankton trajectories between impact and reference lakes, but presumably different predation regimes (i.e. fish population dynamics and predation intensity) and different overall water quality, suggests that the ultimate mechanism is not related solely to top-down or bottom-up forces (Davidson et al. 2010; Armstrong and Kurek 2018). The notable changes in the intermediate trophic level we report are likely derived from shifts in the epilimnion between mixing periods. Thermal stratification in northwestern New Brunswick is likely now preceded by a progressively shorter ice-cover period (Patterson and Swindles 2014; Lavery et al. 2014). This can affect the pelagic zone through transformations in thermal structure, mixing depth, and possibly enhanced production of organisms (Thompson et al. 2005; Adrian et al. 2009). Given the dominance of the bosminids (between 70 and 90%) compared to other pelagic grazers at all our study lakes in recent times, important ecological thresholds may have been surpassed decades ago. Our observations and interpretations are consistent with the findings of Vogt et al. (2011) that recognizes that energy inputs are disproportionately affecting the pelagic zone across many lakes in recent decades.
In conclusion, despite the a priori defined lake designations as impact or reference, all of our relatively low-nutrient lakes exhibited pronounced shifts in the composition, richness, and body size of cladoceran communities. Sedimentary pigment trends indicated the most striking changes occurred in Lac Unique, and to a much lesser degree at one reference site, First Lake. The broad similarity in recent cladoceran compositional shifts (not the exact timing) across sites provides evidence that conditions in the pelagic zone have changed substantially. We interpret these ecological signals as a result of the indirect effects of climate warming and are able to rule out other potential drivers related to the legacy of regional acidification and dissolved Ca declines observed in some maritime lakes from southern Nova Scotia (Table 1; Harris et al. 2006; Korosi et al. 2013b). Climate change disproportionately impacts the pelagic zone through alterations of the length of the growing season and changes to lake thermal properties. Given the far-reaching effects of this stressor, it is likely that other relatively pristine Canadian maritime lakes are experiencing similar shifts in thermal properties and ecological structure of zooplankton communities (Harris et al. 2006; Korosi et al. 2013b; Armstrong and Kurek 2018). As exhibited by the recent cyanobacteria blooms at Lac Unique, systems that experienced past impacts such as nutrient loading related to watershed disturbance may exhibit more pronounced shifts in trophic structure as climate change intensifies. The ubiquity of the rise of bosminids across systems suggests that this organism may prove to be a useful bioindicator of shifting conditions in the pelagic zone of low-nutrient, hard-water lakes from maritime Canada (Armstrong and Kurek 2018). Overall, our findings suggest that lakes in northwestern NB with good water quality are exhibiting ecological shifts likely associated with post-20th century climate warming.
References
Adamczuk M (2015) Past, present, and future roles of small cladoceran Bosmina longirostris (O. F. Müller, 1785) in aquatic ecosystems. Hydrobiologia 767:1–11
Adrian R, O’Reilly CM, Zagarese H, Baines SB, Hessen DO, Keller W, Livingstone DM, Sommaruga R, Straile D, Van Donk E, Weyhenmeyer GA, Winder M (2009) Lakes as sentinels of climate change. Limnol Oceanogr 54:2283–2297
Appleby PG (2001) Chronostratigraphic techniques in recent sediments. In: Last WM, Smol JP (eds) Tracking environmental change using lake sediments. 1. Basin analysis, coring, and chronological techniques. Springer, Dordrecht, pp 171–203
Armstrong Z, Kurek J (2018) Sensitivity and response of low-nutrient lakes to post twentieth century environmental change in New Brunswick, Canada. J Paleolimnol. https://doi.org/10.1007/s10933-018-0046-8
Bennett KD (1996) Determination of the number of zone in a biostratigraphical sequence. New Phytol 132:155–170
Berström SE et al (2000) Habitat distribution of zooplankton in relation to macrophytes in a eutrophic lake. Internationale Verinigung fur Theoretische und Angewandte Limnologie Verhandlungen 27:2861–2864
Cottingham KL, Ewing HA, Greer ML, Carey CC, Weathers KC (2015) Cyanobacteria as biological drivers of lake nitrogen and phosphorus cycling. Ecosphere 6:1–19
Curry A (2013) New Brunswick lake ecosystems: building a comprehensive provincial monitoring programme. Final report 2013 NB Environmental Trust Fund
Curry A (2014) Future state of NB lakes. Final report 2014 NB Environmental Trust Fund
Daufresne M, Lengfellner K, Sommer U (2009) Global warming benefits the small in aquatic ecosystems. Proc Natl Acad Sci 106:12788–12793
Davidson TA, Sayer CD, Perrow M, Bramm M, Jeppesen E (2010) The simultaneous inference of zooplanktivorous fish and macrophyte density from sub-fossil cladoceran assemblages: a multivariate regression tree approach. Fresh Biol 55(3):546–564
DeMott WR, Kerfoot CW (1982) Competition among cladocerans: nature of the interaction between Bosmina and Daphnia. Ecology 63:1949–1966
Dodds WK, Perkin JS, Gerken JE (2013) Human impact on freshwater ecosystem services: a global perspective. Environ Sci Technol 47:9061–9068
Dufrene M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Glew J (1988) A portable extruding device for close interval sectioning of unconsolidated core samples. J Paleolimnol 1:235–239
Glew J (1991) Miniature gravity corer for recovering short sediment cores. J Paleolimnol 5:241–243
Grimm EC (1987) CONISS: a FORTRAN 77 program for stratigraphically constrained cluster analysis by the method of incremental sum of squares. Comput Geosci 13:13–25
Hargan KE, Nelligan C, Jeziorski A, Rühland KM, Paterson AM, Keller W, Smol JP (2016) Tracking the long-term responses of diatoms and cladocerans to climate warming and human influences across lakes of the Ring of Fire in the Far North of Ontario, Canada. J Paleolimnol 56:153–172
Harris MA, Cumming BF, Smol JP (2006) Assessment of recent environmental changes in New Brunswick (Canada) lakes based on paleolimnological shifts in diatom species assemblages. Can J Bot 84:151–162
Jeziorski A, Yan N (2006) Species identity and aqueous calcium concentrations as determinants of calcium concentrations of freshwater crustacean zooplankton. Can J Fish Aquat Sci 63:1007–1013
Jiang X, Yang W, Zhang L, Chen L, Niu Y (2014) Predation and cyanobacteria jointly facilitate competitive dominance of small-bodied cladocerans. J Plankton Res 36(4):956–965
Juggins S (2009) Rioja: analysis of Quaternary science data. R package version 0.5-6
Korhola A, Rautio M (2001) tracking environmental change using lake sediments. Zool Ind 4:5–41
Korosi JB, Smol JP (2012a) An illustrated guide to the identification of cladoceran subfossils from lake sediments in northeastern North America: part 1—the Daphniidae, Leptodoridae, Bosminidae, Polyphemidae, Holopedidae, Sididae, and Macrothricidae. J Paleolimnol 48:571–586
Korosi JB, Smol JP (2012b) An illustrated guide to the identification of cladoceran subfossils from lake sediments in northeastern North America: part 2—the Chydoridae. J Paleolimnol 48:587–622
Korosi JB, Kurek J, Smol JP (2013a) A review on utilizing Bosmina size structure archived in lake sediments to infer historic shifts in predation regimes. J Plankton Res 35:444–460
Korosi JB, Ginn BK, Cumming BF, Smol JP (2013b) Establishing past environmental conditions and tracking long-term environmental change in the Canadian Maritime provinces using lake sediments. Environ Rev 21:15–27
Kurek J, Korosi JB, Jeziorski A, Smol JP (2010) Establishing reliable minimum count sizes for cladoceran subfossils sampled from lake sediments. J Paleolimnol 44:603–612
Kurek J, Weeber RC, Smol JP (2011) Environment trumps predation and spatial factors in structuring cladoceran communities from Boreal Shield lakes. Can J Fish Aquat Sci 68:1408–1419
Labaj AL, Kurek J, Weeber RC, Smol JP (2013) Long-term changes in invertebrate size structure and composition in a boreal headwater lake with a known minnow introduction. J Limnol 72(2):215–226
Labaj AL, Kurek J, Jeziorski A, Smol JP (2015) Elevated metals concentrations inhibit biological recovery of Cladocera in previously acidified boreal lakes. Fresh Biol 60:347–359
Lavery JM, Kurek J, Rühland KM, Gillis CA, Pisaric MFJ, Smol JP (2014) Exploring the environmental context of recent Didymosphenia geminate proliferation in Gaspésie, Quebec, using Paleolimnology. Can J Fish Aquat Sci 71:616–626
Leavitt PR, Hodgson DA (2001) Practical methods for analysis of sedimentary pigments. In: Smol JP, Last WM (eds) Developments in palaeoenvironmental research: tracking environmental changes using lake sediments, biological techniques and indicators, vol 3. Kluwer, Dordrecht
Luoto TP, Nevalainen L (2012) Ecological responses of aquatic invertebrates to climate change over the past ∼ 400 years in a climatically ultra-sensitive lake in the Niedere Tauern Alps (Austria). Fundam Appl Limnol 181:169–181
Maaref A (2014) Rapport Final du FFE—Projet 130251: plan de lutte contre la propogation des algues bleu-vert dans le basin verstan du Lac Unique. Government of New Brunswick ETF
Magnuson JJ, Benson BJ, Kratz TK (1990) Temporal coherence in the limnology of a suite of lakes in Wisconsin, USA. Fresh Biol 23:145–159
Manca M, Sm Nocentini, Belis CA, Comoli P, Cormbella L (1996) Invertebrate fossil remains as indicators of late Quaternary environmental changes in Latium crater lakes (L. Albana and L. Nemi). Memorie dell’Istuto Italiano di Idrobiologia 55:149–176
Nevalainen L, Ketola M, Korosi JB, Manca M, Kurmayer R, Koinig K, Psenner R, Luoto TP (2014) Zooplankton (Cladocera) species turnover and long-term decline of Daphnia in two high mountain lakes in the Austrian Alps. Hydrobiologia 722:75–91
Oksanen J, Guillaume Blanchet F, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) vegan: community ecology package. R package version 2.0-4
Paerl HW, Huisman J (2008) Blooms like it hot. Science 320:57–58
Paerl HW, Hall NS, Calandrino ES (2011) Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci Total Environ 409:1739–1745
Patoine A, Leavitt P (2006) Century-long synchrony of fossil algae in a chain of Canadian prairie lakes. Ecology 87:1710–1721
Patterson RT, Swindles GT (2014) Influence of ocean-atmosphere oscillations on lake ice phenology in eastern North America. Clim Dyn 45:2293–2308
Pick FR (2016) Blooming algae: a Canadian perspective on the rise of toxic cyanobacteria. Can J Fish Aquat Sci 73:1149–1158
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Reynolds CS (2006) The ecology of freshwater phytoplankton. Cambridge University Press, New York
Roberts DW (2007) labdsv: ordination and multivariate analysis for ecology. R package version 1.1
Schelske CL, Peplow A, Brenner M, Spencer CN (1994) Low-background gamma counting: applications for 210Pb dating of sediments. J Paleolimnol 10:115–128
Schulz KL, Sterner RW (1999) Phytoplankton phosphorus limitation and food quality for Bosmina. Limnol Oceanogr 44:1549–1556
Sheridan JA, Bickford D (2011) Shrinking body size as an ecological response to climate change. Nat Clim Change 1:401–406
Smol JP (2010) The power of the past: using sediments to track the effects of multiple stressors on lake ecosystems. Freshw Biol 55:43–59
Sturtevant R (2006) Cladoceran field guide—sizes. Great Lakes Environmental Research Laboratory (GLERL). http://www.glerl.noaa.gov/seagrant/GLWL/Zooplankton/Cladocera/CladoceraKeySizeTable.html
Taranu ZE, Gregory-Eaves I, Leavitt PR, Bunting L, Buchaca T, Catalan J, Domaizon I, Guilizzoni P, Lami A, McGowan S, Moorhouse H, Morabito G, Pick FR, Stevensen MA, Thompson PL, Vinebrooke RD (2015) Acceleration of cyanobacterial dominance in north temperate-subarctic lakes during the Anthropocene. Ecol Lett 18:375–384
Thienpont JR, Korosi JB, Cheng ES, Deasley K, Pisaric MFJ, Smol JP (2015) Recent climate warming favours more specialized cladoceran taxa in western Canadian Arctic lakes. J Biogeogr 42:1553–1565
Thompson R, Kamenik C, Schmidt R (2005) Ultra-sensitive Alpine lakes and climate change. J Limnol 64:139–152
Vogt RJ, Rusak JA, Patoine A, Leavitt PR (2011) Differential effects of energy and mass influx on the landscape synchrony of lake ecosystems. Ecology 92:1104–1114
Wagner C, Adrian R (2009) Cyanobacteria dominance: quantifying the effects of climate change. Limnol Oceanogr 54:2460–2468
Walseng B, Halvorsen G (2016) Freshwater Crustaceans in Norway. Norwegian Institute for Nature Research (NINA). http://www.nina.no/english/Environmental-monitoring/Freshwater-crustaceans
Woolway RI, Dokulil MT, Marszelewski W, Schmid M, Bouffard D, Merchant CJ (2017) Warming of Central European lakes and their response to the 1980s climate regime shift. Clim Change. https://doi.org/10.1007/s10584-017-1966-4
Yvon-Durocher G, Montoya JM, Trimmer M, Woodward G (2011) Warming alters the size spectrum and shifts the distribution of biomass in freshwater ecosystems. Glob Change Biol 17:1681–1694
Zapata M, Rodríguez F, Garrido JL (2000) Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar Ecol Prog Ser 195:29–45
Acknowledgements
Funding for this research was provided by a 2015–2016 New Brunswick Environmental Trust Fund (NB ETF) Grant and a 2015 Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to JK. MD was supported by an Independent Student Research Grant (ISRG) from Mount Allison University. IGE acknowledges generous support from the Canada Research Chairs program. We also thank Paul MacKeigan for field and lab assistance, Leanne Elchyshyn for processing pigment samples and Allen Curry for data sharing and study site suggestions. Two reviewers and Andrew Labaj provided helpful comments that improved this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Daly, M., Kurek, J., Gregory-Eaves, I. et al. Reorganization of aquatic communities from low-nutrient lakes in northwestern New Brunswick, Canada. J Paleolimnol 61, 185–200 (2019). https://doi.org/10.1007/s10933-018-0052-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-018-0052-x