Abstract
Densities of two sympatric amphidromous grazing fishes, Plecoglossus altivelis and Sicyopterus japonicus, and gross primary production of benthic algae were investigated at the second, third and fourth-order sites located, respectively, 7.0, 6.7 and 3.6 km from the mouth of the Choshi River, Japan. Gross primary production estimated by multiplying gross photosynthetic rates by hours of insolation on the streambed increased downstream with decreasing canopy cover by valley walls and trees standing along the river. Density of P. altivelis was greatest at the fourth-order site with the higher primary production, while the distribution pattern of S. japonicus differed between adult (> ca. 7 cm in total length) and young individuals. Density of adult of S. japonicus was greater at the third-order site, although the young were more abundant at the fourth-order site. Aggressive acts against conspecifics and different species were observed only by adults of S. japonicus and most frequently observed at the third-order site. The upstream shift in the distribution of the adults of S. japonicus would mitigate interference competition with P. altivelis and contribute to their coexistence in the river.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Upstream migrations of amphidromous fish involve habitat shifts from marine to freshwater environments, which derive from active dispersal behavior to find food and grow prior to reproduction (McDowall, 1988). The distribution of migrants in a river is affected both by their abilities to move upstream and the various environmental factors that affect their growth and survival. For example, in tropical and subtropical streams some species of amphidromous grazing goby are more abundant in habitats with lower vegetation cover and many stony substrates consisting of bedrock, boulders and cobbles (Nelson et al., 1997; Abe et al., 2003). These environmental factors are related to the abundance and renewal rates of algal food for the grazing fish. Thus, spatial variation in food availability seems to be one of the most important factors affecting the distribution of amphidromous fish in a river.
In general a temperate river system, except a turbid river, has a downstream gradient of benthic algal productivity and thereby food availability for stream grazers (Vannote et al., 1980). As the stream size increases from headwaters to the mouth of the river, the shading effects of riparian vegetation and valley walls are mitigated. As a result, the photosynthetic activities by benthic algae increase with stream size owing to an increase in the total amount of light reaching the streambed (Naiman & Sedell, 1980). The gradient of the algal productivity will affect upstream movement of amphidromous grazing fish that take their basic energetic source from benthic algae. It is expected that the abundance of amphidromous grazing fish will increase with stream size and algal productivity.
In the Japanese archipelago, two amphidromous grazing fish, Plecoglossus altivelis Temminck & Schlegel and Sicyopterus japonicus (Tanaka), are distributed sympatrically within the range from 30° to 36° N. Juveniles of P. altivelis and S. japonicus migrate upstream in spring to early summer and live in the freshwater areas through their life. P. altivelis live for 1 year and spawn once time in late autumn (Kawanabe, 1958), while S. japonicus spend for 4–5 years in the river and mature after growing up to ca. 7 cm in total length during about 2 years (Dôtu & Mito, 1955). In the streams and rivers P. altivelis and S. japonicus feed on benthic diatoms and cyanobacteria attached to stony substrates and often exhibit intra- and interspecific aggression to use algal food exclusively. Particularly, P. altivelis make a feeding territory that has been shown to vary in size between individuals and the density of P. altivelis, ranging from 0.48 to 7.04 m2 (Iguchi & Hino, 1996). The similar food requirements and interference behaviors for food intake suggest competitive relationship between P. altivelis and S. japonicus, although the potential ways that such amphidromous grazing fish coexist have not been investigated. The differential use of habitats is one of the important ways that potentially competing species can find the opportunity to avoid competitive exclusion and thereby coexist (Connell, 1980). Many studies indicated that stream fish with similar ecological requirements often exploited different habitats or microhabitat patches at different times (Mizno et al., 1979, Greenberg, 1991) as a consequence of competitive interaction (Fausch & White, 1981; Nakano et al., 1988; Sone et al., 2001) or of the specific difference in habitat preference (Schlosser & Toth, 1984).
In this study we investigated the longitudinal distribution of two amphidromous grazing fish, P. altivelis and S. japonicus, in relation to the spatial variation in benthic algal productivity in order to test the hypothesis that the abundance of amphidromous grazing fish would increase with stream size and benthic algal productivity. Furthermore, the distribution patterns of P. altivelis and S. japonicus were compared to assess differential use of habitats by these sympatric fish species.
Methods
Study area
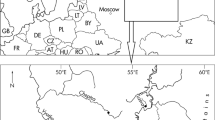
This study was conducted on 12–14 September 2002 in the Choshi River (34°10′ N, 136°12′ E) which flows into Owase Bay on the east shore of the Kii Peninsula, Japan (Fig. 1). In the Choshi River estuary extended 1.05 km up from the mouth. Three study sites were established in the different stream order channels defined by Strahler (1963) and located in freshwater area of the river (Fig. 1). Second-order site was established in a small tributary and located 7.0 km away from the mouth. Third and fourth-order sites were established in the main course of the river and located, respectively, 6.7 and 3.6 km away from the mouth. At all study sites the substrate was composed mainly of cobble and boulders. Discharge was estimated in each study site by multiplying the wetted stream width, mean depth and mean water velocity. Water velocity was measured using a currentmeter (CR-7, Kosumo Rikken, Osaka, Japan). Amount of canopy cover by the valley walls and trees standing along the river and duration of insolation on the streambed were estimated using the computer program CanopOn version 1.10 (http://www.takenaka-akio.cool.ne.jp/etc/canopon/) from photographs of the sky that were taken vertically adjacent to the river surface with a digital camera (C-900 Zoom, Olympus Optical Co., Ltd., Tokyo, Japan) equipped with a fish-eye lens (FCON-02, Olympus Optical Co., Ltd., Tokyo, Japan) at six points in each study site. As made by Kobayasi (1961), amount of canopy cover was calculated as a percentage of the occluded area of the sky on the photograph. Duration of insolation on the streambed was determined as the hours on the sun course within the area of the open sky on the photograph by reading of transparent overlay of sun course on 13 September at 34° N. For measurement of the concentration of dissolved nitrate (NO3) and orthophosphate (PO4), a water sample (1,000 ml) was collected three times a day from each study site at 9:00–10:00, 12:00–13:00 and 15:00–16:00. These water samples were filtrated and then analyzed using an autoanalyzer (TRAACS 800, Bran + Luebbe K.K., Tokyo, Japan).
Measurement of gross primary production
Net photosynthetic rate (NR) and community respiration rate (CR) were measured using the method developed by Stewart (1987). NR corresponded to the accumulation rate of organic materials in algal biomass and was expressed as oxygen production in the light, while CR included the metabolism by heterotrophic organisms such as microbes and insects as well as algae and was expressed as oxygen loss in the dark. The six cobbles were collected randomly from each study site and placed individually in a sealed plastic bag (Ziploc, Asahi Kasei Life and Living Corp., Tokyo, Japan) filled with stream water (2.7–3.0 l in volume). These bags were then put back into the respective site of the stream and incubated for 1 h under natural light conditions. After that, water in the bags was replaced with a fresh sample of water taken from the stream and the substrates in the bags covered by a black vinyl sheet were incubated for 1.5 h under dark conditions. The concentration of dissolved oxygen was measured using an oxygen meter (U-21, Horiba Ltd., Kyoto, Japan) at the beginning and end of the incubation period. Light intensity on the water surface of the river was monitored with a light meter (Model LI-189, LI-COR, Inc., Lincoln, USA) during 10:00–14:00 and varied from 409 to 1,377 μmol m−2 s−1. After the measurement, algae on the substrate were collected using a nylon brush. The surface area of each substrate was traced on aluminum foil and measured using NIH Image version 1.62 (http://www.rsb.info.nih.gov/nih-image/). The algal samples were filtered with a pre-ashed glass fiber filter (Whatman GF/C, Whatman International Ltd., Maidstone, UK). Algal biomass expressed as ash-free dry mass (AFDM) was measured by drying the filters at 80°C for 24 h and then oxidizing at 600°C for 24 h. NP and CR were estimated from the change in the oxygen concentration under the light and dark conditions, respectively. The amount of oxygen production in the light and loss in the dark were divided by the incubation period and surface area of the substrate to express NR and CR, respectively. Gross primary production (GP) corresponded to the total amount of organic materials synthesized by benthic algae during a day and was expressed by the total amount of oxygen production per day. The equation to calculate GP was as follows.
where GP, NP, CR and t were gross primary production, net photosynthetic rate, community respiration rate and hours of insolation on streambed, respectively.
Measurements of the density of fish
The density of P. altivelis and S. japonicus was measured by snorkeling census in each study site. Before the start of the observation, six points were established along the longitudinal line (about 50 m long) in each study site. An observer approached each point slowly from the downstream side and then established a 2 × 2 m quadrate using the bank and boulders in the stream as landmarks. After that, the observer lay motionless at about 1 m away from the quadrate for several minutes to allow fishes to resume their normal behaviors (e.g. getting out of refuges and feeding). The species and numbers of fishes in a quadrate were recorded at each point. The number of S. japonicus were recorded, dividing the fish into adult (>7 cm) and young (<7 cm in total length) individuals by eye. The young individuals are also characterized by their coloration; they are more bright-colored and have distinct vertical bars as compared with the adult individuals. P. altivelis could not be divided into the adult and young individuals because these fish live for only 1 year. After the recording of the species and numbers of fishes, any aggressive behavior shown by P. altivelis and S. japonicus, which included the approaching and chasing of conspecifics and specimens of different fish species, were counted during a 5 min period. All observations were conducted by an expert who had been trained for the visual size estimation of fish before this study. The training included the underwater size estimation of sticks that were cut in different length. After the training, the size of fish can be estimated visually within an error of about 3 mm (Ito & Yanagisawa, 2003). After the observation, the size of each quadrate was checked using a scale and water velocity and water depth were measured at each point.
Statistical analysis
Differences in the environmental factors, gross primary production and fish densities between the three study sites were examined using an analysis of variance and a post-hoc test of Student–Newman–Keuls procedure. To reduce non-normality and non-homogeneity of variance in the data, the portion of canopy cover and the fish density were arcsine-square-root and log transformed, respectively. Differences in the number of the aggressive acts during the observation periods between the three study sites were analyzed using nonparametric statistical tests, Kruskal–Wallis test and Tukey–Kramer multiple comparison test.
Results
Environmental gradient
Environmental conditions in the second, third and fourth-order sites are summarized in Table 1. There were significant differences in the portion of canopy cover, duration of insolation on streambed and nitrate concentration between the three sites. The differences in the canopy cover and duration of insolation were recognized between each pair of the three study sites and the nitrate concentration was lower in the second-order site than in the third and fourth-order sites. Significant differences were also recognized in the benthic algal biomass (F 2,15 = 4.469, P < 0.05, Fig. 2a) and gross primary production (F 2,15 = 13.218, P < 0.001, Fig. 2b) between these sites. The algal biomass was greater in the second-order site than in the third and fourth-order sites, while the gross primary production was greater in the fourth-order site than in the second and third-order sites (P < 0.05).
Densities of amphidromous grazing fish
In this study 444 individuals of stream fish were observed in total. P. altivelis and S. japonicus comprised 66% of the total number of fish (82 individuals of P. altivelis and 212 individuals of S. japonicus) and dominated the diurnal fish fauna in the Choshi River. Other fish observed were Moroco jouyi (Jordan & Snyder), Oncorhynchus masou macrostomus Günther, Rhinogobius sp., Tridentiger obscurus (Temminck & Schlegel), Zacco platypus (Temminck & Schlegel), Z. temminckii (Temminck & Schlegel). Water velocity at the observation points did not differ significantly between the second (mean ± SE, 34.4 cm s−1 ± 4.4), third (52.0 cm s−1 ± 9.2) and fourth-order (54.0 cm s−1 ± 7.7) sites (F 2,15 = 2.159, P > 0.1), although water depth at the observation points differed between the three study sites (F 2,15 = 36.744, P < 0.0001). The differences were recognized between the second (39.0 cm ± 3.2) and third-order (105.7 cm ± 6.3) sites (P < 0.05) and between the second and fourth-order (106.2 cm ± 8.5) sites (P < 0.05).
Significant differences were recognized in the density of P. altivelis (F 2,15 = 14.529, P < 0.001, Fig. 3a) and S. japonicus (F 2,15 = 31.328, P < 0.0001, Fig. 3b) between the three study sites. The density of P. altivelis was greater in the fourth-order site than in the second and third-order sites, while the S. japonicus was more abundant in the third and fourth-order sites than in the second-order site (P < 0.05). There were significant differences in the density of the young (F 2,15 = 24.126, P < 0.0001) and adult (F 2,15 = 7.801, P < 0.005) individuals of S. japonicus between these sites (Fig. 3c). The density of young decreased from the fourth to second-order site, although the adults were more abundant in the third-order site than the second and fourth-order sites (P < 0.05).
Aggressive behavior of fish
In this study aggressive behavior was observed only in the adult individuals of S. japonicus. Aggressive acts of P. altivelis and the young individuals of S. japonicus were not observed during the observations. Of 53 aggressive acts in total during the 90 min observations, 12, 34 and 7 acts were aggressive interference against P. altivelis, the adults of S. japonicus and the young of S. japonicus, respectively. The number of aggressive acts during the 5 min observation period differed between the three study sites (P < 0.005), and was greater in the third-order site (P < 0.05) than the other study sites (Fig. 4).
Discussion
In the Choshi River, the benthic algal production increased downstream with the decrease in canopy cover and increase in duration of insolation as predicted by the River continuum concept (Vannote et al., 1980). The distribution of the two amphidromous grazing fish corresponded to the gradient of the algal production. P. altivelis and S. japonicus were more abundant in the fourth-order site with the higher algal production. Spatial variation in the algal production is an important factor affecting the distribution of grazing fish exploring available habitats (Power, 1984; Abe et al., 2003). However there was no obvious evidence to conclude that the distribution pattern of P. altivelis and S. japonicus was a direct result from exploration of habitats. Random dispersion would also explain this pattern because these fish disperse into the river upstream. In either case, high algal production in downstream areas would create a profitable environment for amphidromous grazing fish by providing plentiful growth habitats in the lower area in a river.
In this study, algal biomass showed an inverse response to the density of amphidromous grazing fish against the downstream increase in the algal production. In general, algal biomass is reduced in the presence of grazers (Steinman, 1996) and a vast reduction in algal biomass occurs at high density of grazers (Steinman et al., 1987, Colletti et al., 1987). In the third and fourth-order sites a large abundance of grazing fish occurred as compared with that in the second-order site and hence their intensive grazing would reduce algal biomass to lower levels.
The distribution of S. japonicus along the river differed between adults and young individuals. The adults were more abundant in the upper area (third-order site), while the density of the young was greater in the lower area (the fourth-order site). This pattern of the distribution was also observed in other species of amphidromous goby, S. lagocephalus and Rhinogobius sp. (Keith, 2003). An upstream increase in the adult individuals could result from the upstream movement or site-specific difference in mortality. In the Choshi River the mortality of the adult S. japonicus would be caused by exhaustion from aggressive acts and/or predation by diving and wading birds. However the aggressive acts were frequently observed in the third-order site as compared with the fourth-order site. Furthermore, water depth, which is an important determinant of the vulnerability to avian predators (Power et al., 1989), did not differ significantly between the third and fourth-order sites. Thus, the results suggested that S. japonicus would gradually move upstream with growth. An upstream increase in the average body size was reported in some species of amphidromous goby (Gobiomorphus) in New Zealand, which results from the upstream movements of adult fish (McDowall, 1988). The upstream movements of the adults would mitigate exploitation competition with P. altivelis and the young of S. japonicus. In the lower areas, the algal production was enhanced by prolonged sunlight periods, although many juveniles of P. altivelis and S. japonicus migrate into this area each year and exploit the benthic algal food intensively.
The behavior of the gradual upstream movements of S. japonicus, furthermore, would be related to the coexistence with P. altivelis. The adults of S. japonicus were extremely aggressive against conspecifics and other fish feeding on the benthic algae. Competition theory expects that two species sharing a single limited resource are unable to coexist in the same habitats (Hardin, 1960) and their coexistence is possible on the condition that the effects of intraspecific competition are greater than that of interspecific competition (Case, 2000). Upstream movements of the aggressive individuals of S. japonicus from the lower areas would contribute to mitigate interspecific competition with P. altivelis.
Conclusion
Densities of sympatric amphidromous grazing fish, P. altivelis and S. japonicus, increased downstream and the pattern of their distribution along a river corresponded to the downstream increase in gross primary production by benthic algae. In the S. japonicus, adult individuals were highly aggressive and their distribution was shifted upstream, compared with P. altivelis and young individuals of S. japonicus. The upstream shift in the distribution of adults of S. japonicus would contribute to their coexistence with P. altivelis by mitigating their interspecific competition.
References
Abe, S., K. Iguchi, S. Ito, Y. Uchida, H. Ohnishi & K. Ohmori, 2003. Habitat use of the grazing goby (Sicyopterus japonicus) in response to spatial heterogeneity in riparian shade. Journal of Freshwater Ecology 18: 161–167.
Case, T. J., 2000. An Illustrated Guide to Theoretical Ecology. Oxford University Press, New York.
Colletti, P. J., D. W. Blinn, A. Pickart & V. T. Wagner, 1987. Influence of different densities of the mayfly grazer Heptagenia criddlei on lotic diatom communities. Journal of North American Benthological Society 6: 270–280.
Connell, J. H., 1980. Diversity and the coevolution of competitors, or the ghost of competition past. Oikos 35: 131–138.
Dôtu, Y. & S. Mito, 1955. Life history of a gobiid fish, Sicydium japonicus Tanaka. Science Bulletin of the Faculty of Agriculture, Kyushu University 15: 213–221 (in Japanese with English summary).
Fausch, K. D. & R. J. White, 1981. Competition between brook trout (Salvelinus fontinalis) and brown trout (Salmo trutta) for position in a Michigan stream. Canadian Journal of Fisheries and Aquatic Science 38: 1220–1227.
Greenberg, L. A., 1991. Habitat use and feeding behavior of thirteen species of benthic stream fishes. Environmental Biology of Fishes 31: 389–401.
Hardin, G., 1960. The competitive exclusion principle. Science 131: 1292–1297.
Iguchi, K. & T. Hino, 1996. Effect of competitor abundance on feeding territoriality in a grazing fish, the ayu Plecoglossus altivelis. Ecological Research 11: 165–173.
Ito, S. & Y. Yanagisawa, 2003. Mate choice and mating pattern in a stream goby of the genus Rhinogobius. Environmental Biology of Fishes 66: 67–73.
Kawanabe, H., 1958. On the significance of the social structure for the mode of density effect in a salmon-like fish, “ayu”, Plecoglossus altivelis Temminck et Schlegel. Memoirs of the College of Science, University of Kyoto, Series B 25: 171–180.
Keith, P., 2003. Biology and ecology of amphidromous Gobiidae of the Indo-Pacific and the Caribbean regions. Journal of Fish Biology 63: 831–847.
Kobayasi, H., 1961. Productivity in sessile algal community of Japanese mountain river. Botanical Magazine of Tokyo 74: 331–341.
McDowall, R. M., 1988. Diadromy in Fishes. Migrations between Freshwater and Marine Environments. Croom Helm, London.
Mizuno, N., S. Uehara & M. Maki, 1979. Studies on a freshwater fish, Rhinogobius brunneus (Gobiidae) IV. Habitat segregation among sympatric populations of 4 colour types. Japanese Journal of Ecology 29: 137–147 (in Japanese with English abstract).
Naiman, R. J. & J. R. Sedell, 1980. Relationships between metabolic parameters and stream order in Oregon. Canadian Journal of Fisheries and Aquatic Science 37: 834–847.
Nakano, S., S. Kitano, K. Nakai & K. D. Fausch, 1988. Competitive interactions for foraging microhabitat among introduced brook charr, Salvelinus fontinalis, and native bull charr, S. confluentus, and westslope cutthroat trout, Oncorhynchus clarki lewisi, in a Montana stream. Environmental Biology of Fishes 52: 345–355.
Nelson, S. G., J. E. Parham, R. B. Tibbatts, F. A. Camacho, T. Leberer & B. D. Smith, 1997. Distribution and microhabitats of the amphidromous gobies in streams of Micronesia. Micronesica 30: 83–91.
Power, M. E., 1984. Habitat quality and distribution of algae-grazing catfish in a Panamanian stream. Journal of Animal Ecology 53: 357–374.
Power, M. E., T. L. Dudley & S. D. Cooper, 1989. Grazing catfish, fishing birds, and attached algae in a Panamanian stream. Environmental Biology of Fishes 26: 285–294.
Schlosser, I. J. & L. A. Toth, 1984. Niche relationships and population ecology of rainbow (Etheostoma caeruleum) and fantail (E. flabellare) darters in a temporally variable environment. Oikos 42: 229–238.
Sone, S., M. Inoue & Y. Yanagisawa, 2001. Habitat use and diet of two stream gobies of the genus Rhinogobius in south-western Shikoku, Japan. Ecological Research 16: 205–219.
Strahler, A. N. 1963, The Earth Sciences. Harper & Roe, New York.
Steinman, A. D. 1996. Effects of grazers on freshwater benthic algae. In Stevenson, R. J., M. L. Bothwell & R. L. Lowe (eds), Algal Ecology, Freshwater Benthic Ecosystems. Academic Press, San Diego, 341–373.
Steinman, A. D., C. D. McIntire, S. V. Gregory, G. A. Lamberti & L. R. Ashkenas, 1987. Effects of herbivore type and density on taxonomic structure and physiognomy of algal assemblages in laboratory streams. Journal of North American Benthological Society 6: 175–188.
Stewart, A. J., 1987. Responses of stream algae to grazing minnows and nutrients: A field test for interactions. Oecologia 72: 1–7.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell, & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Science 37: 130–137.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: K. Martens
Rights and permissions
About this article
Cite this article
Abe, Si., Yodo, T., Matsubara, N. et al. Distribution of two sympatric amphidromous grazing fish Plecoglossus altivelis Temminck & Schlegel and Sicyopterus japonicus (Tanaka) along the course of a temperate river. Hydrobiologia 575, 415–422 (2007). https://doi.org/10.1007/s10750-006-0389-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-006-0389-4