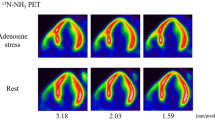
Abstract
Positron emission tomography (PET) is a versatile imaging technology that allows assessment of myocardial perfusion, both at a spatially relative scale and also in absolute terms, thereby enabling noninvasive evaluation of myocardial blood flow (MBF) and coronary flow reserve (CFR). Assessment of MBF using FDA-approved PET isotopes, such as 82Rb and 13N-ammonia, has been well validated, and several software packages are currently available, thereby allowing for MBF evaluation to be incorporated into routine workflow in contemporary nuclear laboratories. Incremental diagnostic and prognostic information provided with the knowledge of MBF has the potential for widespread applications. Improving the ability to identify the true burden of obstructive epicardial coronary stenoses and allowing for noninvasive assessment of coronary micro circulatory function can be achieved with MBF assessment. On the other hand, attenuated CFR has been shown to predict adverse cardiovascular prognosis in a variety of clinical settings and patient subgroups. With expanding applications of MBF, this tool promises to provide unique insight into the integrity of the entire coronary vascular bed beyond what is currently available with relative perfusion assessment. This review intends to provide an in-depth discussion of technical and clinical aspects of MBF assessment with PET as it relates to patients with ischemic heart disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Positron emission tomography (PET) is emerging as an indispensable tool in contemporary nuclear cardiology laboratories and is no longer considered merely a research tool. Utilization of PET for cardiac indications is rapidly growing, facilitated by wider availability of radiotracers and accessibility of PET scanners [1]. In addition, this promising technology has the advantage of improving efficiency and throughput in laboratories routinely incorporating PET in their workflow [2]. Moreover, preferential use of PET for myocardial perfusion imaging (MPI) has been advocated, when available, as an approach towards enhancing patients’ safety with radionuclide imaging due to the shorter half-life of PET radiotracers [3, 4]. As such, PET technology is uniquely positioned as a versatile noninvasive modality with the potential for different cardiac applications [5].
There are many advantages of cardiac PET including the evaluation of relative and absolute myocardial perfusion for detection of flow-limiting coronary artery disease (CAD) [6] with superior image quality and diagnostic accuracy compared to conventional single-photon emission computed tomography (SPCET) [7, 8]. It also affords the ability to perform quantitative assessment of myocardial blood flow (MBF) in absolute units (ml/min/g). Furthermore, cardiac PET is reliable for evaluation of myocardial viability [9] and is emerging as a promising tool for imaging of atherosclerosis, arterial wall inflammation, and detection of cardiac involvement in systemic diseases, such as sarcoidosis [10, 11].
The interest in noninvasive quantification of MBF is not new and has long been sought as a means for better understanding of coronary physiology [12]. The ability to measure MBF, both at rest and under hyperemic conditions, and thereby determining coronary flow reserve (CFR), allows for systematic evaluation of the integrity of the entire coronary vascular bed, beyond the ordinary assessment of obstructive lesions in epicardial vessels. The feasibility of performing flow quantification in clinical settings using 82Rb, 13N-ammonia, and 15O-water and the reproducibility of such measurements allow for easier incorporation of MBF in routine practice [13]. This enhanced ability to combine relative MPI with MBF measurements is arguably a major advantage of cardiac PET, further enhancing the diagnostic [14] and prognostic value of cardiac PET MPI [15,16,17].
The aim of this review is to focus on the basic concepts, technical considerations, and clinical applications of noninvasive assessment of MBF/CFR using PET. The review will mainly focus on the applications of noninvasive MBF/CFR in patients with CAD and ischemic heart disease. Other applications will be reviewed in other papers in this issue.
Technical considerations
Tracers
The high spatial and temporal resolutions, along with the low radiation dosimetry, all make PET an ideal test for noninvasive evaluation of MBF and CFR. Currently, three PET radiotracers are available for MBF evaluation: 15O-water, 13N-ammonia, and 82Rb. Both 15O-water and 13N-ammonia have been validated against radioactive microsphere in animal model [18, 19], whereas 82Rb has been mainly validated in comparison to 13N-ammonia [20, 21].
15O-water is considered the gold standard for flow evaluation owing to its ideal properties. It is inert, freely diffusible and has a linear relation to MBF with first pass extraction of tissue approaching unity [19, 22]. In addition, its short half-life and low radiation allow for repetitive measurements in relatively short time [23]. However, low signal-to-noise ratio resulting from free diffusion of the tracer between target tissue and background leads to inadequate image quality for relative perfusion assessment. Additionally, 15O-water requires onsite cyclotron and has a short half-life, thereby limiting its use in routine clinical practice [19, 22, 23].
In comparison, the two widely used tracers (13N-ammonia and 82Rb) have a nonlinear relation to MBF with a roll-off phenomenon. The first-pass retention fraction of 13N-ammonia and 82Rb at rest is 85 and 65%, respectively, with declining rates at higher blood flow [1, 24, 25]. 13N-ammonia diffuses through capillary and interstitial tissue to the myocytes, and a portion of the retained tracer diffuses back to the blood, while another portion remains trapped in the metabolic glutamine pool [26]. The superb quality of the relative perfusion imaging of 13N-ammonia allows for the evaluation of ischemia, and the relatively longer half-life, almost 10 min, allows for potential use of exercise as a stress modality in addition to vasodilators. However, its use is hurdled by the need for onsite cyclotron for production.
82Rb is a potassium analogue and therefore requires an active Na-K ATPase transporter and is generator-produced, making it more attractive for widespread clinical use [27]. It has similar diagnostic accuracy to 13N-ammonia [28], but its exceptionally short half-life (76 s) allows for performance of stress and rest imaging in almost identical situations [29]. The characteristics of the different radiotracers are summarized in Table 1.
Imaging techniques
MBF is quantified from the dynamic PET images through application of mathematical models. Dynamic imaging is a rapid image acquisition which usually starts 10 s before the injection of the radioactive tracer to track the initial transport and the exchange of the tracer between the blood and myocardium. Dynamic images are performed at rest and maximum hyperemia to quantify the MBF and subsequently calculate the CFR by dividing the stress MBF over the rest MBF.
Yoshida et al. validated a simplified two-tissue compartment model for quantification of MBF by 13N-ammonia and 82Rb accounting for transport kinetics of each tracer [28]. This model has also proven feasible in humans using 82Rb [30]. However, unlike 13N-ammonia, 82Rb has no radioactive metabolite and it does not bind to plasma protein, and therefore, a one-tissue compartment model maybe utilized for 82Rb-derived MBF assessments. One-tissue compartment model has been validated against 13N-ammonia in human subjects by Lortie et al. [21] and has been further validated in two additional studies against 15O-water [31, 32]. Currently, 82Rb is the most widely used tracer in clinical practice since due to its ease of delivery being generator-produced. There are several mathematical extraction models available for MBF quantification with 82Rb, among which the one-tissue compartment model is the simplest and most widely used. Most of these models result in slightly different estimates of rest and peak MBF; however, these differences in CFR are relatively small (Fig. 1a–c). In addition, correlations between CFR and stress MBF measures made with the same input function were high, regardless of extraction model used as was shown in a large cohort of patients (n = 2783) referred for PET MPI [33]. Furthermore, the same investigators found that the prognostic value with CFR, and to a much lesser extent stress MBF, was persistent regardless of the utilized extraction model.
Panel a shows myocardial blood flow and flow reserve with a one-compartment model of 82Rb kinetics and a nonlinear extraction function while panels b and c show myocardial blood flow and flow reserve estimates from different two-compartment models. Please note that that myocardial blood flow showed significant differences between the different methods while the flow reserve showed smaller difference
It is worth mentioning that there is a linear relationship between the resting MBF and rate pressure product (RPP) [34]. Since the CFR is the ratio of stress MBF to rest MBF, it is essential to correct resting MBF to the baseline RPP to avoid erroneous low CFR. The correction is calculated according to the formula Corrected MBF = MBF × (mean RPP at rest in PET study/ideal RPP) [35].
Correlation between CFR and FFR
Fractional flow reserve (FFR) is an invasive measure of hemodynamic significance of epicardial coronary stenoses. FFR represents the ratio between intracoronary pressure distal to a luminal stenosis under hyperemic conditions and central aortic pressure. Lesions with FFR values <0.8 or <0.75 were associated with inducible ischemia and, therefore, are considered hemodynamically significant [36, 37]. A strategy of FFR-guided coronary interventions was superior to angiography-guided interventions and to medical therapy alone in terms of preventing future cardiovascular events [38, 39], and use of FFR in the invasive catheterization laboratory for evaluation of indeterminate coronary lesions is currently recommended [40, 41].
In comparison to FFR, CFR measures global flow augmentation in response to vasodilation, whereas FFR provides lesion-specific measure of severity as a function of change in coronary pressure. In addition, FFR is independent of heart rate or blood pressure, unlike CFR which is corrected for both parameters [42]. Comparative studies showed good correlation between CFR and FFR, mainly among patients with single-vessel CAD [43]. Yet, discrepancies between CFR and FFR do exist, and they point to the differences in pathophysiology invoked with focal stenotic lesions in epicardial vessels (leading to an abnormal FFR) and that seen in diffuse disease of large conduit vessels or impaired microcirculatory function (resulting in attenuated CFR) [44]. Combining CFR and FFR not only helps advance our understanding of coronary physiology but also provides an opportunity to improve patients’ outcomes through identifying novel targets for medical therapies and interventional options [45].
Clinical applications
Noninvasive CFR has been shown to be useful in multiple clinical scenarios. It aids in the diagnosis of diffuse CAD and adds incremental prognostic value to readily available clinical information.
Diagnosis of multivessel or left main CAD
Even though a large body of evidence has accumulated over the years to support the role of single-photon emission computed tomography (SPECT) perfusion imaging in risk stratification and guiding revascularization in patients with suspected CAD [46, 47], the concern about underestimating the true severity of CAD remains the Achilles’ heel of relative myocardial perfusion assessment [48]. This is predominantly attributed to the potential for missing perfusion abnormalities in situations with “balanced” flow reduction in epicardial vessels, such as severe left main stenosis or the presence of hemodynamically significant stenoses in all three coronary territories [49]. In a study of 101 patients with angiographic left main CAD (≥50% stenosis) and no prior myocardial infarction or coronary revascularization that underwent SPECT MPI, only 56% of patients had a high-risk scan. Combining visual perfusion data and nonperfusion variables, especially transient ischemic dilation, 83% of patients were identified as high risk [50].
Detection of left main coronary disease may be improved with the incorporation of nonperfusion and gated functional findings to qualitative assessment of relative perfusion scans [50]. Additionally, determining “left ventricular ejection fraction reserve”—which is feasible during PET MPI since assessment of left ventricular systolic function occurs at peak stress—provides incremental diagnostic advantage for detecting left main or three-vessel disease [51]. Overall, PET appears to have superior sensitivity in detecting multivessel CAD when compared to SPECT [52].
The potential complementary role of MBF to relative MPI in detecting multivessel CAD was examined in a cohort of 120 patients without prior CAD who underwent 82Rb PET MPI and subsequent invasive coronary angiography [14]. The majority of patients (88%) with multivessel CAD had reduced CFR (<2). Moreover, CFR added incremental predictive power to relative MPI in detecting presence of multivessel CAD. Thus, preserved CFR predicts a low likelihood of multivessel CAD. On the other hand, while there is higher likelihood of multivessel CAD when CFR is impaired, not all patients with low CFR have three-vessel CAD; other causes of low CFR need to be entertained including endothelial dysfunction and diffuse coronary disease.
These data suggest that global CFR quantification has the potential to improve diagnostic accuracy for detection of multivessel CAD when combined with relative MPI, especially given the limited utility of SPECT in this patient population [53]. Similar data were also seen using 15O-water among 104 patients with intermediate pretest likelihood of CAD, where the absolute quantification of MBF was found to have superior diagnostic accuracy compared to relative perfusion analysis in detecting patients with multivessel disease using invasive coronary angiography as a gold standard [54].
Similarly, Naya et al. found that normal CFR excluded high-risk CAD in patients who underwent both rest/stress 82Rb PET MPI and subsequent invasive coronary angiography [55]. In this investigation of 290 patients without prior history of CAD, a preserved CFR (>1.93) with normal or mildly-moderately abnormal MPI (<10% of left ventricular mass) excluded the presence of multivessel or left main coronary disease with a negative predictive value of 97%.
Detection of microvascular dysfunction and subclinical CAD
Qualitative or semiquantitative MPI identifies stress-induced perfusion defects, which indicates the presence of flow-limiting coronary stenoses. If only relative perfusion assessment is used, the case in patients undergoing SPECT MPI, the opportunity to diagnose early atherosclerosis is commonly missed. Studies have shown that subclinical CAD, manifesting as coronary artery calcification or endothelial dysfunction, is prevalent among patients with normal perfusion patterns [15, 56]. Attenuation of hyperemic MBF is seen among patients with cardiovascular risk factors [57, 58], and CFR measurements (using 13N-ammonia PET) were lower among patients without overt CAD who had higher coronary heart disease risk [59]. Hence, CFR can be considered as a noninvasive marker of coronary microvascular health.
Such patients with impaired CFR appear to be at increased risk for adverse cardiovascular events [15,16,17], even in the setting of angiographically normal epicardial coronary arteries [60] (see “Prognosis and risk stratification” section). This ability to uncover CAD in its subclinical stages afforded by noninvasive CFR assessment helps identify subsets of patients at risk for future cardiac events who may benefit from early institution of aggressive risk factor control [61]. Furthermore, response to preventative medications can be assessed on serial measurements over time, demonstrating favorable changes in CFR with proper interventions [62, 63].
Differentiating diffuse epicardial CAD from microvascular dysfunction
Combining available noninvasive parameters of left ventricular perfusion (relative perfusion pattern, presence of transient cavity dilation, hyperemic MBF and CFR) and function (gated wall motion analysis at peak stress and LVEF reserve) allows enhanced ability to differentiate between various phenotypes of CAD [64]. Patients with focal stenoses (or severe diffuse disease) affecting multiple epicardial vessels and those with pronounced global microvascular disease may not be adequately differentiated on the basis of relative perfusion analysis alone; stress MBF and CFR play a complementary role (Fig. 2). However, in many different clinical situations, assessment of coronary anatomy by either coronary calcium scoring, CT angiography [65], or invasive angiography is necessary to make this distinction.
Schematic representation of differences in myocardial perfusion between patients with severe focal stenosis and patients with severe diffuse disease. Reproduced with permission from Al-Mallah et al. Patients with severe focal stenosis demonstrate a perfusion defect by relative perfusion assessment and also have attenuated MBF/CFR in the territory of the affected vessel (top) while patients with severe diffuse disease may have normal (or near normal with base-apex gradient described by Gould et al.) relative perfusion pattern but will have global reduction in CFR (bottom)
Prognosis and risk stratification
Several studies have established the prognostic utility of PET-determined MBF and CFR measurements in predicting future major adverse cardiac events (MACE) and their ability to offer incremental risk stratification beyond traditional markers of risk (Table 2) [15,16,17, 60, 66,67,68,69,70,71,72,74].
Herzog et al. were the first to demonstrate abnormal CFR (<2) using 13N-ammonia PET to be independently predictive of future cardiac death, nonfatal MI, late revascularization, or cardiac hospitalization (HR 2.9, 95%CI [1.2–6.6], p < 0.05) [15]. Furthermore, impaired CFR provided further risk stratification among patients with normal qualitative regional perfusion within the first 3 years after the test, with lower event rates seen among patients with normal CFR (6.3 vs 1.4%, p < 0.05). These findings were the first steps on the road towards establishing the prognostic value of MBF and CFR [75].
Consequently, the prognostic utility of CFR using 82Rb was evaluated prospectively [17]. In this study by Ziadi et al., abnormal CFR was shown to be a predictor of cardiac death and myocardial infarction, regardless of the status of relative perfusion scan. Incidence of MACE was lower in patients with normal CFR relative to those with low CFR, whether MPI was normal (3.8 vs 9%, p = 0.003), or abnormal (9 vs 24%, p < 0.001). Figure 3 summarizes prognostic utility of impaired CFR in predicting MACE.
Similar findings to those shown were further corroborated by data in patients with chronic kidney disease [68], diabetes [69], and end-stage renal disease on dialysis [73]; in patients referred for coronary revascularization [71]; and in women [70]. Moreover, reclassification indexes have specifically demonstrated the ability of noninvasive markers of coronary vascular dysfunction to reclassify patients across the continuum of cardiovascular risk [68, 69, 73]. In addition, recent data suggested that peak MBF may also be used in the risk stratification of patients undergoing PET MPI [76].
PET MBF/CFR assessment may also be of help in other clinical scenarios. Taqueti et al. found that elevation of cardiac troponin among 761 patients without overt flow-limiting CAD to be associated with impaired global CFR, where a CFR <2 was associated with a 2-fold increase in the risk of having abnormal troponin level (HR 2.2, 95%CI [1.4–3.5], p = 0.0015) [72]. Additionally, CFR modified the effect of troponin elevation on the study endpoint of cardiac death, nonfatal MI, and late revascularization. Among patients with elevated troponin, the event rate was significantly lower for those with intact CFR compared to those with impaired CFR (0.9 vs 7.4%, p = 0.046). In fact, event rates were not statistically different between those with or without troponin elevation as long as CFR was ≥2 (0.9 vs 1.7%, p = 0.58).
Moreover, data on the role of PET-determined flow measurements and gender-based differences in cardiovascular risk are particularly enlightening. In a cohort of consecutive patients (n = 324) referred for invasive coronary angiography following rest/stress 82Rb PET and a median follow up of 3 years, women were found to have a significantly higher risk of adverse cardiac events in spite of a lower burden of obstructive disease on invasive coronary angiography (HR 2.05, 95%CI [1.05–4.02], p = 0.03) [70]. Interestingly, this observed excess risk in women was no longer seen after adjusting for CFR (HR 1.81, 95%CI [0.91–3.59], p = 0.10), and only women with attenuated CFR (<1.6) demonstrated higher rates of cardiac events. As such, the authors concluded that CFR was responsible for a significant proportion of cardiovascular risk observed in women and that impaired CFR needs to be addressed as “hidden biological” risk factor for future cardiac events, which may represent a novel target for cardiac risk reduction.
MBF/CFR in special populations
In addition, CFR and MBF have been shown to provide incremental risk stratification in patient subgroups with increased cardiac risk. Table 3 summarizes the prognostic utility of CFR in such special populations.
Diabetes
The prognostic value of MPI in diabetics is well documented [77]. In addition, Murthy and colleagues studied 2783 consecutive patients (1172 diabetics and 1611 nondiabetics) who underwent quantification of CFR and were followed up for a median of 1.4 years, Impaired CFR was associated with an adjusted 3.2- and 4.9-fold increase in the rate of cardiac death for diabetics and nondiabetics, respectively (p = 0.0004). It is important to note that diabetic patients without known CAD with impaired CFR experienced an annualized rate of cardiac death comparable to that for nondiabetic patients with known CAD (2.8 vs 2.0%; p = 0.33). Conversely, diabetics without known CAD and preserved CFR had very low annualized cardiac mortality, which was similar to patients without known CAD or diabetes mellitus and normal stress perfusion and systolic function (0.3 vs 0.5%; p = 0.65).
Chronic kidney disease
Since cardiovascular mortality is the main cause of death in this population, accurate risk stratification is essential [78, 79]. SPECT is an important modality to assess these patients. However, there are still patients who experience event despite being labeled a low risk by SPECT. A study of end-stage renal disease (ESRD) clinically referred for myocardial perfusion PET imaging found that the addition of global CFR in ESRD patient resulted in risk reclassification in 27% of patients. Thus, global CFR may provide independent and incremental risk stratification for all-cause and cardiovascular mortality in this patient population [73].
Women
The multicenter PET registry demonstrated in 6037 women and men that stress Rb-82 PET provides significant and clinically meaningful effective risk stratification of women and men, supporting this modality as an alternative to comparative imaging modalities [80]. Rb-82 PET findings were particularly helpful at identifying high-risk, older women. Furthermore, among patients who undergo PET MPI with CFR assessment and eventually undergo coronary angiography, women have higher pretest likelihood, a significantly lower burden of obstructive CAD in comparison with men and higher event rate (adjusted hazard ratio, 2.05; 95% confidence interval, 1.05–4.02; p = 0.03). This higher excess cardiovascular risk in women was independently associated with impaired CFR [70]. Thus, CFR may be a helpful marker in women with nonobstructive disease who have higher event rate despite low-risk angiograms.
Cardiac allograft vasculopathy
Alterations in coronary vasomotor function have been described previously in cardiac transplant recipients and blunted vasodilator capacity, assessed using invasive Doppler flow measurements, predicted development of cardiac allograft vasculopathy, and cardiac death in post-transplant patients [81]. Similarly, PET-determined CFR demonstrated an inverse correlation with plaque volume as assessed using intravascular ultrasound in a group of 27 heart transplant recipients followed longitudinally post-transplant [82].
MBF/CFR in the guidelines
Given its clinical value, the most recent PET guidelines from the American Society of Nuclear Cardiology suggested that quantitative absolute MBF measurements with PET appear most helpful [9] in patients without known prior history of cardiac disease who present with symptoms suspicious for myocardial ischemia, patients with known CAD, in whom more specific physiological assessment is desired, patients with suspected multivessel CAD or microvascular dysfunction, and patients with suspected transplant vasculopathy. In contrast, there are particular patients for whom reporting hyperemic blood flow or flow reserve may not add diagnostic value or can be ambiguous or misleading, including patients post-CABG who can have diffuse reduction on MBF despite patent grafts, patients with large transmural infarcts, and patients with advanced severe chronic renal dysfunction and/or severe LV dysfunction. While the diagnostic value of CFR in these patients is not clear, the prognostic value of CFR in these patients has been demonstrated before [73, 83].
Conclusion
The increasing availability of PET MPI in many contemporary nuclear cardiology practices has provided us with robust noninvasive tools to evaluate the integrity of the coronary tree. In particular, MBF and CFR lend themselves as sources of valuable diagnostic and prognostic information in patients with known or suspected CAD. Current literature supports a strong role for MBF and CFR in improving the yield of MPI studies beyond relative perfusion analysis. Additionally, the ability to routinely assess MBF and CFR provides unique insight about coronary physiology and helps offer new and novel therapeutic alternatives to patients at risk for or with established CAD.
Abbreviations
- CAD:
-
Coronary artery disease
- CFR:
-
Coronary flow reserve
- MBF:
-
Myocardial blood flow
- MPI:
-
Myocardial perfusion imaging
- PET:
-
Positron emission tomography
References
Al-Mallah MH, Sitek A, Moore SC, Di Carli M, Dorbala S (2010) Assessment of myocardial perfusion and function with PET and PET/CT. J Nucl Cardiol 17:498–513
Bateman TM (2012) Advantages and disadvantages of PET and SPECT in a busy clinical practice. J Nucl Cardiol 19(Suppl 1):S3–11
Cerqueira MD, Allman KC, Ficaro EP, Hansen CL, Nichols KJ, Thompson RC, Van Decker WA, Yakovlevitch M (2010) Recommendations for reducing radiation exposure in myocardial perfusion imaging. J Nucl Cardiol 17:709–718
Depuey EG, Mahmarian JJ, Miller TD, Einstein AJ, Hansen CL, Holly TA, Miller EJ, Polk DM, Samuel WL (2012) Patient-centered imaging. J Nucl Cardiol 19:185–215
Bateman TM, Dilsizian V, Beanlands RS, DePuey EG, Heller GV, Wolinsky DA (2016) American Society of Nuclear Cardiology and Society of Nuclear Medicine and Molecular Imaging joint position statement on the clinical indications for myocardial perfusion pet. J Nucl Med 57:1654–1656
Ritchie J, Bateman TM, Bonow RO, Crawford MH, Gibbons RJ, Hall RJ, O’Rourke RA, Parisi AF, Verani MS (1995) Guidelines for clinical use of cardiac radionuclide imaging. A report of the American Heart Association/American College of Cardiology Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures, Committee on Radionuclide Imaging, developed in collaboration with the American Society of Nuclear Cardiology. Circulation 91:1278–1303
Holly TA, Abbott BG, Al-Mallah M, Calnon DA, Cohen MC, DiFilippo FP, Ficaro EP, Freeman MR, Hendel RC, Jain D (2010) Single photon-emission computed tomography. J Nucl Cardiol 17:941–973
Mc Ardle BA, Dowsley TF, deKemp RA, Wells GA, Beanlands RS (2012) Does rubidium-82 PET have superior accuracy to SPECT perfusion imaging for the diagnosis of obstructive coronary disease?: a systematic review and meta-analysis. J Am Coll Cardiol 60:1828–1837
Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, Gropler RJ, Knuuti J, Schelbert HR, Travin MI (2016) ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol 23:1187–1226
Bucerius J, Hyafil F, Verberne HJ, Slart RH, Lindner O, Sciagra R, Agostini D, Ubleis C, Gimelli A, Hacker M (2016) Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur J Nucl Med Mol Imaging 43:780–792
Youssef G, Leung E, Mylonas I, Nery P, Williams K, Wisenberg G, Gulenchyn KY, Dekemp RA, Dasilva J, Birnie D, Wells GA, Beanlands RS (2012) The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med 53:241–248
Soufer R, Zaret BL (1990) Positron emission tomography and the quantitative assessment of regional myocardial blood flow. J Am Coll Cardiol 15:128–130
Nesterov SV, Deshayes E, Sciagra R, Settimo L, Declerck JM, Pan XB, Yoshinaga K, Katoh C, Slomka PJ, Germano G, Han C, Aalto V, Alessio AM, Ficaro EP, Lee BC, Nekolla SG, Gwet KL, deKemp RA, Klein R, Dickson J, Case JA, Bateman T, Prior JO, Knuuti JM (2014) Quantification of myocardial blood flow in absolute terms using (82)Rb PET imaging: the RUBY-10 Study. JACC Cardiovasc Imaging 7:1119–1127
Ziadi MC, Dekemp RA, Williams K, Guo A, Renaud JM, Chow BJ, Klein R, Ruddy TD, Aung M, Garrard L, Beanlands RS (2012) Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? J Nucl Cardiol 19:670–680
Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, Burkhard N, Wyss CA, Kaufmann PA (2009) Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol 54:150–156
Farhad H, Dunet V, Bachelard K, Allenbach G, Kaufmann PA, Prior JO (2013) Added prognostic value of myocardial blood flow quantitation in rubidium-82 positron emission tomography imaging. Eur Heart J Cardiovasc Imaging 14:1203–1210
Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, Ruddy TD, Sarveswaran N, Tee RE, Beanlands RS (2011) Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 58:740–748
Bellina CR, Parodi O, Camici P, Salvadori PA, Taddei L, Fusani L, Guzzardi R, Klassen GA, L’Abbate AL, Donato L (1990) Simultaneous in vitro and in vivo validation of nitrogen-13-ammonia for the assessment of regional myocardial blood flow. J Nucl Med 31:1335–1343
Bergmann SR, Fox KA, Rand AL, McElvany KD, Welch MJ, Markham J, Sobel BE (1984) Quantification of regional myocardial blood flow in vivo with H215O. Circulation 70:724–733
El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, Fischman A, Coughlan M, Yasuda T, Di Carli MF (2009) Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. J Nucl Med 50:1062–1071
Lortie M, Beanlands RS, Yoshinaga K, Klein R, Dasilva JN, DeKemp RA (2007) Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging 34:1765–1774
Bergmann SR, Herrero P, Markham J, Weinheimer CJ, Walsh MN (1989) Noninvasive quantitation of myocardial blood flow in human subjects with oxygen-15-labeled water and positron emission tomography. J Am Coll Cardiol 14:639–652
Camici PG, Gropler RJ, Jones T, L’Abbate A, Maseri A, Melin JA, Merlet P, Parodi O, Schelbert HR, Schwaiger M, Wijns W (1996) The impact of myocardial blood flow quantitation with PET on the understanding of cardiac diseases. Eur Heart J 17:25–34
Schelbert HR, Phelps ME, Huang SC, MacDonald NS, Hansen H, Selin C, Kuhl DE (1981) N-13 ammonia as an indicator of myocardial blood flow. Circulation 63:1259–1272
Hsu B (2013) Pet tracers and techniques for measuring myocardial blood flow in patients with coronary artery disease. J Biomed Res 27:452–459
Bergmann SR, Hack S, Tewson T, Welch MJ, Sobel BE (1980) The dependence of accumulation of 13NH3 by myocardium on metabolic factors and its implications for quantitative assessment of perfusion. Circulation 61:34–43
Gould KL (1989) Clinical cardiac PET using generator-produced Rb-82: a review. Cardiovasc Intervent Radiol 12:245–251
Yoshida K, Mullani N, Gould KL (1996) Coronary flow and flow reserve by PET simplified for clinical applications using rubidium-82 or nitrogen-13-ammonia. J Nucl Med 37:1701–1712
Demer LL, Gould KL, Goldstein RA, Kirkeeide RL, Mullani NA, Smalling RW, Nishikawa A, Merhige ME (1989) Assessment of coronary artery disease severity by positron emission tomography. Comparison with quantitative arteriography in 193 patients. Circulation 79:825–835
El Fakhri G, Sitek A, Guerin B, Kijewski MF, Di Carli MF, Moore SC (2005) Quantitative dynamic cardiac 82Rb PET using generalized factor and compartment analyses. J Nucl Med 46:1264–1271
Prior JO, Allenbach G, Valenta I, Kosinski M, Burger C, Verdun FR, Bischof Delaloye A, Kaufmann PA (2012) Quantification of myocardial blood flow with 82Rb positron emission tomography: clinical validation with 15O-water. Eur J Nucl Med Mol Imaging 39:1037–1047
Germino M, Ropchan J, Mulnix T, Fontaine K, Nabulsi N, Ackah E, Feringa H, Sinusas AJ, Liu C, Carson RE (2016) Quantification of myocardial blood flow with (82)Rb: validation with (15)O-water using time-of-flight and point-spread-function modeling. EJNMMI Res 6:68
Murthy VL, Lee BC, Sitek A, Naya M, Moody J, Polavarapu V, Ficaro EP, Di Carli MF (2014) Comparison and prognostic validation of multiple methods of quantification of myocardial blood flow with 82Rb PET. J Nucl Med 55:1952–1958
Czernin J, Muller P, Chan S, Brunken RC, Porenta G, Krivokapich J, Chen K, Chan A, Phelps ME, Schelbert HR (1993) Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation 88:62–69
Marinho NV, Keogh BE, Costa DC, Lammerstma AA, Ell PJ, Camici PG (1996) Pathophysiology of chronic left ventricular dysfunction. New insights from the measurement of absolute myocardial blood flow and glucose utilization. Circulation 93:737–744
Melikian N, De Bondt P, Tonino P, De Winter O, Wyffels E, Bartunek J, Heyndrickx GR, Fearon WF, Pijls NH, Wijns W, De Bruyne B (2010) Fractional flow reserve and myocardial perfusion imaging in patients with angiographic multivessel coronary artery disease. JACC Cardiovasc Interv 3:307–314
Schelbert HR (2012) FFR and coronary flow reserve: friends or foes? JACC Cardiovasc Imaging 5:203–206
Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’t Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF (2009) Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 360:213–224
De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Mobius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engstrom T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Juni P, Fearon WF (2012) Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 367:991–1001
Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A (2014) 2014 ESC/EACTS Guidelines on Myocardial Revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 35:2541–2619
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH (2011) 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 58:e44–122
de Bruyne B, Bartunek J, Sys SU, Pijls NH, Heyndrickx GR, Wijns W (1996) Simultaneous coronary pressure and flow velocity measurements in humans. Feasibility, reproducibility, and hemodynamic dependence of coronary flow velocity reserve, hyperemic flow versus pressure slope index, and fractional flow reserve. Circulation 94:1842–1849
De Bruyne B, Baudhuin T, Melin JA, Pijls NH, Sys SU, Bol A, Paulus WJ, Heyndrickx GR, Wijns W (1994) Coronary flow reserve calculated from pressure measurements in humans. Validation with positron emission tomography. Circulation 89:1013–1022
Johnson NP, Kirkeeide RL, Gould KL (2012) Is discordance of coronary flow reserve and fractional flow reserve due to methodology or clinically relevant coronary pathophysiology? JACC Cardiovasc Imaging 5:193–202
Johnson NP, Gould KL, Di Carli MF, Taqueti VR (2016) Invasive FFR and noninvasive CFR in the evaluation of ischemia: what is the future? J Am Coll Cardiol 67:2772–2788
Navare SM, Mather JF, Shaw LJ, Fowler MS, Heller GV (2004) Comparison of risk stratification with pharmacologic and exercise stress myocardial perfusion imaging: a meta-analysis. J Nucl Cardiol 11:551–561
Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS (2003) Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 107:2900–2907
Beller GA (2008) Underestimation of coronary artery disease with SPECT perfusion imaging. J Nucl Cardiol 15:151–153
Bourque JM, Beller GA (2011) Stress myocardial perfusion imaging for assessing prognosis: an update. JACC Cardiovasc Imaging 4:1305–1319
Berman DS, Kang X, Slomka PJ, Gerlach J, de Yang L, Hayes SW, Friedman JD, Thomson LE, Germano G (2007) Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. J Nucl Cardiol 14:521–528
Dorbala S, Vangala D, Sampson U, Limaye A, Kwong R, Di Carli MF (2007) Value of vasodilator left ventricular ejection fraction reserve in evaluating the magnitude of myocardium at risk and the extent of angiographic coronary artery disease: a 82Rb PET/CT study. J Nucl Med 48:349–358
Bateman TM, Heller GV, McGhie AI, Friedman JD, Case JA, Bryngelson JR, Hertenstein GK, Moutray KL, Reid K, Cullom SJ (2006) Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol 13:24–33
Knuuti J, Saraste A (2012) Advances in clinical application of quantitative myocardial perfusion imaging. J Nucl Cardiol 19:643–646
Kajander SA, Joutsiniemi E, Saraste M, Pietila M, Ukkonen H, Saraste A, Sipila HT, Teras M, Maki M, Airaksinen J, Hartiala J, Knuuti J (2011) Clinical value of absolute quantification of myocardial perfusion with (15)O-water in coronary artery disease. Circ Cardiovasc Imaging 4:678–684
Naya M, Murthy VL, Taqueti VR, Foster CR, Klein J, Garber M, Dorbala S, Hainer J, Blankstein R, Resnic F, Di Carli MF (2014) Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J Nucl Med 55:248–255
Berman DS, Wong ND, Gransar H, Miranda-Peats R, Dahlbeck J, Hayes SW, Friedman JD, Kang X, Polk D, Hachamovitch R, Shaw L, Rozanski A (2004) Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol 44:923–930
Prior JO, Quinones MJ, Hernandez-Pampaloni M, Facta AD, Schindler TH, Sayre JW, Hsueh WA, Schelbert HR (2005) Coronary circulatory dysfunction in insulin resistance, impaired glucose tolerance, and type 2 diabetes mellitus. Circulation 111:2291–2298
Schindler TH, Cardenas J, Prior JO, Facta AD, Kreissl MC, Zhang XL, Sayre J, Dahlbom M, Licinio J, Schelbert HR (2006) Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol 47:1188–1195
Dorbala S, Hassan A, Heinonen T, Schelbert HR, Di Carli MF (2006) Coronary vasodilator reserve and Framingham risk scores in subjects at risk for coronary artery disease. J Nucl Cardiol 13:761–767
Britten MB, Zeiher AM, Schachinger V (2004) Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coron Artery Dis 15:259–264
Schindler TH, Schelbert HR, Quercioli A, Dilsizian V (2010) Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging 3:623–640
Quercioli A, Montecucco F, Pataky Z, Thomas A, Ambrosio G, Staub C, Di Marzo V, Ratib O, Mach F, Golay A, Schindler TH (2013) Improvement in coronary circulatory function in morbidly obese individuals after gastric bypass-induced weight loss: relation to alterations in endocannabinoids and adipocytokines. Eur Heart J 34:2063–2073
Schindler TH, Cadenas J, Facta AD, Li Y, Olschewski M, Sayre J, Goldin J, Schelbert HR (2009) Improvement in coronary endothelial function is independently associated with a slowed progression of coronary artery calcification in type 2 diabetes mellitus. Eur Heart J 30:3064–3073
Patel MB, Bui LP, Kirkeeide RL, Gould KL (2016) Imaging microvascular dysfunction and mechanisms for female-male differences in CAD. JACC Cardiovasc Imaging 9:465–482
Al-Mallah MH, Aljizeeri A, Villines TC, Srichai MB, Alsaileek A (2015) Cardiac computed tomography in current cardiology guidelines. J Cardiovasc Comput Tomogr 9:514–523
Fukushima K, Javadi MS, Higuchi T, Lautamaki R, Merrill J, Nekolla SG, Bengel FM (2011) Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med 52:726–732
Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF (2011) Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 124(20):2215–2224. doi:10.1161/CIRCULATIONAHA.111.050427
Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Dorbala S, Charytan DM, Blankstein R, Di Carli MF (2012) Coronary vascular dysfunction and prognosis in patients with chronic kidney disease. JACC Cardiovasc Imaging 5:1025–1034
Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbala S, Blankstein R, Di Carli MF (2012) Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation 126:1858–1868
Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF (2017) Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation 135:566–577
Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF (2015) Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 131:19–27
Taqueti VR, Everett BM, Murthy VL, Gaber M, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF (2015) Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation 131:528–535
Shah NR, Charytan DM, Murthy VL, Skali Lami H, Veeranna V, Cheezum MK, Taqueti VR, Kato T, Foster CR, Hainer J, Gaber M, Klein J, Dorbala S, Blankstein R, Di Carli MF (2016) Prognostic value of coronary flow reserve in patients with dialysis-dependent ESRD. J Am Soc Nephrol 27:1823–1829
Al-Mallah M, Ahmed A, Aljizeeri A, Suleiman I (2015) Incremental prognostic value of noninvasive coronary flow reserve. Eur Heart J 36:857–857
Beanlands RS, Ziadi MC, Williams K (2009) Quantification of myocardial flow reserve using positron emission imaging the journey to clinical use. J Am Coll Cardiol 54:157–159
Al-Mallah MH, Aljizeeri A, Ahmed D, Alfaris M, Suleiman A, Suliman I (2015) Peak myocardial blood flow versus noninvasive coronary flow reserve for prediction of future events
Kang X, Berman DS, Lewin HC, Cohen I, Friedman JD, Germano G, Hachamovitch R, Shaw LJ (1999) Incremental prognostic value of myocardial perfusion single photon emission computed tomography in patients with diabetes mellitus. Am Heart J 138:1025–1032
Ahmed AM, Qureshi WT, O’Neal WT, Khalid F, Al-Mallah MH (2017) Incremental prognostic value of SPECT-MPI in chronic kidney disease: a reclassification analysis. J Nucl Cardiol:1–16
Al-Mallah MH, Hachamovitch R, Dorbala S, Di Carli MF (2009) Incremental prognostic value of myocardial perfusion imaging in patients referred to stress single-photon emission computed tomography with renal dysfunction clinical perspective. Circ Cardiovasc Imaging 2:429–436
Kay J, Dorbala S, Goyal A, Fazel R, Di Carli MF, Einstein AJ, Beanlands RS, Merhige ME, Williams BA, Veledar E (2013) Influence of sex on risk stratification with stress myocardial perfusion Rb-82 positron emission tomography: results from the PET (positron emission tomography) prognosis multicenter registry. J Am Coll Cardiol 62:1866–1876
Hollenberg SM, Klein LW, Parrillo JE, Scherer M, Burns D, Tamburro P, Oberoi M, Johnson MR, Costanzo MR (2001) Coronary endothelial dysfunction after heart transplantation predicts allograft vasculopathy and cardiac death. Circulation 104:3091–3096
Wu YW, Chen YH, Wang SS, Jui HY, Yen RF, Tzen KY, Chen MF, Lee CM (2010) Pet assessment of myocardial perfusion reserve inversely correlates with intravascular ultrasound findings in angiographically normal cardiac transplant recipients. J Nucl Med 51:906–912
Ahmed AM, Sulaiman I, Alfaris M, Ahmed D, Aljizeeri A, Alsaileek A, Sulaiman A, Sakr S, Al-Mallah M (2017) Non-invasive coronary flow reserve predicts major adverse cardiac events in patients with prior coronary artery bypass grafting. J Am Coll Cardiol 11:1400
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Firas Al Badarin is a speaker of Lantheus Medical Imaging.
Ahmed Aljizeeri declares that he has no conflict of interest.
Fatimah Almasoudi declares that she has no conflict of interest.
Mouaz Al-Mallah declares that he has no conflict of interest.
Rights and permissions
About this article
Cite this article
Al Badarin, F., Aljizeeri, A., Almasoudi, F. et al. Assessment of myocardial blood flow and coronary flow reserve with positron emission tomography in ischemic heart disease: current state and future directions. Heart Fail Rev 22, 441–453 (2017). https://doi.org/10.1007/s10741-017-9625-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-017-9625-4