Abstract
Although congestion is considered to be the main reason for hospital admission in patients with acute heart failure, a simplistic view considering idro saline retention and total body volume accumulation did not provide convincing data. Clinical congestion occurrence is often the tip of the iceberg of several different mechanisms ranging from increased filling pressure to extravascular fluid accumulation and blood flow redistribution. Therefore, the clinical evaluation is often restricted to a simple physical examination including few and inaccurate signs and symptoms. This superficial approach has led to contradictory data and patients have not been evaluated according to a more realistic clinical scenario. The integration with new diagnostic ultrasonographic and laboratory tools would substantially improve these weaknesses. Indeed, congestion could be assessed by following the most recognized HF subtypes including primitive cardiac defect, presence of right ventricular dysfunction, and organ perfusion. Moreover, there is a tremendous gap regarding the interchangeable concept of fluid retention and redistribution used with a univocal meaning. Overall, congestion assessment should be revised, considering it as either central, peripheral, or both. In this review, we aim to provide different evidence regarding the concept of congestion starting from the most recognized pathophysiological mechanisms of AHF decompensation. We highlight the fact that a better knowledge of congestion is a challenge for future investigation and it could lead to significant advances in HF treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Congestion is the main reason for hospital admission in patients with acute heart failure (AHF). Around 90% of patients admitted for an episode of HF experience some signs of congestion. Volume overload and fluid accumulation are considered hallmarks of acute HF. The two main characteristics of congestion are sudden redistribution of blood volume from systemic to pulmonary districts and intravascular fluid retention. Unfortunately, a simplistic view taking into account idro saline retention, total body volume accumulation, and euvolemia restoration by common diuretic treatment did not provide enough convincing data or potential solutions. Currently, a universally recognized diagnostic algorithm which can grade congestion is lacking [1,2,3]. In different studies, diverse clinical and diagnostic tools have been used and the methods employed for its evaluation are generally too broad. Current discrepancies could lead to a different prognostic impact and management of residual congestion, although most trials agree in recognizing its prognostic value. Congestion is traditionally defined as an increase of LV diastolic filling pressure associated with typical signs and symptoms of HF [4, 5]. Clinical congestion appearance is the tip of the iceberg of cardiac dysfunction leading to increased filling pressure backward transmitted to the pulmonary circulation and central venous system. For this reason, its recognition by simple clinical examination is often delayed and the use of some new diagnostic tools appears mandatory for an early identification. Therefore, it could be classified in central and peripheral in relation to the involved organ damage [5, 6]. The interchangeable concept of volume overload and congestion should be addressed and specific monitoring which accounts for the intravascular or extra-vascular compartment and the main site of fluid retention are worth considering. These simple assumptions suggest that the congestion cascade should be re-examined in a more integrated model that recognizes systemic and cardiac congestion as two distinct processes. Moreover, the congestion occurrence may be analyzed looking at different HF subtypes, the clinical presentation and the temporal trend (i.e., worsening HF vs de novo HF).
The prognostic role of congestion
Although congestion is related to poor outcome, its precise clinical assessment is often misleading, and in the case of evaluation, this is not performed with a systematic method because a validated score at admission and before discharge is still not endorsed. Despite the different methods employed, many trials confirmed the prognostic role of congestion. Indeed, it is one of the principal goals during treatment of the acute phase: in the ACTIVE HF trial, when evaluating the prognostic impact of vasopressin-2 antagonist, patients who experience increased jugular venous pressure, dyspnea at rest, and edema have a 3-fold increased risk of hospital readmission and death during a short time follow-up period [7]. In the more recent PROTECT trial, by evaluating adenosine-2 receptor antagonist Rolofilline, the authors graded dyspnea and those subjects with early dyspnea relief and greater weight loss showed a reduced mortality with respect to patients with persistent dyspnea after 3 days of treatment [8]. A more detailed post hoc analysis from the EVEREST trial evaluated congestion by clinical assessment and severity signs. The authors divided congestion among quartiles and patients with higher scores had a poor prognosis. A current study revealed that around 40% of patients were discharged with some signs of residual congestion [9]. The EURO survey program confirmed such a trend, showing that during acute phases, only 24% of patients had a complete congestion resolution, and their mean weight loss was at least 2 kg [10]. This feature is not surprising when assuming that residual congestion is inversely related to the length of hospital stay and most likely to be caused by incomplete treatment and drug titration. These current findings have been recently replaced in patients with either reduced systolic function (HFrEF) and preserved systolic function (HFpEF) over a 3-month follow-up period [11]. Finally, a post hoc analysis by the CARESS and DOSE trials, respectively, conducted in patients submitted to ultrafiltration and furosemide treatments, residual congestion was related to poor outcome at 90 days [12]. Despite this data, congestion remains difficult to assess, especially when symptoms are mild and patients are prone to recurrent episodes. Therefore, an evaluation by means of clinical signs is superficial and it could hide effective cardiac filling pressure increase. For these reasons, numerous scores based on clinical evaluation, imaging tools, and biological tests are available to assist physicians in ascertaining and quantifying congestion [13,14,15]. Overall, congestion recognition at an early stage by an integrated clinical laboratory and ultrasonographic assessment remains one of the principal goals for future research [16].
Congestion modality assessment
Over recent years, several authors proposed a few models including echocardiographic parameters, lung ultrasound, chest x-ray, and additional clinical assessment, to optimize and grade congestion [14,15,16]. Despite advances in diagnostic tools, clinical examination still appears to be fundamental for an acute HF classification and initial management. Although the clinical approach has some weaknesses, it remains central for HF categorization following the Stevenson criteria. During a clinical visit, it is possible to establish patients’ volume status and peripheral perfusion [17]. However, a standardized protocol indicating the parameters to be included and the evaluation timing course is still lacking. Recently, a position paper by the ESC HF group suggested the use of a flow chart including either clinical, echocardiographic, and lung ultrasound measurements to detect congestion more precisely [18]. Most studies are restricted to the clinical evaluation: when assessed, the traditionally recognized signs are dyspnea, ortopnea jugular vein distension, epatojugular reflex hepatomegaly, peripheral edema, and third heart sound; chest radiography, although included in the initial diagnosis and evaluation of AHF, is considered in only a few studies. The traditional evidence of cardiomegaly, venous hypertension, and fluid lung redistribution until the interstitial edema is not believed to be specific targets [19, 20]. The ADHERE registry confirmed approximately one of every five patients admitted from the Emergency Department with acute decompensated heart failure had no signs of congestion on chest radiography [21]. This indicates that physicians should not rule out subjects with negative radiographic signs and a careful clinical examination needs to be undertaken on patients presenting with dyspnea. Alternative signs of congestion are pulmonary rales, body weight changes, Valsalva manoeuvre and dyspnea on exertion. All these markers include some weaknesses due to scarce specificity and inter-observer variation leading to a modest accuracy.
Although the above cited signs are acknowledged as universal parameters for congestion detection, other protocols take into consideration different factors: in the PROTECT trial, the heart rate, blood pressure, respiratory rate, natriuretic peptides, and the NYHA class were considered as the primary end point for good a good response to treatment [8]. In the ESCAPE trial, besides the pulmonary catheterization measurement, the included congestion signs were only dyspnea, edema, jugular venous pressure, and body weight [22]. Conversely, in the post hoc analysis from the ASCEND-HF trial, the authors indicated body weight gain, blood urea nitrogen (BUN) increase, and low BNP level reduction as the congestion measurements most related to an adverse outcome [23]. More recently, Harjola et al. from the ESC-AHF committee suggested a score based on organ injury due to congestion. In this document, the authors intend to provide a better identification of target organ damage by an assessment of heart, kidney, lung, abdomen, and brain [24]. However, the evaluation of organ deterioration is not only limited to a physical examination as it also includes laboratory markers, echocardiography, lung and abdominal ultrasound. In line with these assumptions, and with the concept of organ crosstalk between different organs, mediated via mechanical, soluble, and cellular mechanisms, a cardio-pulmonary renal syndrome has recently been described [25]. This crosstalk could be reordered by congestion, starting from increased cardiac filling pressure towards pulmonary venous congestion, increased right atrial and central vein pressure, up to renal vein elevation and kidney congestion. The wide range of the adopted criteria leads to a dishomogeneous evaluation during the physical examination, and an unmet need of criteria standardization appears mandatory, both in terms of parameters and in terms of the timing course evaluation. Both aspects could potentially create an enormous bias and they must be clarified. Therefore, specific guidelines should be devised to standardize this topic. As suggested by the recent position paper, the simple application of a clinical congestion score based only on symptoms and signs has limited value and it should be integrated by new diagnostic features [18]. These would probably include ultrasonographic parameters associated with available laboratory markers including natriuretic peptide, osmolarity, and hemoconcentration. Despite all these theories, congestion classification and assessment in HF (by universally recognized and easily detectable criteria) remain an unmet need and a goal for future studies.
Different congestion scenario in heart failure subtypes
Acute HF presentation encompasses a wide spectrum in relation to the prevalent underlying pathophysiological mechanisms, organ perfusion, and congestion. Acute HF can occur as hypertensive HF, pulmonary edema, right HF, HF associated with acute coronary syndrome (ACS), and cardiogenic shock. Besides, on the basis of temporal criteria, acute HF could be divided into de novo heart failure or worsening decompensated chronic HF [26]. Data from the ESC registry showed that most patients (61%) presented with acute decompensated HF, 13% had pulmonary edema, 14% experienced ACS, and only 3% had cardiogenic shock, confirming the heterogenous picture of acute HF syndrome [27]. The OPTIME HF demonstrated that most episodes are related to congestion and only 10% to hypoperfusional defect. Moreover, 42% experienced some precipitating factors causing clinical deterioration status, and among the initial causes, around half can be attributed to extracardiac reasons [28]. Another classification, beyond the timing course, focused attention on the presence or absence of CAD and the main cardiac defect in terms of preserved or reduced ejection fraction. Accordingly, data from the ADHERE registry showed that patients with HFpEF were more likely female, had a history of hypertension, less coronary artery disease, and a lower risk of inpatient death but a higher likelihood of deterioration in renal function during hospitalization [29]. All these phenotypes could appear in completely different modalities: this depends on congestion severity and variety of congested systemic organs. Therefore, the primitive cardiac dysfunction, left or right congestion, and normal or low cardiac output play an important role in congestion status. Overall, a greater pathophysiological understanding of the different congestion features of the various AHFS is needed in order to identify targets for therapy and research. This includes not only idro saline retention but also hemodynamics, neurohormonal overdrive, inflammatory activation, and the cardiorenal crosstalk.
Looking at the post hoc analyses of DOSE and CARESS, no significant differences in terms of the dyspnea score, ortopnea, peripheral edema between HFpEF and HFrEF were demonstrated [30]. Therefore, any significant discrepancies regarding echo findings and laboratory parameters between the two groups have been revealed [31]. However, both studies did not measure congestion following the more recent suggested criteria and the evaluation was restricted to the dyspnea score, Jugular venous pressure, pheripheral edema, and NP measurement. Only the Van Aelst partially analyzed echocardiographic parameters regarding pulmonary pressure and cava vein.
Conversely, congestion appearance is different in isolated right HF with respect to left HF. In the former, congestion is mainly due to increased central venous pressure leading to epathomegaly gut congestion and pheripheral edema. In the latter, congestion is the consequence of increased filling pressure with pulmonary hypertension and lung congestion. The current picture could vary in relation to the timing of increased pulmonary pressure, presence of contemporary right ventricular dysfunction, and systemic conditions in terms of stroke volume and mean arterial pressure [32].
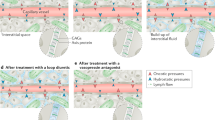
Similar divergencies regarding lung involvement in terms of extravascular water accumulation and pleural effusion may be found in either HFpEF and HFrEF [15, 33]. Current odds have been updated in a cluster analysis identifying three main phenotypes with different clinical characteristics: cluster 1 had the highest average systolic blood pressure at admission and lung congestion. Cluster 2 represented subjects with both “cardiac and renal failure” low EF and poor renal function. Cluster 3 comprised mostly older patients with the high prevalence of atrial fibrillation and preserved EF [34]. In line with these theories, when a panel of 37 different biomarkers were analyzed in the PROTECT trial, the authors revealed that subjects with HFrEF had an increased value of biomarkers related to cardiac stretch and volume overload whereas in HFpEF, biomarker interactions were mostly related to inflammatory activation and fibrosis [35]. Accordingly, the BIOSTAT network analysis showed that biomarker profiles specific for HFrEF are related to cellular proliferation and metabolism, whereas biomarker profiles specific for HFpEF are related to inflammation and extracellular matrix reorganization [36]. The current differences reflect the end of life and death modalities in both subtypes: in HFrEF, the main causes of death are low output state, end stage worsening congestion status, and sudden death due to ventricular tachiarrythmia; in HFpEF, a consistent percentage of deaths are related to non-cardiovascular reasons, to systemic vascular accidents, and to right heart failure [37] (Fig. 1).
Many other factors distinguish systolic and diastolic HF: HFpEF is associated with impaired LV diastolic function and significant ventricular and aortic stiffening. The degree of aortic stiffness and reduced arterial compliance suggests central and peripheral vascular derangements as two main factors in HFNEF-pulse wave velocity measured in the arterial arm, which was significantly higher in HF-PSF subjects than in both HF-RSF. Conversely, venous capacitance was higher in HFrEF subjects compared with HFPEF subjects [38]. The Swedish heart failure registry has recently confirmed a close association between PP and HF that increases linearly with EF [39]. Current divergences could be emphasized during exercise stress in around 50% of HFpEF patients developing pulmonary hypertension. Reduction in arterial elastance and ventricular-arterial interaction was attenuated owing to a minor elastance increase in HFpEF [40].
Qualitative studies have also documented that patients with HFrEF develop more severe symptoms than those with HFpEF and are associated with more significant exercise intolerance, frequent hospitalizations, right heart failure, and reduced survival. The current clinical scenario may be partially explained by baseline and demographic characteristics in the two subtypes: patients with systolic dysfunction are more frequently younger males with prevalent CAD and normal or low blood pressure value, mostly with a history of recurrent episodes, whereas subjects with diastolic dysfunction are more frequently older females with several associated metabolic disorders, with higher blood pressure values with respect to the previous group. All these items could explain the different manifestations of congestion volume overload that appear predominant in patients with acute HFrEF where weight gain associated with a significant degree of systemic venous congestion is typical. Otherwise, patients presenting with normal or elevated blood pressure (HFpEF) exhibit much more pulmonary congestion rather than systemic congestion and little weight increase [41]. Further differences are related to pulmonary hypertension occurrence and pulmonary vascular remodeling [42]: in a retrospective study, Guazzi et al. found that despite similar levels of wedge pressure in HFpEF and HFrEF, pulmonary circulation is stiffer in patients with HFpEF-PH than in patients with HFrEF, leading to a higher diastolic pulmonary gradient [43]. The same group previously demonstrated a different echocardiographic pattern significance of right ventricular (RV) dysfunction that correlates with the prognosis in patients with reduced (HFrEF), mid-range (HFmrEF), or preserved (HFpEF) left ventricular ejection fraction [44]. Although a specific association between the pathophysiological mechanisms of congestion and left ventricular ejection fraction is obtained only through clinical signs, no studies have demonstrated specific hemodynamic and neurohormonal models in different subtypes [45]. Based on these assumptions and evidence, the Cotter theory, indicating cardiac and vascular congestion, needs to be updated: acute decompensated HF resulting from decreased myocardial contractility and impairment of previous deteriorated cardiac performance leading to fluid retention; the second form is characterized by rapid increase of systemic pressure and systemic vascular resistance, altered ventriculo-arterial coupling with afterload mismatch and superimposed left diastolic dysfunction [46]. Thus, the primitive cardiac defect instead of simple fluid accumulation may play a fundamental role and it could create a different congestion scenario.
Central and peripheral congestion: two sides of the same coin?
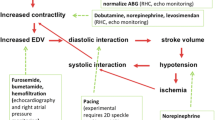
The existing paradigm for understanding congestion in acute HF focused primarily on volume overload and left ventricular filling pressure elevation. The traditional model regards cardiac dysfunction with inadequate cardiac output for metabolic and systemic organ function as the main factor. This primitive failure leads to peripheral vasoconstriction and initial increase of venous return mediated by neurohormonal activation to maintain adequate organ perfusion. The initial beneficial effects of these mechanisms become harmful over a long time period; the idro saline retention and fluid volume overload with consequent blood volume expansion are the main drivers of more advanced stages [47]. The mismatch occurring among cardiac contractility, increased stiffness, augmented afterload, and reduced preload causes a further increase in left ventricular filling pressure with both forward and backward HF. The latter dysfunction creates a left atrial pressure increase, reduced pulmonary vein return, and capacitance associated with post capillary hypertension. Pulmonary congestion is the final situation that occurs due to blood flowing upstream into the left cardiac chambers and to increased pressure in pulmonary capillaries that are not compensated by lymphatic drainage [48, 49]. The increase of the fluid filtration rate from the interstitium and altered drainage capacity shifts fluids from vascular to the interstitial bed. Capillary pulmonary permeability, vascular endothelial properties, and pulmonary arterial vessel thickening could develop a contemporary pre capillary hypertension [50]. All together, these factors contribute to an increased mean and systolic pulmonary pressure elevation, wedge pressure increase with eventual right ventricle remodeling and dysfunction. Maladaptive RV remodeling appears to be a consequence of continuous RV pressure overload, leading to tricuspid regurgitation and subsequent systo-diastolic dysfunction [51, 52]. These mechanisms also lead to RV dissynchrony, which depends on RV myocytes that prolong their contraction time delaying the systolic leftward septal movement. Another causal factor of maladaptive remodeling is ventriculo-arterial uncoupling, which represents the lack of relationship between RV contractility and afterload [53, 54]. Persistent RV deterioration can cause increased central venous pressure, reduced venous return, and pre-load condition, with peripheral edema and jugular venous distension occurrence.
On the other side, increased RAA and sympathetic activities enhance water and sodium reabsorption into the kidney, to maintain adequate filtraction fraction. Unfortunately, the persistent neuro endocrine activity induces detrimental effects on both the heart and kidney. In the cardiovascular system, LV hypertrophy and remodeling are developed; it accelerates fibrosis and apoptosis processes and peripheral vasoconstriction occurs in order to increase plasma volume. At kidney level, it provides intrarenal blood flow redistribution, reduced medullary blood flow, glomerulosclerosis, tubular fibrosis, and efferent and afferent arterial vasoconstriction [55]. Sympathetic stimulation causes peritubular capillary oncotic pressure elevation and it reduces peritubular capillary hydrostatic pressure with a consequent increase in sodium resorbtion in the proximal tubule. Angiotensin II also stimulates sodium resorption via two mechanisms: a direct effect at medullary level stimulating the synthesis of renin by the macula densa and indirectly by the aldosterone production, which in turn activates Na K exchange in the distal collector duct. These features result in interstitial fluid accumulation with a reduction in effective circulating blood volume [56, 57].
Because around 40% of blood volume physiologically resides in the arterial district and this percentage is reduced during HF, a substantial volume expansion is necessary to preserve organ perfusion. This is obtained by an altered blood volume and interstitial volume expansion that become the main drivers of clinical and organ congestion. Another actor to be accounted in this framework is the venous district: it contains approximately 70% of total blood volume and it is much more compliant than the arterial system. Therefore, a relevant numbers of α1 and α2 adrenergic receptors are lodged in splanchnic veins, making them highly sensitive to adrenergic stimulation [58]. These anatomic findings imply that for a given sympathetic stimulus, the veins respond to a much greater degree than the arteries. The final result is that sympathetic activation reduces venous capacitance increasing both central and peripheral vein pressure. Current mechanism leads to a reduced venous return accelerating capillary permeability and interstitial fluid accumulation. Taken together, all these mechanisms cause a fluid shift from the venous vessels to the extravascular volume expansion, culminating in the syndrome of congestion (Fig. 2) [59].
These assumptions mean that two distinct mechanisms contribute separately and mutually to the congestion and we can clearly distinguish a central congestion from a peripheral congestion. This current concept was confirmed by the ESCAPE data revealing that worsening renal function was not related to cardiac output, pulmonary wedge pressure, and systemic vascular resistance. Conversely, there was a weak correlation with right atrial pressure [60]. Current findings suggest that in patients with invasive monitorization, the relationship between heart failure and renal dysfunction is more complex than hemodynamics alone. Therefore, they confirmed that some discordances exist between cardiac congestion and liquid retention [61]. Despite the fact that continuous monitorization in both HFrEF and HFpEF found a close relation between increased pulmonary pressure and events recurrence in intensive care ward patients, other data did not show any type of relationship existing between central venous pressure and total blood volume. Interestingly, by using albumin-labeled radioiodate injections, Miller et al. demonstrated a wide distribution of TBV plasma volume and red cell volume [62]. Therefore, after diuretic administration, any differences were observed in terms of delta TBV and PV before and after treatment. Current data confirm the heterogeneity of body fluid accumulation and blood redistribution during HF condition.
The role of the third space: the intrestitium site
Despite the consideration of volume overload as a hallmark of acute HF, the pathophysiology of fluid accumulation and redistribution remains understood only in part. Similarly, although it is known that hemodynamic congestion precedes pulmonary and peripheral congestion, there is no concordance between body weight gain and invasive measurement. Such discordance could be partially explained by taking into account the extravascular bed: the balance occurring between intravascular and interstitial space is normally modulated by Starling forces across the capillary wall integrity that guarantees an equilibrium resulting in stable no net movement of fluid in steady-state conditions [63]. However, the decrease in capillary hydrostatic pressure as occurred in HF with impaired cardiac output results in a shift from interstitial fluid into the intravascular space in an attempt to restore effective circulating BV and maintain normal organ perfusion. This reserve capacity of the interstitial fluid compartment provides a compensatory mechanism to support PV expansion in patients with HF. Despite these recognized mechanisms, there is a wide heterogeneity because of multiple confounding factors that influence a uniform answer: differences in systemic systolic blood pressure, opposing oncotic forces, changes in capillary permeability, lymphatic drainage, the degree of neurohormonal activation, renal function, and tubular Na resorption are highly variable and, therefore, make the extent of BV expansion highly variable [63, 64].
Secondly, 30–35% of total BV is in the arterial district 50% in the vein bed and the resting 15% in the interstitium. At capillary level, a shift in the distribution of body fluid between the interstitial and the intravascular fluid compartments could occur. This is caused by transcapillary oncotic increase and hydrostatic pressure reduction promoting transudation in the interstitium whereas low interstitial compliance opposes fluid accumulation [65]. Thirdly, the extent of interstitial volume expansion and, therefore, BV expansion depends on the severity of HF, which in turn is related to neurohormonal activation, vasopressin activity, and renal sodium avidity [66]. In this picture, interstitial glycosaminoglycan integrity plays a considerable role in the permeability regulation. Recent insights suggest that Na+ is not distributed in the body solely as a free cation, but that it is also bound to large interstitial glycosaminoglycan (GAG) networks in different tissues. The GAG structure consists of elastin fibers and collagen, in which the equilibrium is maintained by electrostatic forces depending on the Na concentration. For each increase of Na, from both dietary intake as well as retention depending on tubular reabsorption, we have structural damage and architectural destabilization linked to a sudden increase of Na. This increase alters the electrostatic equilibrium with a rise in interstitial oncotic pressure, a reduction in hydrostatic pressure, and further shifting of liquid from the intravascular to the extravascular bed [64]. Therefore, endothelial glycocalyx (eGC) consists of a network of different types of soluble proteoglycans and glycoproteins that are connected to the endothelial cell membrane through adhesion molecules. A dynamic equilibrium exists between the eGC and flowing blood, which continuously affects its composition and electrostatic gradient. In normal conditions, it reduces vascular permeability, restricts molecules from reaching the endothelium, and avoids platelet adhesion. Most importantly, the endothelial GAG network acts as a Na+ buffer by binding positively charged Na+ cations. As a result, the eGC buffer allows the gradual passage of Na+ from the blood into the space between the eGC and endothelium [67]. Na+ can subsequently enter the endothelial cell through apical endothelial Na+ channels. Then, sodium-potassium adenosine triphosphatease restores cell homeostasis by creating a transcellular passage for Na+ into the interstitium. However, most Na+ is transported between endothelial cells along its electrochemical gradient via the paracellular pathway. Any damage occurring at the endothelial glycocalyx (eGC) leads to increased vascular permeability, diminished sodium (Na+) buffer capacity, and disturbed mechanotransduction [68].
Based on these hallmarks, the congestion cascade could be re-examined in a more systematic model, looking at the integrity and composition of the interstitium. Overall, the intra and extravascular congestion by specific diagnostic tools measuring the entity and variety of fluid accumulation is worth investigating.
New application for grading congestion
Because the diagnostic accuracy of congestion based on clinical signs, dyspnea score, and chest radiography is quite inaccurate, a non-invasive algorithm has been recently updated and it accounted echocardiography, chest echography, and laboratory tools [5, 18]. This statement proposed a series of applicable measurements easily detectable in clinical practice.
Echocardiography
Comprehensive echo examination provides useful information about cardiac structure and function: by cardiac ultrasound examination, it is possible to establish the whole systolic function, kinetic abnormalities, presence of valve disease, and myocardial mass. Thus, echocardiography appears mandatory during the early evaluation phases, because of its accuracy in recognizing the main patho-physiological mechanisms of AHF: the most traditional parameter of systolic function detection is ejection fraction by which we categorize the type of HF in reduced (EF < 40%), preserved (EF > 50%), or mid-range HF (EF between 40 and 50%) [69]. This achievement is relevant to identify the primitive cardiac function defect and may be matched with blood pressure value in order to recognize systemic organ perfusion and cardio vascular coupling interaction. Another important item we can obtain by cardiac ultrasound is the RV condition and adaptation. Although reproducibility and correlation with true RV volumes invasively measured are often misleading, its assessment by echocardiography demonstrated a close relation with prognosis. Indeed, echo modality has got some intrinsic limitations related to acoustic window and the inability to completely detect the whole RV volume by traditional approach. Thus, formula applicable for systolic LV evaluation cannot be reproduced for RV: its different shape, contraction modality, free wall characteristics, and the lack of specific anatomic references points, make it difficult to extend calculations validated for LV [70]. RV systolic function can be also evaluated using multiple methods: tricuspid anular peak systolic excursion (TAPSE) is the simpler and more accurate parameter measuring lateral anulus movement in M-mode reflecting longitudinal RV function but even the effective systolic function. It is easily applicable with best reproducibility and it provides useful information of RV status among other echo parameters. Because of these, characteristic is recommended for evaluation and assessment in patients with primitive or secondary pulmonary hypertension. Systolic wave velocity (S′) obtained by TDI signal is the measure of free wall contraction at basal level obtained by the average of three cardiac cycles. It is prone to loading condition and heart rate as well as RV strain and strain rate calculated as percentage of RV free wall systolic shortening by speckle tracking modality. The analysis of LV filling pressure by transmitral flow and early diastolic tissue wave (E/e1) ratio provides useful information and details on intracardiac LV pressure and overload. Another important feature available by Doppler ultrasound is pulmonary systolic pressure by the tricuspidal regurgitation using Bernoulli equation. Recently, the combination of TAPSE and PASP ratio has been proposed as a surrogate marker of RV-vascular coupling. This new variable showed a strong relationship with prognosis in chronic HF patients with both reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF) [71, 72]. Finally, the right atrial pressure could be estimated by the collapsibility of inferior vena cava (VCI) during respiratory excursions. Despite several non-invasive diagnostic methods have been described to assess PH and RVD in chronic conditions, poor data are reported in acute setting during early hospitalization phase. Future researches may confirm whether these parameters will be related to outcome in acute setting and whether a decrease of VCI, PASP, and E/e1 ratio after acute treatment will be really associated with effective adverse event reduction.
Lung echography
Ultrasound lung comets (LUS) assessment is a simple, accurate, fast, and economic tool to assess pulmonary congestion and detect milder degrees of congestion that might benefit from an intensification of therapy. The number of B-lines is directly proportional to the severity of lung congestion. B-lines measured were also related to clinical evidence of pulmonary congestion, a raised plasma BNP, increased echocardiographic measures of left ventricular filling pressure, left atrial dilatation, raised pulmonary artery pressure, and inferior vena cava distension [15, 73]. Accordingly, B-lines appear useful, in addition to B-type natriuretic peptide (BNP) and chest X-ray, for the differential diagnosis of suspected AHF as suggested by current guidelines. [74] Residual pulmonary congestion evaluated by LUS before discharge identifies patients with greater unmet needs who might benefit from experimental interventions [75, 76]. Again, it must be emphasized that dyspnea disappearance should not be considered the same as resolution of pulmonary congestion. In this setting, LUS assessment seems to be superior with respect to chest radiography, with real-time, low-cost, and radiation-free advantages. Indeed, chest radiography could be negative in 20–30% of patients particularly those with chronic pulmonary vein hypertension [77]. More than three B-lines in each intercostal space are suggestive of interstitial edema.
Natriuretic peptides
The most known laboratory markers employed in HF diagnosis are natriuretic peptides (NP). Both BNP and its precursor NT-pro-BNP are related with LV end diastolic pressure and pulmonary pressure [78]. Therefore, in acute setting, significant BNP level increase is a reliable sign of severe diastolic dysfunction confirmed by the relationship with high E/e1 ratio and signs of pulmonary congestion [79, 80]. From the OPTIMIZE data, discharge BNP compared with other clinical variables was the most important factor for 1-year adverse event prediction [81]. A meta-analysis including 19 studies significant levels decrease of both BNP and NTproBNP from admission to discharge was associated with reduced risk of readmission and worsening HF [82]. Therefore, in a group of patients, candidates for heart transplantation, the BNP-guided management combined with peak exercise oxygen consumption test was indicative for cardio surgery and a cut off value less than 506 pg/ml showed an identical course with respect to survival transplanted patients [83]. Despite these findings, some concerns are recently raised about the natriuretic peptide (NP)-guided treatment and prognostic role of NP in HFpEF: indeed, in the TIME-CHF trials, NTproBNP-guided therapy did not improve HF hospitalization free survival [84]. Moreover, the GUIDE IT study conducted to optimize BNP level below 100 and NTproBNP below 1000 pg/mL in patients with different NYHA class, showed equivalent outcome in active arm vs standard treatment in terms of both adverse events and quality of life [85]. Beyond specific studies, some unresolved concerns deserve specific attention and NP measurement needs to be contextualized thinking of the following question: NP levels have got similar diagnostic and prognostic powerful across the whole spectrum of HF? Which is the best NP threshold for guiding management? What is the best timing for blood samples and optimal delta biomarker-guided HF care? Which is the significance of “non responders”? [86, 87] Finally, NP measurement should be evaluated accounting for sex, age, body mass, chronic kidney diseases, and inflammatory conditions.
Adrenomedullin
Adrenomedullin (AM) is a new biomarker expressed in the adrenal glands, heart, lungs, and kidneys, vascular endothelium with vasodilatory action originally discovered in human pheochromocytoma tissue. AM receptors are distributed throughout the cardiovascular system and have been identified in the heart and lungs [88]. AM acts as an autocrine and/or paracrine peptide to play a key role in the regulation of water and sodium homeostasis [89]. Given to its vasoactive properties, AM levels are physiologically elevated during pregnancy and its precursor proADM is blunted in severe preeclampsia [90]. Both AM proAM are all higher in patients with heart failure than healthy subjects in proportional to the disease severity. The BACH study revealed that MR-proANP levels have additional diagnostic significance compared to BNP levels in patients with intermediate BNP levels. MR-proANP is as useful as BNP for AHF diagnosis in dyspneic patients and may provide additional clinical utility in subgroup with grey zone BNP value [91]. Despite these positive results, current biomarkers should be still tested in a randomized trial study and the precise mechanism of increase needs to be furtherly investigated.
Hemoconcentration
Increased hemoglobin (HB) and hematoctitc (Hct) levels over treatment for HF are now accounted as indirect markers of decongestion. Several studies described a relation existing between hemoconcentration and favorable outcome: in a post hoc analysis from the EVEREST trial Hct increase from admission to discharge was associated with less congestion and decreased mortality despite renal impairment occurrence during hospitalization [92]. Similarly, a single center study showed around 38% of patients experienced any type of concentration and this trend occurred in both HFrEF and HFpEF. Therefore, distinguishing between transient or persistent hemoconcnetration, only the latter was related to improved outcome [93]. Aggressive diuretic therapy and good diuretic response are markers for better fluid removal, clinical decongestion leading to hemoconcentration. Additionally, WRF occurrence in the setting of hemoconcentration is not related with adverse events and it may simply identify those groups with increased fluid depletion or higher vulnerability to rapid fluid changes [94]. (Table 1).
Tumor marker antigen carbohydrate 125
Tumor marker antigen carbohydrate 125 (CA-125) levels have shown a correlation with the severity of fluid overload and the risk of mortality and readmission. CA-125 is a glycoprotein widely used for ovarian cancer and abdominal diffusion monitoring, and it has emerged as a potential biomarker of heart failure (HF). Plasma CA-125 correlates with clinical and echocardiographic parameters related to increased central venous pressure, abdominal, and pleural effusion and liver congestion [95, 96]. Particularly interesting is the correlation with symptoms and signs of right HF and inflammatory markers. Indeed, high levels of this glycoprotein have shown to be present in most patients admitted for AHF and independently related to mortality and subsequent admission for AHF [97]. Therefore, CA-125 levels appear reduced during a aggressive decongestive therapy, for this reason could be of potential interest to monitor the reduction of liquid in the sierose membranes during acute treatment [98].
Plasma osmolality
Plasma osmolality is an indirect marker of intravascular liquid overload and it is directly related to sodium, blood urea nitrogen (BUN), and glucose. It has been suggested that low discharge serum osmolality was independently predictive of postdischarge mortality and readmission [99]. In the EVEREST trial, plasma osmolality was reduced in the active arm, whereas in the placebo group, it tends to increase throughout hospitalization. However, this effect on osmolality declined in the early post-discharge period; its reduction was associated with improvement of congestion signs [100] Whether vasopressin (AVP) release in response to both osmotic and nonosmotic stimuli and its consequences on water fluctuation has a clinical impact in HF must be investigated by cross-sectional researches.
All together, these biomarkers assay associated with careful clinical and echo examination could provide a better idea of extravascular and intravascular fluid retention, organ congestion much more involved and hemodynamic status. This method could help physicians in the decision making and better individualized strategy in relation to the congestion scenario.
Conclusions
Congestion is the main feature of acute decompensated HF and it is often anticipated by several signs behind the traditional dyspnea. By a combined clinical laboratory and imaging examinations, we are now able to identify cardiac and systemic congestion through an integrated analysis [101, 102]. Unfortunately, by now, a wide range of criteria conduced to a lack of uniformity in the decision making. Thus, an unmet need of criteria standardization appears mandatory both in terms of adopted parameters as in terms of timing course evaluation. Indeed, persistence of sub-clinical congestion quantified by combined ultrasound and NP assessment might help in guiding treatment, facilitate personalized therapy, and avoid future events. In conclusion, it could be time to take a step back and give much more importance to the congestion typology and fluid accumulation occurrence that could differ in the different HF scenario. A universal acknowledgement of clinical and cardiac congestion, vascular redistribution, and intravascular fluid overload appears mandatory to better stratify our patients. Current topics will become an increasingly significant area of interest and a challenge for future research.
References
Gheorghiade M, Filippatos G, De Luca I, Burnett J (2006) Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med 119:S3–S10
O’Connor CM, Stough WG, Gallup DS, Husselblad V, Gheorghiade M (2005) Demographic clinical characteristics and outcome of patients hospitalized for decompensated heart failure: observation from the IMPACT-HF registry. J Card Fail 11:200–205
Pang P, Cleland JG, Teerlink JR, Collins SP, Lindsell CJ, Sopko G, Peacock WF, Fonarow GC, Aldeen AZ, Kirk JD, Storrow AB, Tavares M, Mebazaa A, Roland E, Massie BM, Maisel AS, Komajda M, Filippatos G, Gheorghiade M, Acute Heart Failure Syndromes International Working Group (2008) A proposal to standardize dyspnoea measurement in clinical trials of acute heart failure syndromes: the need for a uniform approach. Eur Heart J 29:816–824
Mentz RJ, Kjeldsen K, Rossi GP, Voors AA, Cleland JG, Anker SD, Gheorghiade M, Fiuzat M, Rossignol P, Zannad F, Pitt B, O'Connor C, Felker GM (2014) Decongestion in acute heart failure. Eur J Heart Fail 16:471–482. https://doi.org/10.1002/ejhf.74
Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G (2010) Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 12:423–433. https://doi.org/10.1093/eurjhf/hfq045
Danziger J, Chen K, Cavender S, Lee J, Feng M, Mark RG, Mukamal KJ, Celi LA (2016) Admission peripheral edema, central venous pressure, and survival in critically ill patients. Ann Am Thorac Soc 13:705–711. https://doi.org/10.1513/AnnalsATS.201511-737OC
Gheorghiade M, Gattis WA, O'Connor CM, Adams KF Jr, Elkayam U, Barbagelata A, Ghali JK, Benza RL, McGrew FA, Klapholz M, Ouyang J, Orlandi C, Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Congestive Heart Failure (ACTIV in CHF) Investigators (2004) Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA 291:1963–1971
Metra M, O'Connor CM, Davison BA, Cleland JG, Ponikowski P, Teerlink JR, Voors AA, Givertz MM, Mansoor GA, Bloomfield DM, Jia G, DeLucca P, Massie B, Dittrich H, Cotter G (2011) Early dyspnoea relief in acute heart failure: prevalence, association with mortality, and effect of rolofylline in the PROTECT study. Eur Heart J 32:1519–1534. https://doi.org/10.1093/eurheartj/ehr042
Ambrosy AP, Vaduganathan M, Huffman MD, Khan S, Kwasny MJ, Fought AJ, Maggioni AP, Swedberg K, Konstam MA, Zannad F, Gheorghiade M (2012) Clinical course and predictive value of liver function tests in patients hospitalized for worsening heart failure with reduced ejection fraction: an analysis of the EVEREST trial. Eur J Heart Fail 14:302–311. https://doi.org/10.1093/eurjhf/hfs007
Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Leiro MC, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors A, Nielsen OW, Zannad F, Tavazzi L (2010) EURObservational research Programme: the heart failure pilot survey (ESC-HF pilot). Eur J Heart Fail 12:1076–1084. https://doi.org/10.1093/eurjhf/hfq154
Rubio-Gracia J, Demissei BG, Ter Maaten JM, Cleland JG, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison BA, Givertz MM, Bloomfield DM, Dittrich H, Damman K, Pérez-Calvo JI, Voors AA (2018) Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int J Cardiol 258:185–191. https://doi.org/10.1016/j.ijcard.2018.01.067
Lala A, McNulty SE, Mentz RJ, Dunlay SM, Vader JM, AbouEzzeddine OF, DeVore AD, Khazanie P, Redfield MM, Goldsmith SR, Bart BA, Anstrom KJ, Felker GM, Hernandez AF, Stevenson LW (2015) Relief and Recurrence of Congestion During and After Hospitalization for Acute Heart Failure: Insights From Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ Heart Fail 8:741–748. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001957
Galas A, Krzesiński P, Gielerak G, Piechota W, Uziębło-Życzkowska B, Stańczyk A, Piotrowicz K, Banak M (2018) Complex assessment of patients with decompensated heart failure: the clinical value of impedance cardiography and N-terminal pro-brain natriuretic peptide. Heart Lung S0147-9563(18):30290–30295. https://doi.org/10.1016/j.hrtlng.2018.10.004
Öhman J, Harjola VP, Karjalainen P, Lassus J (2018) Focused echocardiography and lung ultrasound protocol for guiding treatment in acute heart failure. ESC Heart Fail 5:120–128. https://doi.org/10.1002/ehf2.12208
Palazzuoli A, Ruocco G, Beltrami M, Nuti R, Cleland JG (2018) Combined use of lung ultrasound, B-type natriuretic peptide, and echocardiography for outcome prediction in patients with acute HFrEF and HFpEF. Clin Res Cardiol 107:586–596. https://doi.org/10.1007/s00392-018-1221-7
Girerd N, Seronde MF, Coiro S, Chouihed T, Bilbault P, Braun F, Kenizou D, Maillier B, Nazeyrollas P, Roul G, Fillieux L, Abraham WT, Januzzi J Jr, Sebbag L, Zannad F, Mebazaa A, Rossignol P (2018) Integrative assessment of congestion in heart failure throughout the patient journey. JACC Heart Fail 6:273–285. https://doi.org/10.1016/j.jchf.2017.09.023
Thibodeau JT, Drazner MH (2018) The role of clinical examination in patients with heart failure. JACC Heart Fail 6:543–551. https://doi.org/10.1016/j.jchf.2018.04.005
Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, Testani JM, Tang WHW, Orso F, Rossignol P, Metra M, Filippatos G, Seferovic PM, Ruschitzka F, Coats AJ (2019) The use of diuretics in heart failure with congestion - a position statement from the heart failure Association of the European Society of cardiology. Eur J Heart Fail 21:137–155. https://doi.org/10.1002/ejhf.1369
Kelder JC, Cramer MJ, van Wijngaarden J, van Tooren R, Mosterd A, Moons KG, Lammers JW, Cowie MR, Grobbee DE, Hoes AW (2011) The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation 124:2865–2873. https://doi.org/10.1161/CIRCULATIONAHA.111.019216
Mahdyoon H, Klein R, Eyler W, Lakier JB, Chakko SC, Gheorghiade M (1989) Radiographic pulmonary congestion in end-stage congestive heart failure. Am J Cardiol 63:625–627
Collins SP, Lindsell CJ, Storrow AB, Abraham WT, ADHERE Scientific Advisory Committee, Investigators and Study Group (2006) Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann Emerg Med 47:13–18
Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW (2005) Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 294:1625–1633
Ambrosy AP, Cerbin LP, Armstrong PW, Butler J, Coles A, DeVore AD, Dunlap ME, Ezekowitz JA, Felker GM, Fudim M, Greene SJ, Hernandez AF, O'Connor CM, Schulte P, Starling RC, Teerlink JR, Voors AA, Mentz R (2017) Body weight change during and after hospitalization for acute heart failure: patient characteristics, Markers of Congestion, and Outcomes: Findings From the ASCEND-HF Trial. JACC Heart Fail 5:1–13. https://doi.org/10.1016/j.jchf.2016.09.012
Harjola VP, Mullens W, Banaszewski M, Bauersachs J, Brunner-La Rocca HP, Chioncel O, Collins SP, Doehner W, Filippatos GS, Flammer AJ, Fuhrmann V, Lainscak M, Lassus J, Legrand M, Masip J, Mueller C, Papp Z, Parissis J, Platz E, Rudiger A, Ruschitzka F, Schäfer A, Seferovic PM, Skouri H, Yilmaz MB, Mebazaa A (2017) Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the acute heart failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 19:821–836. https://doi.org/10.1002/ejhf.872
Husain-Syed F, McCullough PA, Birk HW, Renker M, Brocca A, Seeger W, Ronco C (2015)Cardio-pulmonary-renal interactions: a multidisciplinary approach. J Am Coll Cardiol 65:2433–2448. https://doi.org/10.1016/j.jacc.2015.04.024
Filippatos G, Zannad F (2007) An introduction to acute heart failure syndromes: definition and classification. Heart Fail Rev 12:87–90
Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo-Leiro MG, Laroche C, Seferovic PM, Anker SD, Ferrari R, Ruschitzka F, Lopez-Fernandez S, Miani D, Filippatos G, Maggioni AP (2017) Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC heart failure long-term registry. Eur J Heart Fail 19:1242–1254. https://doi.org/10.1002/ejhf.890
Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy CW, Young JB (2008) Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med 168:847–854. https://doi.org/10.1001/archinte.168.8.847
Sweitzer NK, Lopatin M, Yancy CW, Mills RM, Stevenson LW (2008) Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (> or =55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced (<40%) fractions. Am J Cardiol 101:1151–1156. https://doi.org/10.1016/j.amjcard.2007.12.014
Ambrosy AP, Bhatt AS, Gallup D, Anstrom KJ, Butler J, DeVore AD, Felker GM, Fudim M, Greene SJ, Hernandez AF, Kelly JP, Samsky MD, Mentz RJ (2017) Trajectory of Congestion Metrics by Ejection Fraction in Patients With Acute Heart Failure (from the Heart Failure Network). Am J Cardiol 120:98–105. https://doi.org/10.1016/j.amjcard.2017.03.249
Van Aelst LNL, Arrigo M, Placido R, Akiyama E, Girerd N, Zannad F, Manivet P, Rossignol P, Badoz M, Sadoune M, Launay JM, Gayat E, Lam CSP, Cohen-Solal A, Mebazaa A, Seronde MF (2018) Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur J Heart Fail 20:738–747. https://doi.org/10.1002/ejhf.1050
De Luca L, Fonarow GC, Adams KF Jr, Mebazaa A, Tavazzi L, Swedberg K, Gheorghiade M (2007) Acute heart failure syndromes: clinical scenarios and pathophysiologic targets for therapy. Heart Fail Rev 12:97–104
Horiuchi Y, Tanimoto S, Latif AHMM, Urayama KY, Aoki J, Yahagi K, Okuno T, Sato Y, Tanaka T, Koseki K, Komiyama K, Nakajima H, Hara K, Tanabe K (2018) Identifying novel phenotypes of acute heart failure using cluster analysis of clinical variables. Int J Cardiol 262:57–63. https://doi.org/10.1016/j.ijcard.2018.03.098
Dwyer KH, Merz AA, Lewis EF, Claggett BL, Crousillat DR, Lau ES, Silverman MB, Peck J, Rivero J, Cheng S, Platz E (2018) Pulmonary congestion by lung ultrasound in ambulatory patients with heart failure with reduced or preserved ejection fraction and hypertension. J Card Fail 24:219–226. https://doi.org/10.1016/j.cardfail.2018.02.004
Tromp J, Khan MAF, Mentz RJ, O'Connor CM, Metra M, Dittrich HC, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JGF, Givertz MM, Bloomfield DM, Van Veldhuisen DJ, Hillege HL, Voors AA, van der Meer P (2017) Biomarker profiles of acute heart failure patients with a mid-range ejection fraction. JACC Heart Fail 5:507–517. https://doi.org/10.1016/j.jchf.2017.04.007
Tromp J, Westenbrink BD, Ouwerkerk W, van Veldhuisen DJ, Samani NJ, Ponikowski P, Metra M, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Lang CC, Ng LL, Zannad F, Zwinderman AH, Hillege HL, van der Meer P, Voors AA (2018) Identifying pathophysiological mechanisms in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 72:1081–1090. https://doi.org/10.1016/j.jacc.2018.06.050
Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M, Butler J (2017) Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol 69:556–569. https://doi.org/10.1016/j.jacc.2016.10.078
Balmain S, Padmanabhan N, Ferrell WR, Morton JJ, McMurray JJ (2007) Differences in arterial compliance, microvascular function and venous capacitance between patients with heart failure and either preserved or reduced left ventricular systolic function. Eur J Heart Fail 9:865–871
Teng TK, Tay WT, Dahlstrom U, Benson L, Lam CSP, Lund LH (2018) Different relationships between pulse pressure and mortality in heart failure with reduced, mid-range and preserved ejection fraction. Int J Cardiol 254:203–209. https://doi.org/10.1016/j.ijcard.2017.09.187
Obokata M, Nagata Y, Kado Y, Kurabayashi M, Otsuji Y, Takeuchi M (2017)Ventricular-arterial coupling and exercise-induced pulmonary hypertension during low-level exercise in heart failure with preserved or reduced ejection fraction. J Card Fail 23:216–220. https://doi.org/10.1016/j.cardfail.2016.10.001
Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M, Butler J (2017) Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol 69:556–569. https://doi.org/10.1016/j.jacc.2016.10.078
Lai YC, Wang L, Gladwin MT (2019) Mechanisms leading to pulmonary vascular remodelling in HFpEF, a summary of pre-clinical models of HFpEF and PH-HFpEF. J Physiol 597:1143–1156. https://doi.org/10.1113/JP275858
Adir Y, Guazzi M, Offer A, Temporelli PL, Cannito A, Ghio S (2017) Pulmonary hemodynamics in heart failure patients with reduced or preserved ejection fraction and pulmonary hypertension: similarities and disparities. Am Heart J 192:120–127. https://doi.org/10.1016/j.ahj.2017.06.006
Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E, Temporelli PL, Rossi A, Faggiano P, Traversi E, Vriz O, Dini FL (2017) Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail 19:873–879. https://doi.org/10.1002/ejhf.664
Bistola V, Polyzogopoulou E, Ikonomidis I, Parissis J (2018) Congestion in acute heart failure with reduced vs. preserved left ventricular ejection fraction: differences, similarities and remaining gaps. Eur J Heart Fail 20:748–750. https://doi.org/10.1002/ejhf.1115
Cotter G, Felker MG, Kirkwood AF, Milo-Cotter O, O’Connor CM (2008) The pathophysiology of acute heart failure. It is all about fluid accumulation? Am Heart J 155:9–18
Kemp CD, Conte JV (2012) The pathophysiology of heart failure. Cardiovasc Pathol 21:365–371. https://doi.org/10.1016/j.carpath.2011.11.007
Vagnarelli F, Corsini A, Lorenzini M, Pacini D, Ferlito M, Bacchi Reggiani L, Longhi S, Nanni S, Norscini G, Cinti L, Bugani G, Pasquale F, Biagini E, Grigioni F, Di Bartolomeo R, Marini M, Perna GP, Melandri G, Rapezzi C (2015) Acute heart failure in patients with acute aortic syndrome: pathophysiology and clinical-prognostic implications. Eur J Heart Fail 17:917–924. https://doi.org/10.1002/ejhf.325
Mentz RJ, O'Connor CM (2016) Pathophysiology and clinical evaluation of acute heart failure. Nat Rev Cardiol 13:28–35. https://doi.org/10.1038/nrcardio.2015.134
Miller WR (2016) Fluid volume overload and congestion in heart failure time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail 9:e002922. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002922
Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S, Torbicki A, Voelkel NF, Hassoun PM (2013) Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 62:D22–D33
Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA (2014) Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 35:3452–3462
Dixon DD, Trivedi A, Shah SJ (2016) Combined post- and pre-capillary pulmonary hypertension in heart failure with preserved ejection fraction. Heart Fail Rev 21:285–297. https://doi.org/10.1007/s10741-015-9523-6
Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB, National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure (2006) Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 114:1883–1891
Brewster UC, Setaro JF, Perazella MA (2003) The renin angiotensin-aldosterone system: cardiorenal effects and implications for renal and cardiovascular disease states. Am J Med Sci 326:15–24
Sinkeler SJ, Damman K, van Veldhuisen DJ, Hillege H, Navis G (2012) A reappraisal of volume status and renal function impairment in chronic heart failure: combined effect of pre renal failure and venous congestion on renal function. Heart Fail Rev 17:263–270
Palazzuoli A, Ruocco G (2018)Heart-kidney interactions in Cardiorenal syndrome type 1. Adv Chronic Kidney Dis 25:408–417. https://doi.org/10.1053/j.ackd.2018.08.013
Birch DJ, Turmaine M, Boulos PB, Burnstock G (2008) Sympathetic innervation of human mesenteric artery and vein. J Vasc Res 45:323–332
Fallick C, Sobotka PA, Dunlap ME (2011) Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail 4:669–675. https://doi.org/10.1161/CIRCHEARTFAILURE.111.961789
Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA (2008) Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol 51:1268–1274
Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, Piovanelli B, Carubelli V, Bugatti S, Lombardi C, Cotter G, Dei Cas L (2012) Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail 5:54–62
Miller WL, Mullan BP (2014) Understanding the heterogeneity in volume overload and fluid distribution in decompensated heart failure is key to optimal volume management: role for blood volume quantitation. JACC Heart Fail 2:298–305
Starling EH (1896) On the absorption of fluids from the connective tissue spaces. J Physiol 19:312–326. https://doi.org/10.1113/jphysiol.1896.sp000596
Anand IS, Ferrari R, Kalra GS, Wahi PL, Poole-Wilson PA, Harris PC (1989) Edema of cardiac origin. Studies of body water and sodium, renal function, hemodynamic indexes, and plasma hormones in untreated congestive cardiac failure. Circulation. 80:299–305. https://doi.org/10.1161/01.CIR.80.2.299
Nijst P, Verbrugge FH, Grieten L, Dupont M, Steels P, Tang WHW, Mullens W (2015) The pathophysiological role of interstitial sodium in heart failure. J Am Coll Cardiol 65:378–388. https://doi.org/10.1016/j.jacc.2014.11.025
Mullens W, Verbrugge FH, Nijst P, Tang WHW (2017) Renal sodium avidity in heart failure: from pathophysiology to treatment strategies. Eur Heart J 38:1872–1882. https://doi.org/10.1093/eurheartj/ehx035
Tarbell JM, Pahakis MY (2006) Mechano transduction and the glycocalyx. J Intern Med 259:339–350
Oberleithner H, Peters W, Kusche-Vihrog K, Korte S, Schillers H, Kliche K, Oberleithner K (2011) Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Arch 462:519–528
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16(2015):233–270
Mercurio V, Palazzuoli A, Correale M, Lombardi C, Passantino A, Ravera A, Ruocco G, Sciatti E, Triggiani M, Lagioia R, Scrutinio D, Tocchetti CG, Nodari S (2018) Right heart dysfunction: from pathophysiologic insights to therapeutic options: a translational overview. J Cardiovasc Med (Hagerstown) 19:613–623. https://doi.org/10.2459/JCM.0000000000000700
Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ et al (2013) Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 62(25 Suppl):D22–D33
Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ (2003) A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol 41(6):1021–1027
Gargani L, Frassi F, Soldati G, Tesorio P, Gheorghiade M, Picano E (2008) Ultrasound lung comets for the differential diagnosis of acute cardiogenic dyspnoea: a comparison with natriuretic peptides. Eur J Heart Fail 10:70–77
Platz E, Lewis EF, Uno H, Peck J, Pivetta E, Merz AA, Hempel D, Wilson C, Frasure SE, Jhund PS, Cheng S, Solomon SD (2016) Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur Heart J 37:1244–1251
Cogliati C, Casazza G, Ceriani E, Torzillo D, Furlotti S, Bossi I, Vago T, Costantino G, Montano N (2016) Lung ultrasound and short-term prognosis in heart failure patients. Int J Cardiol 218:104–108
Coiro S, Rossignol P, Ambrosio G, Carluccio E, Alunni G, Murrone A, Tritto I, Zannad F, Girerd N (2015) Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur J Heart Fail 17:1172–1181
Jambrik Z, Monti S, Coppola V, Agricola E, Mottola G, Miniati M, Picano E (2004) Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol 93:1265–1270
Mueller T, Gegenhuber A, Poelz W, Haltmayer M (2005) Diagnostic accuracy of B type natriuretic peptide and amino terminal proBNP in the emergency diagnosis of heart failure. Heart. 91:606–612
Palazzuoli A, Beltrami M, Ruocco G, Franci B, Campagna MS, Nuti R (2016) Diagnostic utility of contemporary echo and BNP assessment in patients with acute heart failure during early hospitalization. Eur J Intern Med 30:43–48. https://doi.org/10.1016/j.ejim.2015.11.031
Chen AA, Wood MJ, Krauser DG, Baggish AL, Tung R, Anwaruddin S, Ianuzzi J (2006)NT-proBNP levels, echocardiographic findings, and outcomes in breathless patients: results from the ProBNP investigation of Dyspnoea in the emergency department (PRIDE) echocardiographic substudy. Eur Heart J 27:839–845
Kociol RD, Horton JR, Fonarow GC, Reyes EM, Shaw LK, O'Connor CM, Felker GM, Hernandez AF (2011) Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail 4:628–636. https://doi.org/10.1161/CIRCHEARTFAILURE.111.962290
Savarese G, Musella F, D'Amore C, Vassallo E, Losco T, Gambardella F, Cecere M, Petraglia L, Pagano G, Fimiani L, Rengo G, Leosco D, Trimarco B, Perrone-Filardi P (2014) Changes of natriuretic peptides predict hospital admissions in patients with chronic heart failure: a meta-analysis. JACC Heart Fail 2:148–158. https://doi.org/10.1016/j.jchf.2013.11.007
Kato TS, Collado E, Khawaja T, Kawano Y, Kim M, Farr M, Mancini DM, Schulze PC (2013) Value of peak exercise oxygen consumption combined with B-type natriuretic peptide levels for optimal timing of cardiac transplantation. Circ Heart Fail 6:6–14. https://doi.org/10.1161/CIRCHEARTFAILURE.112.968123
Sanders-van Wijk S, Maeder MT, Nietlispach F, Rickli H, Estlinbaum W, Erne P, Rickenbacher P, Peter M, Pfisterer MP, Brunner-La Rocca HP (2014)Long-term results of intensified, N-terminal-pro-B-type natriuretic peptide-guided versus symptom-guided treatment in elderly patients with heart failure: five-year follow-up from TIME-CHF. Circ Heart Fail 7:131–139. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000527
Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston-Miller N, Januzzi JL Jr, Mark DB, Piña IL, Passmore G, Whellan DJ, Yang H, Cooper LS, Leifer ES, Desvigne-Nickens P, O'Connor CM (2017) Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 318:713–720. https://doi.org/10.1001/jama.2017.10565
Kubánek M, Goode KM, Lánská V, Clark AL, Cleland JG (2009) The prognostic value of repeated measurement of N-terminalpro-B-type natriuretic peptide in patients with chronic heart failure due to left ventricular systolic dysfunction. Eur J Heart Fail 11:367–377
Gaggin HK, Truong QA, Rehman SU, Mohammed AA, Bhardwaj A, Parks KA, Sullivan DA, Chen-Tournoux A, Moore SA, Richards AM, Troughton RW, Lainchbury JG, Weiner RB, Baggish AL, Semigran MJ, Januzzi JL (2013) Characterization and prediction of natriuretic peptide "nonresponse" during heart failure management: results from the ProBNP Outpatient Tailored Chronic Heart Failure (PROTECT) and the NT-proBNP-Assisted Treatment to Lessen Serial Cardiac Readmissions and Death (BATTLESCARRED) study. Congest Heart Fail 19:135–142
Kremer D, Ter Maaten JM, Voors AA (2018)Bio-adrenomedullin as a potential quick, reliable, and objective marker of congestion in heart failure. Eur J Heart Fail 20:1363–1365. https://doi.org/10.1002/ejhf.1245
Morbach C, Marx A, Kaspar M, Güder G, Brenner S, Feldmann C, Störk S, Vollert JO, Ertl G, Angermann CE (2017) Prognostic potential of midregional pro-adrenomedullin following decompensation for systolic heart failure: comparison with cardiac natriuretic peptides. Eur J Heart Fail 19:1166–1175. https://doi.org/10.1002/ejhf.859
Matson BC, Corty RW, Karpinich NO, Murtha AP, Valdar W, Grotegut CA, Caron KM (2014) Midregional pro-adrenomedullin plasma concentrations are blunted in severe preeclampsia. Placenta. 35:780–783. https://doi.org/10.1016/j.placenta.2014.07.003
Möckel M, Searle J, Hartmann O, Anker SD, Peacock WF, Wu AH, Maisel A, BACH Writing group (2013)Mid-regional pro-adrenomedullin improves disposition strategies for patients with acute dyspnoea: results from the BACH trial. Emerg Med J 30:633–637. https://doi.org/10.1136/emermed-2012-201530
Greene SJ, Gheorghiade M, Vaduganathan M, Ambrosy AP, Mentz RJ, Subacius H, Maggioni AP, Nodari S, Konstam MA, Butler J, Filippatos G (2013) Haemoconcentration, renal function, and post-discharge outcomes among patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Eur J Heart Fail 15:1401–1411. https://doi.org/10.1093/eurjhf/hft110
van der Meer P, Postmus D, Ponikowski P, Cleland JG, O'Connor CM, Cotter G, Metra M, Davison BA, Givertz MM, Mansoor GA, Teerlink JR, Massie BM, Hillege HL, Voors AA (2013) The predictive value of short-term changes in hemoglobin concentration in patients presenting with acute decompensated heart failure. J Am Coll Cardiol 61:1973–1981. https://doi.org/10.1016/j.jacc.2012.12.050
Breidthardt T, Weidmann ZM, Twerenbold R, Gantenbein C, Stallone F, Rentsch K, Rubini Gimenez M, Kozhuharov N, Sabti Z, Breitenbücher D, Wildi K, Puelacher C, Honegger U, Wagener M, Schumacher C, Hillinger P, Osswald S, Mueller C (2017) Impact of haemoconcentration during acute heart failure therapy on mortality and its relationship with worsening renal function. Eur J Heart Fail 19:226–236. https://doi.org/10.1002/ejhf.667
D'Aloia A, Vizzardi E, Metra M (2016) Can carbohydrate Antigen-125 be a new biomarker to Guide heart failure treatment?: the CHANCE-HF trial. JACC Heart Fail 4:844–846. https://doi.org/10.1016/j.jchf.2016.09.001
Núñez J, Núñez E, Bayés-Genís A, Fonarow GC, Miñana G, Bodí V, Pascual-Figal D, Santas E, Garcia-Blas S, Chorro FJ, Rizopoulos D, Sanchis J (2017)Long-term serial kinetics of N-terminal pro B-type natriuretic peptide and carbohydrate antigen 125 for mortality risk prediction following acute heart failure. Eur Heart J Acute Cardiovasc Care 6:685–696. https://doi.org/10.1177/2048872616649757
Huang F, Zhang K, Chen J, Cai Q, Liu X, Wang T, Lv Z, Wang J, Huang H (2013) Elevation of carbohydrate antigen 125 in chronic heart failure may be caused by mechanical extension of mesothelial cells from serous cavity effusion. Clin Biochem 46:1694–1700. https://doi.org/10.1016/j.clinbiochem.2013.09.008
Núñez J, Miñana G, Núñez E, Chorro FJ, Bodí V, Sanchis J (2014) Clinical utility of antigen carbohydrate 125 in heart failure. Heart Fail Rev 19:575–584. https://doi.org/10.1007/s10741-013-9402-y
Kaya H, Yücel O, Ege MR, Zorlu A, Yücel H, Güneş H, Ekmekçi A, Yılmaz MB (2017) Plasma osmolality predicts mortality in patients with heart failure with reduced ejection fraction. Kardiol Pol 75:316–322. https://doi.org/10.5603/KP.a2016.0168
Vaduganathan M, Goldsmith SR, Senni M, Butler J, Gheorghiade M (2016) Contrasting acute and chronic effects of tolvaptan on serum osmolality in the EVEREST trial. Eur J Heart Fail 18:185–191. https://doi.org/10.1002/ejhf.415
Pellicori P, Shah P, Cuthbert J, Urbinati A, Zhang J, Kallvikbacka-Bennett A, Clark AL, Cleland JGF (2019) Prevalence, pattern and clinical relevance of ultrasound indices of congestion in outpatients with heart failure. Eur J Heart Fail. https://doi.org/10.1002/ejhf.1383
Aimo A, Vergaro G, Giannoni A, Emdin M (2018) Wet is bad: residual congestion predicts worse prognosis in acute heart failure. Int J Cardiol 258:201–202. https://doi.org/10.1016/j.ijcard.2018.02.018
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Palazzuoli, A., Evangelista, I. & Nuti, R. Congestion occurrence and evaluation in acute heart failure scenario: time to reconsider different pathways of volume overload. Heart Fail Rev 25, 119–131 (2020). https://doi.org/10.1007/s10741-019-09868-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-019-09868-0