Abstract
Over 2.5 million patients in the USA suffer from heart failure with preserved ejection fraction (HFpEF), and pulmonary hypertension (PH) is present in the majority of these patients. PH represents an adverse prognostic factor in HFpEF and has been identified as a potential therapeutic target to improve symptoms and outcomes. The recognition and investigation of a subset of patients with superimposed pulmonary vascular disease (on top of pulmonary venous hypertension) has led to further subclassification of PH due to left heart disease (PH-LHD) into two categories: isolated post-capillary PH and combined post- and pre-capillary PH (CpcPH). In this review, we (1) describe the evolution of the diagnostic criteria of PH-LHD; (2) identify the diagnostic modalities that can be utilized for the identification of patients with CpcPH-HFpEF; (3) review the literature on the prevalence, clinical characteristics, and prognostic factors of CpcPH-HFpEF; (4) discuss recent and ongoing clinical trials investigating the effectiveness of selective pulmonary vasodilators in PH-LHD; and (5) propose future areas for further investigation of the etiology and pathophysiological mechanisms contributing to the development of CpcPH and highlight important considerations in the design of future trials to promote better characterization of this clinical entity. CpcPH-HFpEF is a distinct subset within HFpEF and one that may respond to targeted therapeutics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over 650,000 new cases of heart failure (HF) are diagnosed each year, and the prevalence of HF is estimated to be 5.1 million in the USA [1, 2]. HF with preserved ejection fraction (HFpEF) represents 50 % of all HF cases [3]. Patients with HFpEF have been characterized as older, more often female, and obese with higher rates of hypertension and atrial fibrillation in comparison with patients with HF with reduced ejection fraction (HFrEF) [4]. As the population ages and the obesity and diabetes epidemics advance, HFpEF will become increasingly common. Paired with the sobering fact that effective evidence-based treatments for HFpEF have not yet been clearly identified, HFpEF represents a major therapeutic challenge and public health burden.
Pulmonary hypertension (PH) is a highly prevalent comorbidity in patients with HFpEF, a finding that has been demonstrated in several different patient populations and clinical settings. Non-invasively, in the first community-based investigation of PH in HFpEF (PH-HFpEF), Lam et al. [5] found a PH prevalence of 83 % as evidenced by elevated pulmonary artery systolic pressure (PASP > 40 mmHg) on echocardiography. Invasively, Gerges et al. [6] reported a 54.4 % prevalence of PH (defined as mean pulmonary artery pressure >25 mmHg) among patients with HFpEF undergoing initial diagnostic right heart catheterizations at a tertiary referral center in Vienna, Austria.
Given its prevalence, it is of particular importance that PH is associated with worse outcomes in HF. In a substudy of the EchoCardiography and Heart Outcome Study (ECHOS), elevated right ventricular systolic pressure on echocardiography was an independent predictor of both short- and long-term mortality in HF patients with reduced or preserved ejection fraction followed for a mean of 2.8 years [7]. Similarly, in a community-based HFpEF population with a median PASP of 48 mmHg on echocardiography, patients with PASP ≥ 48 mmHg had significantly worse survival compared to those below the median value. Elevated PASP was found to be a strong adverse prognostic factor on unadjusted and adjusted analyses [5]. Increased rates of hospitalization have also been noted in patients with PH-HFpEF [8].
With regard to management of HFpEF, clinical trials investigating the efficacy of beta blockers [9], angiotensin-converting-enzyme inhibitors [10], and angiotensin receptor blockers [11, 12] in HFpEF were found to be negative despite their success in HFrEF. Future treatment of HFpEF may benefit from targeted therapy of pathophysiological abnormalities in these patients instead of a “one-size-fits-all approach” [13]. While PH is common in HFpEF, likely due to passive venous congestion in the setting of elevated left atrial pressure [14, 15], a smaller number of patients with PH-HFpEF have evidence of pulmonary vascular disease (i.e., combined post- and pre-capillary PH [CpcPH] [16]), and these patients specifically may benefit from therapies that improve pulmonary vascular function.
The purpose of this review is to discuss the types of PH associated with HFpEF with a specific focus on CpcPH, previously referred to as “reactive” or “out-of proportion” PH. The diagnostic criteria for CpcPH continue to evolve as new diagnostic modalities are investigated and validated. Targeted therapies based in part on the successful interventions in PAH have been proposed in CpcPH, and several trials are ongoing. Besides providing data on the efficacy of therapies for CpcPH, it is hoped that these ongoing and future clinical trials, along with clinical and epidemiological studies, will further inform the many questions that remain unanswered in the realm of CpcPH and HFpEF.
Classification of pulmonary hypertension in HFpEF
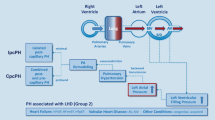
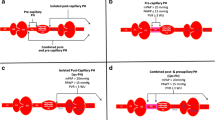
The World Health Organization (WHO) Group 2 PH category (PH due to left heart disease) is subclassified into 3 broad etiologies: left ventricular systolic dysfunction, left ventricular diastolic dysfunction, and left heart valvular dysfunction [17–19]. The predominant manifestation of the pathophysiological mechanisms underlying these three etiologies is elevated left-side filling pressure (i.e., left atrial pressure). Sustained elevations in left atrial pressure cause passive pulmonary venous congestion with elevation of pulmonary pressures [20]. This accurately describes the mechanism behind PH-HFpEF in the majority of patients. The post-capillary PH caused by elevated left atrial pressures has been defined in hemodynamic terms as a mean pulmonary artery pressure (mPAP) ≥25 mmHg, a pulmonary capillary wedge pressure (PCWP) >15 mmHg, and a transpulmonary gradient (TPG [=mPAP − PCWP]) <12 mmHg. In post-capillary PH, pulmonary vascular resistance (PVR [=TPG/cardiac output]) is typically <3.0 Wood units indicating the absence of pulmonary vascular remodeling or disease [19].
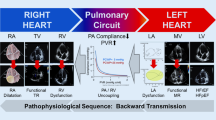
In WHO Group 2 PH, it is now well recognized that a subset of these patients appear to have evidence of intrinsic pulmonary vascular disease with elevations in pulmonary pressures that cannot be solely attributed to passive venous congestion. A histological study of the effects of chronic HF on the pulmonary vasculature (primarily in patients with HFrEF) demonstrated that sustained elevations in left-sided pressures resulted in pathologic changes of the pulmonary arteries characterized by medial hypertrophy, intimal fibrosis, and derangements of elastic fibers [21]. In some patients with WHO Group 2 PH, transmission of venous congestion to the pulmonary capillaries resulting in leakage and damage creates an obstructive vasculopathy such that higher pulmonary pressures are needed to sustain forward flow. Initially, vascular tone may increase, with adequate response to pulmonary vasodilators. However, over time, remodeling occurs, thereby reducing vasodilator responsiveness [17]. Initially, these alterations in pulmonary pressures, thought to reflect the development of a “pre-capillary” component of PH, were characterized diagnostically by a TPG > 12 mmHg and/or PVR > 3.0 Wood units [17, 19]. Recently, the utility of these measures to distinguish between post-capillary and post-capillary plus pre-capillary PH has been called into question.
In an invasive study of the prevalence of pulmonary vascular disease in HFpEF (n = 293), we found that while PH (defined as mPAP > 25 mmHg) is very common (>75 % prevalence), only 18 % had an elevated PVR [22]. On further inspection of these patients, however, it became clear that the high TPG component of the PVR was being driven by an elevated PASP and high PA pulse pressure. Thus, it appears that in elderly patients with HFpEF, a similar type of systolic hypertension occurs in both the systemic and pulmonary vasculatures. Based on anecdotal clinical experience, it is also apparent that the presence of intrinsic pulmonary vascular disease is quite uncommon in HFpEF. Indeed, in our study we found that the diastolic pulmonary gradient (DPG [=pulmonary artery diastolic pressure (PADP) − PCWP]), a sign of intrinsic pulmonary vascular disease, was only elevated (≥7 mmHg) in 7.5 % of patients with HFpEF.
In a retrospective study of patients with HFrEF, Gerges et al. [23] investigated the clinical relevance and prognostic significance of the DPG in patients with PH due to left heart disease. These authors demonstrated that in patients with mPAP ≥ 25 mmHg, PCWP > 15 mmHg, TPG > 12 mmHg, and DPG ≥ 7 mmHg, DPG was associated with more advanced pulmonary vascular remodeling and worse median survival compared to those with a DPG < 7 mmHg. Naeije et al. [24] highlight the fact that TPG is sensitive to pulsatile load and changes in cardiac output and describe several clinical scenarios in which TPG would be considered to be inappropriately elevated or inappropriately normal. These authors conclude that TPG may not accurately identify patients with a “pre-capillary” component and propose the use of the DPG as a better indicator of intrinsic pulmonary vascular disease in the setting of left heart disease. DPG is not sensitive to factors such as pulsatile load and cardiac output, and a DPG > 5 mmHg in the context of the clinical algorithm presented by Naeije and colleagues is superior to TPG for the diagnosis of post-capillary PH with a pre-capillary component [24].
Based on the sensitivity of TPG and PVR to flow and filling pressures, and an initial promising study on DPG, an expert consensus statement was developed to redefine PH due to left heart disease (PH-LHD). In this consensus statement by Vachiéry et al. [16], two types of PH-LHD were defined: isolated post-capillary PH (IpcPH [PCWP > 15 mmHg and DPG < 7 mmHg]) and combined post-and pre-capillary PH (CpcPH [PCWP > 15 mmHg and DPG ≥ 7 mmHg]). Based on these recommendations, terms such as “reactive” or “out of proportion” PH have become obsolete. Recognizing the paucity of evidence on DPG in this context, further investigation of the diagnostic and prognostic value of DPG was encouraged by the authors. As defined, CpcPH identifies a subset of patients with intrinsic pulmonary vascular disease, which, not unlike pulmonary arterial hypertension (PAH), may be responsive to selective pulmonary vasodilators.
It should be noted that the most recent 2015 European Society of Cardiology PH guidelines state that either DPG ≥ 7 mmHg or PVR > 3 Wood units can be used to diagnose CpcPH [25]. However, based on the reasons outlined above, we believe that DPG alone should be used for the diagnosis of CpcPH-HFpEF.
Epidemiology and clinical characteristics of CpcPH and HFpEF
The diastolic dysfunction that characterizes HFpEF and leads to elevated left-sided filling pressures can also result in the development of CpcPH in a subset of HFpEF patients. In a well-characterized prospective HFpEF cohort of 293 patients with invasive hemodynamic data, the prevalence of CpcPH was determined to be 7.5 % [22]. In this cohort, CpcPH was associated with male gender, worse New York Heart Association class, chronic obstructive pulmonary disease, elevated B-type natriuretic peptide, abnormal pulmonary function tests (including low diffusing capacity of carbon dioxide), multiple right ventricular (RV) abnormalities on echocardiography, and notching on pulse-wave Doppler of the RV outflow tract. A study of the epidemiology and predictors of CpcPH in both HFrEF and HFpEF demonstrated a similar prevalence of 12 % CpcPH in each group, respectively [6]. Independent predictors of CpcPH-HFpEF on multivariable analysis included age (≤77 years; Youden index 0.3; AUC of 0.65), valvular heart disease, and tricuspid annular plane systolic excursion/pulmonary artery systolic pressure (TAPSE/PASP) on echocardiography of ≤0.31 mm/mmHg (Youden index 0.5, AUC 0.81).
Although additional studies are needed to further characterize CpcPH-HFpEF and the way in which it differs from isolated pulmonary venous hypertension in HFpEF, these initial studies provide insight into relevant differences among these groups. Furthermore, it should be noted that while CpcPH appears to be relatively uncommon in HFpEF, the overall prevalence of CpcPH-HFpEF is likely to be much higher than WHO Group 1 PAH given the very high prevalence of HFpEF in the general population (>2.5 million in the USA alone).
Diagnostic modalities
Table 1 lists the prevalence and diagnostic criteria of the various forms of PH in the setting of HFpEF, with a comparison to WHO Group 1 PH. Emphasis on standardization and validation of diagnostic criteria will help to identify a more uniform patient population with CpcPH-HFpEF. HFpEF is a heterogeneous syndrome with multiple pathophysiological mechanisms that can contribute to its development [26]. The 2013 ACC/AHA guidelines for the management of HF now specifically define an ejection fraction of ≥50 % as the cut point for HFpEF [1]. This definition will help to reduce the variability in ejection fraction reported among different studies and clinical trials in patients with HFpEF. The consensus statement on PH-LHD also provides uniform terminology and diagnostic criteria for analysis going forward. Creating uniformity in terminology and definitions will help to focus efforts and results on identifying the true difference in pathological mechanisms underlying CpcPH-HFpEF from other forms of HFpEF and PAH. In the sections below, we describe several diagnostic modalities that help diagnose CpcPH-HFpEF.
Cardiac catheterization
The definition for CpcPH is based on invasive hemodynamic parameters determined by cardiac catheterization, which is the gold standard for the diagnosis of PH [16]. The choice to use DPG as a diagnostic parameter has certain limitations with regard to the potential for technical errors in the measurement of the PCWP and PADP. Studies have reported negative DPG values both recently [27] and historically [28] which physiologically should not occur at end-diastole and are the result of technical errors (errors either in the pressures themselves or in the interpretation of the waveforms). Measurement of the PADP can be sensitive to motion artifact depending on the catheter type used, and variations in the method (digitized, cycle averaged or end-expiratory) of measurement of the PCWP also produce errors [27, 29]. Current guidelines recommend the measurement of PCWP at end-expiration to standardize measurements and avoid underestimation due to changes in intrathoracic pressure, but most digitized measurement systems are unable to perform this task [30].
Exercise hemodynamics can also help differentiate CpcPH from those with isolated pulmonary venous hypertension. In patients with HFpEF who undergo right heart catheterization, we routinely perform supine bicycle exercise testing. As shown in Fig. 1, during exercise a patient with CpcPH-HFpEF will have a disproportionate rise in PA pressure compared to PCWP (i.e., PASP, PADP, and mPAP will all rise to a much steeper extent compared to PCWP), whereas patients with isolated HFpEF will have parallel rises in PA pressures and PCWP.
Exercise hemodynamics in heart failure with preserved ejection fraction: isolated pulmonary venous hypertension versus combined post- and pre-capillary pulmonary hypertension. Left panel an example of invasive hemodynamics during exercise in a patient with isolated PVH in the setting of HFpEF. Right panel an example of invasive hemodynamic during exercise in a patient with CpcPH in the setting of HFpEF. In the patient with isolated PVH, PA pressure and PCWP track together as cardiac output increases with exercise. In the patient with CpcPH, PA pressure rises more steeply compared with PCWP as cardiac output increases with exercise. The steeper slope of PA pressure rise compared with PCWP is indicative of intrinsic pulmonary vascular disease
Echocardiography
Comprehensive echocardiography, with Doppler and tissue Doppler imaging, is an important noninvasive tool that can provide several diagnostic clues for CpcPH and is optimal for screening purposes [17]. A PASP > 35 mmHg as detected by Doppler echocardiography can indicate the presence of PH. Increases in PASP occur with normal aging and with obesity; thus, these factors should be taken into account when interpreting the clinical relevance of elevated PASP on echocardiography [31]. Although echocardiography can be useful, there are certain limitations with regard to PASP that should be noted. Determination of PASP on echocardiography is dependent upon sufficient visualization of a TR jet. Some patients will lack a TR jet, and thus, clinical suspicion of PH will have to come from other sources. A study comparing the accuracy of echocardiography versus right heart catheterization for estimation of PASP in patients referred for suspected PH found that despite a moderately strong correlation between PASP on echocardiography and invasive hemodynamic testing, echocardiography-derived PASP was inaccurate in 50.6 % of cases with discrepancies of up to 10 mmHg in either direction [32]. Errors in echocardiographic estimation of PASP can have important diagnostic and management implications; thus, other forms of diagnostic testing should be used to corroborate the echocardiographic estimation of PASP. While right heart catheterization is considered the gold standard for diagnosis for measurement of hemodynamics, performing this test in all patients with HFpEF may not be feasible or cost-effective. Fortunately, the echocardiogram can provide ample additional clues about the diagnosis of pre- versus post-capillary PH in the patient with HFpEF.
The presence of pre-capillary pulmonary vascular disease can lead to derangements in RV function. Tricuspid annular plane systolic excursion (TAPSE), a marker of RV systolic function, has been shown to be an independent predictor of outcomes in patients with HF [33]. Given that PASP is also an important prognostic factor in HF, Guazzi et al. [34] hypothesized that the TAPSE/PASP ratio would be a marker for RV performance and provide enhanced prognostic capability. In their study of patients with HFpEF and HFrEF, these investigators demonstrated that a TAPSE/PASP ratio <0.36 mm/mmHg was associated with increased cardiac-related mortality in both groups. The authors concluded that TAPSE/PASP ratio improved prognostic resolution and may be a valuable index of the RV length/force relationship. In a subsequent study by Gerges et al. [6] that examined RV function in patients with HF and PH, these investigators demonstrated that the TAPSE/PASP ratio, validated against invasive hemodynamics, was a predictor of CpcPH-HFpEF on univariate and multivariate analyses. On receiver operating characteristic analysis, a TAPSE/PASP ratio ≤0.31 mm/mmHg was the ideal cutoff to identify CpcPH-HFpEF. Gerges et al. also demonstrated that RV–pulmonary vascular coupling was worse in patients with CpcPH, further highlighting the role of RV dysfunction as an adverse prognostic indicator. Thus, the use of the TAPSE/PASP ratio can aid in the identification of patients with CpcPH and provide prognostic information.
Right ventricular outflow tract (RVOT) notching patterns seen on pulse-wave Doppler echocardiography can assist in further characterization of pulmonary vascular disease and hemodynamic properties of PH. Arkles et al. [35] examined the echocardiographic RVOT notching patterns, invasive hemodynamics, and clinical data in 88 patients referred for PH and 32 patients with HFrEF and PH. Notching patterns can be classified into three categories (Fig. 2): no notching (defined as a continuous flow-velocity envelope); mid-systolic notching (defined as notching in the initial two-thirds of the flow-velocity envelope, with visualization of two distinct peaks); and late-systolic notching (defined as notching within the terminal third of the flow-velocity envelope, with visualization of two distinct peaks). The notching pattern is created by premature backward wave reflections of the systolic pulmonary arterial wave as blood leaves the right ventricle and enters the pulmonary vascular system [35]. Premature reflections are created when initial systolic flow from the RV encounters vascular obstruction (increased PVR, PA stiffening, or proximal obstruction); the deceleration of forward flow caused by the reflected wave creates a “notch.” The absence of notching was found to be highly prevalent in patients with isolated post-capillary PH. In contrast, mid-systolic notching was indicative of significant right ventricular dysfunction, increased PVR, and decreased pulmonary arterial compliance [35]. Thus, examination of the RVOT pulse-wave Doppler may provide additional information about the presence of pulmonary vascular disease and may assist with the diagnosis of CpcPH-HFpEF. Arkles et al. also recommend additional studies to determine whether RVOT notching can serve as a noninvasive predictor of response to pulmonary vasodilator therapy, which may be especially useful in patients with PH in the setting of HFpEF.
Examples of right ventricular outflow tract flow-velocity envelope notching profiles. Left panel normal RVOT flow-velocity envelope (pulse-wave Doppler tracing) in a patient with isolated pulmonary venous hypertension in HFpEF; Middle panel late-systolic notching in the RVOT flow-velocity envelope in a patient with mild-to-moderate CpcPH-HFpEF; Right panel mid-systolic notching in the RVOT flow-velocity envelope in a patient with severe CpcPH-HFpEF
Besides RVOT notching, there are several additional clues on echocardiography that help differentiate pulmonary venous hypertension (e.g., HFpEF) from PAH, as shown in Fig. 3. CpcPH-HFpEF falls on the spectrum between isolated pulmonary venous hypertension and pure PAH, as shown in Fig. 3. We have found both invasive hemodynamic testing and echocardiography to be complimentary tools that are essential in establishing the final diagnosis in cases of CpcPH-HFpEF.
Echocardiographic and invasive hemodynamic differentiation of pulmonary arterial hypertension, combined post- and pre-capillary pulmonary hypertension, and isolated pulmonary venous hypertension. Left panel prototypical echocardiographic and invasive hemodynamic findings from a patient with PAH. The RA and RV are severely enlarged, and the LV and LA are small and underfilled. The interatrial septum bows from right to left. On mitral inflow, E/A ratio <1 because of underfilling of the LA and decreased compliance of the LV due to extrinsic compression from the enlarged RV. The lateral e’ velocity and lateral E/e’ ratio are normal suggesting normal LV relaxation and filling pressures. There is notching in the RV outflow tract profile on pulse-wave Doppler imaging due to increased PA stiffness. PCWP is normal, and the PADP-PCWP gradient is severely increased. Right panel prototypical echocardiographic and invasive hemodynamic findings from a patient with isolated PVH in the setting of HFpEF. The LA is enlarged and the interatrial septum bows from left to right. On mitral inflow, E/A ratio >1, lateral e’ velocity is reduced, and lateral E/e’ ratio is increased, suggestive of grade 2 diastolic dysfunction with impaired LV relaxation and elevated LV filling pressures. There is no notching in the RV outflow tract profile. PCWP is elevated, and there is no gradient between the PADP and PCWP. Note that although the RV in the right panel is not enlarged, RV enlargement and dysfunction can be present in patients with isolated PVH. Middle panel parameters helpful for differentiating PAH from PVH on echocardiography and invasive hemodynamic testing, and invasive hemodynamic findings in a patient with CpcPH (elevated PCWP and PADP-PCWP gradient). It should be noted that the most challenging patients are in this middle zone (CpcPH-HFpEF), with echocardiographic findings that lie in the middle of the prototypical examples of PAH and PVH shown here. In these patients, careful evaluation of the echocardiogram and invasive hemodynamics will be necessary for an accurate diagnosis. CpcPH combined post- and pre-capillary pulmonary hypertension, E/A ratio of early to late (atrial) mitral inflow velocities, E/e’ ratio of early mitral inflow velocity to early diastolic mitral annular tissue velocity, CO cardiac output, HFpEF heart failure with preserved ejection fraction, LA left atrial, mPAP mean pulmonary arterial pressure, PADP pulmonary artery diastolic pressure, PAH pulmonary arterial hypertension, PCWP pulmonary capillary wedge pressure, PH pulmonary hypertension, PVH pulmonary venous hypertension, PVR pulmonary vascular resistance, RA right atrial, RV right ventricular, RVOT right ventricular outflow tract. Modified with permission from McLaughlin et al. [14] (Illustration by Sanjiv J. Shah, MD)
Treatment of CpcPH in patients with HFpEF
There are no current evidence-based guideline-recommended targeted therapies for CpcPH in the setting of HFpEF. Current management practices involve treatment of the underlying disease and comorbidities that are associated with CpcPH in an individual HFpEF patient. However, there are several ongoing investigations of targeted therapies for CpcPH with the hope that these medications will improve symptoms or outcomes in these patients.
The changes in the pulmonary vasculature attributed to the development of CpcPH are similar to changes seen in PAH. This has led to the hypothesis that selective pulmonary vasodilators may improve symptoms and outcomes in patients with CpcPH-HFpEF. To date, several clinical trials have been conducted to explore this hypothesis. Therapies postulated to be effective for CpcPH in the setting of HFpEF include prostacyclins, endothelial receptor antagonists, phosphodiesterase type 5 (PDE5) inhibitors, ranolazine, and soluble guanylate cyclase (sGC) agonists. Furthermore, several of these drugs have direct myocardial effects and therefore have been studied in the setting of generalized HFpEF or undifferentiated PH-HFpEF (without specifically requiring the presence of CpcPH).
Initial studies of prostacyclins and endothelial receptor antagonists in left heart disease focused on HFrEF, with results that ranged from detrimental to no effect [36–40]. A recent trial of riociguat, a soluble guanylate cyclase agonist, in patients with PH-HFrEF did not meet the primary outcome of a decrease in mPAP at 16 weeks [41]. However, riociguat was well tolerated and reductions in PVR and improvements in quality of life were noted in patients given the highest dose. SilHF, an ongoing study investigating sildenafil versus placebo in PH-HFrEF, examines the effect of PDE5 inhibition on the primary outcomes of patient global assessment and 6-min walk test [42].
Table 2 summarizes ongoing and completed studies of pulmonary vasodilators in HFpEF. In HFpEF, the nitric oxide (NO)–sGC–cyclic guanosine monophosphate (cGMP) pathway has been a major focus of investigation both in isolated post-capillary PH (i.e., pulmonary venous hypertension alone) and in CpcPH. The production of NO is important for maintaining endothelial cell quiescence and preventing endothelial dysfunction. Sildenafil, a PDE5 inhibitor that results in increased availability of cGMP, and riociguat, an agonist of sGC (which generates increased cGMP), have been the drugs investigated to modulate this pathway thus far (a next-generation sGC stimulator, vericiguat, is currently being studied in an ongoing Phase 2 clinical trial in HFpEF [43]).
Guazzi et al. [44] found that PDE5 inhibition improved active pulmonary vasodilation, RV and PA hemodynamics, LV and RV function, and quality of life in a 1-year study of sildenafil versus placebo in patients with HFpEF and PH (n = 44). The RELAX trial of sildenafil versus placebo in patients with HFpEF was a larger study (n = 216) that did not specifically require the presence of PH. In RELAX, 24 weeks of sildenafil therapy did not result in the primary outcome of improved peak oxygen consumption on cardiopulmonary exercise testing. No change in clinical status or exercise capacity was noted with sildenafil in RELAX. The patient populations selected for these two trials and overall differences in trial design are likely significant contributing factors to the differences in results. Importantly, the study by Guazzi and colleagues included patients with significant PH and the mean DPG in that study was in the 8–9 mmHg range; thus, patients with CpcPH may be more likely to respond to PDE5 inhibition.
DILATE-1, a double-blind, randomized, placebo-controlled trial investigated the effect of a single-dose of riociguat versus placebo on change in mPAP in HFpEF patients with isolated pulmonary venous hypertension. There was no reduction in mPAP due to riociguat at any of the investigational doses (0.5, 1, or 2 mg). However, the 2 mg maximal dose resulted in increased stroke volume and reductions in systolic blood pressure, systemic vascular resistance, and right ventricular end-diastolic area. There were no changes in PCWP or PVR. The investigators hypothesized that the lack of reduction in PCWP was due to increased stroke volume and decreased systemic vascular resistance. The lack of change in mPAP may also be due to the effects of riociguat on increasing cardiac output and promoting systemic vasodilation. Overall, favorable hemodynamic effects were noted and riociguat was well tolerated [45], but further studies of sGC stimulators are necessary in PH-HFpEF and CpcPH-HFpEF.
Ongoing clinical trials in PH-HFpEF are investigating the safety and efficacy of endothelin receptor antagonists and prostacyclins. In Safety and Efficacy Trial to Treat Diastolic Heart Failure Using Ambrisentan (NCT00840463), the primary outcome is safety of ambrisentan at 16 weeks with secondary outcomes of 6-min walk test, WHO functional class, and quality of life. Additionally, the Safety and Efficacy of Bosentan in Patients with Diastolic Heart Failure and Secondary PH (BADDHY) trial (NCT00820352) is investigating the hypothesis that bosentan improves exercise capacity, hemodynamics, symptoms, and quality of life in patients with PH-HFpEF. Primary outcomes include change in 6-min walk distance after 12 weeks of bosentan. Neither of these studies specifically sought patients with CpcPH-HFpEF.
Currently, there is only one Phase 2 clinical trial (MELODY-1 [NCT02070991]) that is specifically focused on CpcPH. The goal of the MELODY-1 trial, which is currently still enrolling patients, is to evaluate the safety and tolerability of macitentan 10 mg in patients with CpcPH (associated with either HFpEF or HFrEF). Secondary outcomes include changes in various hemodynamic and echocardiographic variables.
A translational study investigating the role of ranolazine in diastolic dysfunction in isolated RV myocardium from patients undergoing cardiac transplantation found that ranolazine decreased RV diastolic dysfunction by ~30 % [46]. No negative ionotropic effects were noted. Ranolazine has already been FDA approved for anti-anginal indications. In a small pilot study, we found that ranolazine improved RV function at peak exercise in patients with WHO Group I PAH [47]. Thus, ranolazine may have a role in improving RV function in CpcPH-HFpEF.
Prognosis
Overall prognosis of patients with HFpEF who have any PH is poor compared to patients with HFpEF without PH. Elevated PASP has been shown to be a strong independent predictor of all-cause and cardiovascular mortality in patients with HF [48]. Assessment of clinical, echocardiographic, and hemodynamic data from patients with PH-HFpEF was used to create a mortality risk score, which was validated in two independent PH-HFpEF cohorts [49]. Risk factors associated with increased morality included diastolic blood pressure <54 mmHg, PA saturation <55 %, presence of ILD, hypotension (systolic blood pressure <100 mmHg; diastolic blood pressure <70 mmHg), RV hypertrophy on electrocardiography, diffusion capacity of carbon monoxide <35 % of predicted, and serum creatinine ≥1.4 mg/dL.
CpcPH-HFpEF is associated with higher rates of hospitalizations [50] and worse survival, which may be attributable to dysfunctional RV–pulmonary vascular coupling [6]. In a recent study exploring the predictors of mortality in CpcPH-HFpEF, Al-Naamani et al. [51] demonstrated the superior prognostic ability of pulmonary arterial capacitance over DPG, TPG, and PVR. PA capacitance, defined by the ratio of stroke volume to PA pressure, is a determinant of RV afterload; PA capacitance < 1.1 ml/mmHg is associated with worse survival in patients with HFpEF who have PH. In this study, patients with CpcPH did not have worse survival compared to patients with isolated pulmonary venous hypertension in the setting of HFpEF. Whether the presence of CpcPH is associated with worse survival in HFpEF has not yet been fully established. However, regardless of its association with outcomes, CpcPH is a marker for a unique pathophysiology in patients with HFpEF and therefore may be a specific target for therapeutic intervention.
Design of future epidemiology, clinical research, and clinical trial studies in CpcPH-HFpEF
The clinical, laboratory, and echocardiographic characteristics of the patient population represented by CpcPH-HFpEF are still largely undefined. The true prevalence of CpcPH in HFpEF is difficult to determine but has been demonstrated to be ~12 % [6]. Future studies will be needed to deepen our understanding of the pathophysiological mechanisms, genetic contributions, neurohormonal and vasoactive factors, and abnormalities in exercise and gas exchange that are characteristic of this syndrome.
Given the relative rarity of CpcPH-HFpEF compared to HFpEF overall, multicenter registries and observational studies will be necessary to fully characterize the epidemiology, risk factors, and prognosis for CpcPH in HFpEF. For example, derangements in RV–PA coupling have been noted to play an important role in the prognosis of CpcPH-HFpEF [6, 51]. Additional studies further characterizing the deviations from the physiological relationship that are seen in CpcPH may help to identify novel therapies and may clarify patients who are particularly responsive to certain therapies.
Currently, the diagnosis of CpcPH requires right heart catheterization, which may not be feasible in a large number of patients with HFpEF who may be reluctant to undergo invasive hemodynamic testing. The development of a noninvasive diagnostic risk score would greatly aid in identifying patients who may be at increased risk or have CpcPH-HFpEF and can be directed toward appropriate clinical studies and trials. Determining the clinical, echocardiographic, and exercise parameters that define CpcPH-HFpEF could greatly increase the understanding of this disease and identification of patients [6, 52]. An initial noninvasive diagnostic strategy for CpcPH could greatly decrease the demand and cost associated with cardiac catheterizations for large-scale screening purposes in the implementation of new clinical trials.
HFpEF is a heterogeneous syndrome, and the difficulty identifying effective treatments is in part likely due to this fact [13]. For CpcPH-HFpEF clinical trials, optimizing patient selection, feasibility of recruitment, and use of novel study techniques will likely be necessary. Efforts should be made to carefully define selection criteria for future CpcPH-HFpEF trials to optimize homogeneity without sacrificing recruitment. The success of the CHAMPION trial with the use of CardioMEMs to monitor pulmonary pressures presents a novel opportunity for repetitive and frequent monitoring of changes in pulmonary pressures over time, which may provide additional insights into chronic pulmonary vascular changes or response to therapy in CpcPH-HFpEF [50].
Why does CpcPH only occur in a subset of patients with HFpEF? The cardiac and/or vascular substrate that predisposes to CpcPH has not been identified. Genetic mutations or alterations in the production of neurohormonal or vasoactive factors may precipitate the development of CpcPH. The lack of benefit from ACE inhibitors, angiotensin receptor blockers, and beta blockers and the relatively frequent lack of B-type natriuretic peptide elevation suggest the possibility that different molecular pathways are being activated in HFpEF compared to HFrEF. Alternatively, the silencing of protective pathways could be responsible for changes in the cardiac and vascular substrate. Gene expression studies have provided invaluable information about other diseases and may be equally enlightening in CpcPH-HFpEF.
Exercise testing and measures of gas exchange may help to further characterize the mechanisms underlying CpcPH [52]. Additionally, it has been noted that sepsis and tachycardia can induce elevations in DPG [53, 54]. An enhanced understanding of the mechanisms leading to elevations in DPG may provide additional insights in CpcPH.
Conclusions
In summary, CpcPH in HFpEF represents a distinct clinical entity that, while relatively uncommon, is more prevalent than WHO Group I PAH and represents an important cause of morbidity and mortality. Patients with CpcPH-HFpEF appear to have a specific pathophysiological abnormality (intrinsic pulmonary vascular disease) that may be able to be targeted with specific therapies. Selective pulmonary vasodilators may counteract the pulmonary vascular changes associated with CpcPH and result in improved symptoms and outcomes. However, additional studies are needed to enhance the characterization of CpcPH in HFpEF in order to improve the identification of these patients, better understand their underlying pathophysiology, and perform pivotal clinical trials to determine which existing or novel therapies may provide benefit.
Abbreviations
- CpcPH:
-
Combined post- and pre-capillary pulmonary hypertension
- DPG:
-
Diastolic pulmonary gradient
- HFpEF:
-
Heart failure with preserved ejection fraction
- mPAP:
-
Mean pulmonary artery pressure
- PADP:
-
Pulmonary artery diastolic pressure
- PAH:
-
Pulmonary arterial hypertension
- PASP:
-
Pulmonary artery systolic pressure
- PCWP:
-
Pulmonary capillary wedge pressure
- PH-LHD:
-
Pulmonary hypertension due to left heart disease
- PH:
-
Pulmonary hypertension
- PVR:
-
Pulmonary vascular resistance
- RVOT:
-
Right ventricular outflow tract
References
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL (2013) 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128(16):1810–1852
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB (2013) Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 127(1):e6–e245
Oktay AA, Rich JD, Shah SJ (2013) The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep 10(4):401–410
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355(3):251–259
Lam CSP, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM (2009) Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol 53(13):1119–1126
Gerges M, Gerges C, Pistritto AMA, Lang MBM, Trip P, Jakowitsch J, Binder T, Lang IM (2015). Pulmonary hypertension in heart failure: epidemiology, right ventricular function and survival. Am J Respir Crit Care Med 192(10):1234–1246
Kjaergaard J, Akkan D, Iversen KK, Kjoller E, Kober L, Torp-Pedersen C, Hassager C (2007) Prognostic importance of pulmonary hypertension in patients with heart failure. Am J Cardiol 99(8):1146–1150
Klapholz M, Maurer M, Lowe AM, Messineo F, Meisner JS, Mitchell J, Kalman J, Phillips RA, Steingart R, Brown EJ Jr, Berkowitz R, Moskowitz R, Soni A, Mancini D, Bijou R, Sehhat K, Varshneya N, Kukin M, Katz SD, Sleeper LA, Le Jemtel TH (2004) Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol 43(8):1432–1438
Hernandez AF, Hammill BG, O’Connor CM, Schulman KA, Curtis LH, Fonarow GC (2009) Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol 53(2):184–192
Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J (2006) The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 27(19):2338–2345
Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J (2003) Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 362(9386):777–781
Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A (2008) Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 359(23):2456–2467
Shah SJ (2013) Matchmaking for the optimization of clinical trials of heart failure with preserved ejection fraction: no laughing matter. J Am Coll Cardiol 62(15):1339–1342
McLaughlin VV, Shah SJ, Souza R, Humbert M (2015) Management of pulmonary arterial hypertension. J Am Coll Cardiol 65(18):1976–1997
Shah SJ (2012) Pulmonary hypertension. JAMA 308(13):1366–1374
Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galie N, Ghio S, Gibbs JS, Martinez F, Semigran M, Simonneau G, Wells A, Seeger W (2013) Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol 62(25 Suppl):D100–D108
Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G (2009) Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 30(20):2493–2537
McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, Harrington RA, Anderson JL, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Grines CL, Hlatky MA, Jacobs AK, Kaul S, Lichtenberg RC, Lindner JR, Moliterno DJ, Mukherjee D, Pohost GM, Rosenson RS, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Weitz HH, Wesley DJ (2009) ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Associati. Circulation 119(16):2250–2294
Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing Z-C, Krowka MJ, Langleben D, Nakanishi N, Souza R (2009) Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 54(1 Suppl):43–54
Oudiz RJ (2007). Pulmonary hypertension associated with left-sided heart disease. Clin Chest Med 28(1):233–241, x
Delgado JF, Conde E, Sanchez V, Lopez-Rios F, Gomez-Sanchez MA, Escribano P, Sotelo T, Gomez de la Camara A, Cortina J, de la Calzada CS (2005) Pulmonary vascular remodeling in pulmonary hypertension due to chronic heart failure. Eur J Heart Fail 7(6):1011–1016
Dixon DD, Cogswell R, Burke MA, Cuttica MJ, Freed BH, Beussink-Nelson L, Thenappan T, Shah SJ (2015) A non-invasive risk score for the prediction of combined post- and pre-capillary pulmonary hypertension in heart failure with preserved ejection fraction [abstract]. J Heart Lung Transplant 34(4):S118
Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, Maurer G, Lang IM (2013) Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest 143(3):758–766
Naeije R, Vachiery JL, Yerly P, Vanderpool R (2013) The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J 41(1):217–223
Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M (2015). 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. doi:10.1093/eurheartj/ehv317
Shah SJ, Katz DH, Deo RC (2014) Phenotypic spectrum of heart failure with preserved ejection fraction. Heart Fail Clin 10(3):407–418
Tampakakis E, Leary PJ, Selby VN, De Marco T, Cappola TP, Felker MG, Russell SD, Kasper EK, Tedford RJ (2015) The diastolic pulmonary gradient does not predict survival in patients with pulmonary hypertension due to left heart disease. JACC Heart Fail 3(1):9–16
Harvey RM, Enson Y, Ferrer MI (1971) A reconsideration of the origins of pulmonary hypertension. Chest 59(1):82–94
Ryan JJ, Rich JD, Thiruvoipati T, Swamy R, Kim GH, Rich S (2012) Current practice for determining pulmonary capillary wedge pressure predisposes to serious errors in the classification of patients with pulmonary hypertension. Am Heart J 163(4):589–594
Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, Wilkins MR, Badesch DB (2013) Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 62(25 Suppl):D42–D50
Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM (2009) Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation 119(20):2663–2670
Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S (2011) Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest 139(5):988–993
Damy T, Kallvikbacka-Bennett A, Goode K, Khaleva O, Lewinter C, Hobkirk J, Nikitin NP, Dubois-Rande JL, Hittinger L, Clark AL, Cleland JG (2012) Prevalence of, associations with, and prognostic value of tricuspid annular plane systolic excursion (TAPSE) among out-patients referred for the evaluation of heart failure. J Card Fail 18(3):216–225
Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, Temporelli PL, Arena R (2013) Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol 305(9):H1373–H1381
Arkles JS, Opotowsky AR, Ojeda J, Rogers F, Liu T, Prassana V, Marzec L, Palevsky HI, Ferrari VA, Forfia PR (2011) Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am J Respir Crit Care Med 183(2):268–276
Packer M, McMurray J, Massie BM, Caspi A, Charlon V, Cohen-Solal A, Kiowski W, Kostuk W, Krum H, Levine B, Rizzon P, Soler J, Swedberg K, Anderson S, Demets DL (2005) Clinical effects of endothelin receptor antagonism with bosentan in patients with severe chronic heart failure: results of a pilot study. J Card Fail 11(1):12–20
Kalra PR, Moon JC, Coats AJ (2002) Do results of the ENABLE (endothelin antagonist bosentan for lowering cardiac events in heart failure) study spell the end for non-selective endothelin antagonism in heart failure? Int J Cardiol 85(2–3):195–197
Luscher TF, Enseleit F, Pacher R, Mitrovic V, Schulze MR, Willenbrock R, Dietz R, Rousson V, Hurlimann D, Philipp S, Notter T, Noll G, Ruschitzka F (2002) Hemodynamic and neurohumoral effects of selective endothelin A (ET(A)) receptor blockade in chronic heart failure: the heart failure ET(A) receptor blockade trial (HEAT). Circulation 106(21):2666–2672
Califf RM, Adams KF, McKenna WJ, Gheorghiade M, Uretsky BF, McNulty SE, Darius H, Schulman K, Zannad F, Handberg-Thurmond E, Harrell FE Jr, Wheeler W, Soler-Soler J, Swedberg K (1997) A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: the Flolan International Randomized Survival Trial (FIRST). Am Heart J 134(1):44–54
Anand I, McMurray J, Cohn JN, Konstam MA, Notter T, Quitzau K, Ruschitzka F, Luscher TF (2004) Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the Endothelin A Receptor Antagonist Trial in Heart Failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet 364(9431):347–354
Bonderman D, Ghio S, Felix SB, Ghofrani HA, Michelakis E, Mitrovic V, Oudiz RJ, Boateng F, Scalise AV, Roessig L, Semigran MJ (2013) Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation 128(5):502–511
Cooper TJ, Guazzi M, Al-Mohammad A, Amir O, Bengal T, Cleland JG, Dickstein K (2013) Sildenafil in Heart failure (SilHF). An investigator-initiated multinational randomized controlled clinical trial: rationale and design. Eur J Heart Fail 15(1):119–122
Pieske B, Butler J, Filippatos G, Lam C, Maggioni AP, Ponikowski P, Shah S, Solomon S, Kraigher-Krainer E, Samano ET, Scalise AV, Muller K, Roessig L, Gheorghiade M (2014) Rationale and design of the SOluble guanylate Cyclase stimulatoR in heArT failurE studies (SOCRATES). Eur J Heart Fail 16(9):1026–1038
Guazzi M, Vicenzi M, Arena R, Guazzi MD (2011) Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 124(2):164–174
Bonderman D, Pretsch I, Steringer-Mascherbauer R, Jansa P, Rosenkranz S, Tufaro C, Bojic A, Lam CS, Frey R, Ochan Kilama M, Unger S, Roessig L, Lang IM (2014) Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study. Chest 146(5):1274–1285
Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schondube FA, Tirilomis T, Tenderich G, Hasenfuss G, Belardinelli L, Maier LS (2008) Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts—role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol 45(1):32–43
Khan SS, Cuttica MJ, Beussink-Nelson L, Kozyleva A, Sanchez C, Mkrdichian H, Selvaraj S, Dematte JE, Lee DC, Shah SJ (2015) Effects of ranolazine on exercise capacity, right ventricular indices, and hemodynamic characteristics in pulmonary arterial hypertension: a pilot study. Pulm Circ 5(3):547–556
Bursi F, McNallan SM, Redfield MM, Nkomo VT, Lam CS, Weston SA, Jiang R, Roger VL (2012) Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol 59(3):222–231
Agarwal R, Shah SJ, Foreman AJ, Glassner C, Bartolome SD, Safdar Z, Coslet SL, Anderson AS, Gomberg-Maitland M (2012) Risk assessment in pulmonary hypertension associated with heart failure and preserved ejection fraction. J Heart Lung Transplant 31(5):467–477
Benza RL, Raina A, Abraham WT, Adamson PB, Lindenfeld J, Miller AB, Bourge RC, Bauman J, Yadav J (2015) Pulmonary hypertension related to left heart disease: insight from a wireless implantable hemodynamic monitor. J Heart Lung Transplant 34(3):329–337
Al-Naamani N, Preston IR, Paulus JK, Hill NS, Roberts KE (2015) Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail 3(6):467–474
Taylor BJ, Smetana MR, Frantz RP, Johnson BD (2015). Submaximal exercise pulmonary gas exchange in left heart disease patients with different forms of pulmonary hypertension. J Card Fail 21(8):647–655
Enson Y, Wood JA, Mantaras NB, Harvey RM (1977) The influence of heart rate on pulmonary arterial-left ventricular pressure relationships at end-diastole. Circulation 56(4 Pt 1):533–539
Sibbald WJ, Paterson NA, Holliday RL, Anderson RA, Lobb TR, Duff JH (1978) Pulmonary hypertension in sepsis: measurement by the pulmonary arterial diastolic-pulmonary wedge pressure gradient and the influence of passive and active factors. Chest 73(5):583–591
Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E (2013) Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 309(12):1268–1277
Hoendermis ES, Liu LC, Hummel YM, van der Meer P, de Boer RA, Berger RM, van Veldhuisen DJ, Voors AA (2015). Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J 36(38):2565–2573
Zile MR, Bourge RC, Redfield MM, Zhou D, Baicu CF, Little WC (2014) Randomized, double-blind, placebo-controlled study of sitaxsentan to improve impaired exercise tolerance in patients with heart failure and a preserved ejection fraction. JACC Heart Fail 2(2):123–130
Acknowledgments
S.J.S. was supported by the American Heart Association, Dallas, TX (#0835488N) and the National Institutes of Health, Bethesda, MD (R01 HL107557, R01 HL127028), and D.D.D. was supported by the Sarnoff Cardiovascular Research Foundation, Great Falls, VA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.J.S. reports receiving a research grant from Actelion Pharmaceuticals Ltd., consulting fees from Novartis, Bayer, AstraZeneca, and Alnylam; and honoraria from the Pulmonary Hypertension Association, the American Society of Echocardiography, and the American Board of Internal Medicine. D.D.D. and A.T. have no disclosures to report.
Rights and permissions
About this article
Cite this article
Dixon, D.D., Trivedi, A. & Shah, S.J. Combined post- and pre-capillary pulmonary hypertension in heart failure with preserved ejection fraction. Heart Fail Rev 21, 285–297 (2016). https://doi.org/10.1007/s10741-015-9523-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-015-9523-6