Abstract
Pulmonary hypertension (PH) can occur in patients with obstructive sleep apnea (OSA) in the absence of cardiac or lung disease. Data on the development and severity of PH, and the effect of continuous positive airway pressure (CPAP) therapy on pulmonary artery (PA) pressures in these patients have been inconsistent in the literature. We sought to determine whether CPAP therapy affects PA pressures in patients with isolated OSA in this meta-analysis. We searched PubMed, Medline, EMBASE and other databases from January 1980 to August 2015. Studies of patients with OSA, defined as an apnea–hypopnea index >10 events/h, and PH, defined as PA pressure >25 mmHg were included. Two reviewers independently extracted data and assessed risk of bias. A total of 222 patients from seven studies (341.53 person-years) had reported PA pressures before and after treatment with CPAP therapy. 77 % of participants were men, with a mean age of 52.5 years, a mean apnea–hypopnea index of 58 events/h, and mean PA pressure of 39.3 ± 6.3 mmHg. CPAP treatment duration ranged from 3 to 70 months. Using fixed effects meta-analysis, CPAP therapy was associated with a decrease in PA pressure of 13.3 mmHg (95 % CI 12.7–14.0) in our study population. This meta-analysis found that CPAP therapy is associated with a significantly lower PA pressure in patients with isolated OSA and PH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a rising health problem that affects up to 2–10 % of the population [1]. OSA has been associated with obesity, hypertension, and left-sided heart disease [2]. Additionally, there is a known association between OSA and pulmonary hypertension (PH) [3]. OSA patients with PH compared to those without PH have been noted to have higher morbidity and mortality [4]. Patients with OSA tend to have repetitive upper airway collapse and oxygen desaturations leading to hypoxemia [5]. Hypoxemia leads to vasoconstriction of the pulmonary vasculature, which can elevate PA pressures over time [5, 6]. Some suggest that PH does not occur in patients with OSA unless they have clinically significant hypoxemic pulmonary disease [7, 8], while others have found that this is not necessarily the case [9–11]. Repetitive hypoxemia during sleep may lead to pulmonary vascular remodeling, thereby impairing recruitment of pulmonary vessels during times of increased pulmonary flow, and ultimately causing sustained PH during the daytime [9].
Despite these proposed mechanisms, the effect of treatment modalities such as continuous positive airway pressure (CPAP) on PH has not been fully elucidated. CPAP is the primary therapy for patients with obstructive sleep apnea, and it has been hypothesized that CPAP may reduce pulmonary vascular reactivity to hypoxia, with subsequent reduction in PA pressures [1]. However, data on the effect of CPAP therapy on PA pressures in patients with OSA without comorbid lung or heart disease have been inconsistent. Thus, the purpose of our paper is to conduct a systematic meta-analysis to determine the effects of CPAP therapy on PA pressures.
Methods
Literature search
We performed a literature search (TFI, SL and HA) for all relevant publications using Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid Embase, Web of Science, the Wiley Cochrane Library, the Cochrane Database, Google Scholar, and clinicaltrials.gov databases. All databases were searched from January 1980 through August 2015 using the following terms: “obstructive sleep apnea”, “sleep apnea/apnea”, “pulmonary hypertension”, “pulmonary artery pressure”, “continuous positive airway pressure”, and “positive pressure airway”. We reviewed articles found as a result of these searches and references cited in these articles. We additionally hand-searched reference lists and contacted investigators of certain studies when necessary. Efforts were made to also include studies not published in the English language.
Inclusion and exclusion criteria
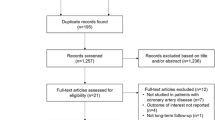
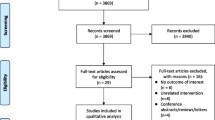
The meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Materials Statement (PRISMA). Two independent reviewers (TFI and SL) screened and extracted data from full text articles, and Cohen’s kappa was calculated to determine inter-rater reliability. Discrepancies were resolved by reaching consensus through discussion. The studies included in this review met the following inclusion criteria: (1) OSA diagnosed by polysomnography, defined as apnea–hypopnea index >10 events/h, (2) no co-morbid cardiac or pulmonary disease (isolated OSA), (3) PA pressure assessed by Doppler echocardiography or right heart catheterization, represented as mean PA pressure, mean systolic PA pressure, or right ventricular systolic pressure, and (4) pulmonary hypertension diagnosed as mean PA pressure greater than 25 mmHg at rest, with a pulmonary capillary wedge pressure <15 mmHg. The process of selecting studies is outlined in Fig. 1.
Studies with participants having the following characteristics were excluded if: (1) the Apnea–Hypopnea Index (AHI) was <10 events/h or was not reported; (2) PA pressure was not reported; or (3) patients had co-morbid conditions such as cardiovascular or pulmonary disease.
Data extraction and quality assessment
The following variables were extracted from each study: authors, year of publication, geographic location, age, gender, body mass index (BMI), sample size, mean AHI, method and results of PA pressure measurements, mean daily CPAP usage time, and duration of CPAP therapy. To determine the extent to which we can be confident about our summary estimate, the quality of the included evidence was assessed according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group tool (Fig. 2). This tool determines the quality of the evidence to be high, moderate, low, or very low based on risk of bias, inconsistency, indirectness, imprecision, and publication bias [12]. Six domains were assessed using GRADE: random sequence generation, allocation concealment, blinding of outcome assessors, incomplete outcome data (whether the investigators reported all outcome data, including attrition and exclusions, and whether missing data were appropriately accounted for), and selective outcome reported (determined by checking published protocols if available or relying on authors’ methods).
Data synthesis and analysis
The studies were evaluated with regards to similarity of baseline patient characteristics, methods of measurement of PA pressures, and duration of follow-up. The primary endpoint was the change in PA pressures pre- and post-CPAP therapy. A fixed effects meta-analysis was used to calculate the weighted mean difference in PA pressures pre- and post-treatment with CPAP therapy. In a secondary analysis, we also used the random effects meta-analysis model to assess the robustness of our findings. Comprehensive Meta-Analysis version 3.0 was used to create the forest plots. Extracted data were exported to Review Manager version 5.3 (Cochrane Collaboration Software) to determine the GRADE risk of bias [12].
Results
A total of 222 patients (341.53 person-years) from seven pooled studies met our inclusion criteria (Fig. 3). The data are presented as mean ± SD unless otherwise specified. Table 1 depicts characteristics of the study participants. Based on all reviewed studies, the prevalence of PH in patients with OSA without overt heart or lung disease was approximately 30.6 % [4, 6, 8, 9, 13–20]. Mean age was 52.5 years, and 77 % of patients were men. The mean AHI was 58 events/h, and mean PA pressure was 39.3 ± 6.3 mmHg. On average, OSA patients with PH had a BMI 3.3 ± 0.7 units higher compared to OSA patients without PH (p < 0.0001). The duration of CPAP treatment ranged from 3 to 70 months. The mean daily CPAP use was at least more than 4.5 h per night for each study. In pooled analysis, CPAP therapy was associated with a 13.3 mmHg reduction in PA pressure (95 % CI 12.7–14.0; p < 0.0001, Fig. 3a), using the fixed model and a 9.6 mmHg reduction in PA pressure (95 % CI 5.5–13.7; p < 0.0001, Fig. 3b) using random effects meta-analysis. Figure 4 depicts the cumulative effect of the mean difference in PA pressure over time. There was low evidence of publication bias (Egger’s test p = 0.31). Overall, the risk of bias was low to moderate, with the majority of the bias revolving around the investigators’ inability to blind the participants due to the nature of the intervention (Fig. 2).
Discussion
This meta-analysis demonstrates that CPAP therapy is associated with a 13.3 mmHg reduction in PA pressure in patients with isolated OSA who have pulmonary hypertension (p < 0.0001). We found no correlation between severity of OSA and the degree of elevation of PA pressures, as also reported by others [9, 21–23].
Previous studies have found mixed results of CPAP therapy in patients with PH and OSA [15, 24, 25]. A decrease in PA pressures with CPAP has been noted, while others have found no change with CPAP treatment [15, 24, 25]. However, some of these studies included patients with coexisting lung disease and compliance of patients on CPAP may have been difficult to monitor objectively. A previous meta-analysis conducted by Sun et al. [22] found a standardized mean difference in PA pressure of −1.34 mmHg (95 % CI −2.33 to −0.34) in all OSA patients receiving CPAP. Although the effect of CPAP reported by Sun et al. is in the same direction as findings from our meta-analysis, the magnitude of effect is much greater in our study than in the previous meta-analysis. There are several plausible explanations for why this might occur. First, the patient population differs between the two meta-analyses. We specifically included patients with isolated OSA, those without other pulmonary or cardiac co-morbid conditions. This is in contrast with Sun et al., who included all patients with OSA regardless of other diagnoses in their analyses. It is possible that CPAP treatment led to some degree of improvement in PH secondary to OSA in these patients, but that their co-morbid pulmonary disease remained unchanged. Co-morbid conditions such as chronic obstructive pulmonary disease and heart failure can also lead to PH in these patients, and thus, the PA pressure may not be altered significantly after CPAP therapy. Second, the previous meta-analysis included some studies that do not meet the current definition of PH as defined by a PA pressure >25 mmHg at rest. Third, we have included two additional studies that were published after the previous meta-analysis was conducted, which may affect the magnitude of the estimate.
Potential biological mechanisms by which OSA may lead to PH include hypoxic pulmonary vasoconstriction, pulmonary vascular remodeling, and endothelial dysfunction [9, 26]. There may be a genetic predisposition to developing pulmonary vascular remodeling, as not all patients with OSA without lung disease have PH [27]. Genetic variability has been reported in sheep and humans who reside at high altitudes [27]. Patients with baseline arteriolar narrowing may have a heightened response to hypoxia, and they may be at risk for pulmonary vascular remodeling with repetitive episodes of apnea [9]. Chronic hypoxia may also impact the transcription of genes involved in vasoconstriction and smooth muscle proliferation [28]. Decreased oxygen tension increases the production of multiple mitogenic factors for endothelial cells [12–15, 29–32]. Endothelin-1 and serotonin, potent vasoconstrictors, had increased expression when placed in a hypoxic environment [33]. However, nitric oxide, a vasodilator [34] and inhibitor of smooth muscle and fibroblast growth [35, 36], has been shown to have decreased transcription during hypoxia [37]. It is postulated that CPAP may reduce pulmonary vascular reactivity to hypoxia and improve pulmonary endothelial function, resulting in decreased PA pressures [38]. CPAP has also been shown to improve cardiovascular remodeling as assessed by serial cardiac biomarkers, transthoracic echocardiography (TTE) and cardiac magnetic resonance imaging [1]. TTE in patients undergoing CPAP therapy has demonstrated an improvement in the degree of PH, and related parameters such as right ventricular end-diastolic diameter, left atrial volume index, and right atrial volume index. Cardiac magnetic resonance imaging has revealed a decrease in left ventricular mass, with progressive improvement over the course of a year [1]. Thus, CPAP therapy may lead to resolution of systolic and diastolic abnormalities and improvement in cardiovascular remodeling in OSA patients with PH. Pulmonary hypertension in patients with OSA also portends a worse prognosis and may lead to complications such as right ventricular failure over time [4, 39]. Thus, aggressive therapy with CPAP may be warranted.
The current study has some limitations. First, we analyzed only seven studies in the meta-analysis since the literature on the effects of CPAP on PA pressure for patients with isolated OSA is currently limited. Second, all studies, with the exception of one, utilized TTE to assess PA pressures on follow-up, whereas right heart catheterization may be a more accurate method. However, when patients were followed over months with monitoring of PA pressure at various time intervals, TTE was widely used as it is less invasive and more easily accessible than right heart catheterization. These two methods used to assess PA pressures could have led to misclassification of PA pressure values. Third, most participants (77 %) were men, thereby limiting generalizability of our results to women.
Despite the above limitations, our study has numerous strengths: studies included in this meta-analysis were conducted at various geographic locations; thus, the overall effect may be generalizable to different populations. In addition, adherence to CPAP therapy was optimal, ranging from 87 % (5.4 h/night) in the Alchanatis study to 91 % (6 h/night) in the Arias trial [6, 9, 19]. To maximize the utilization of published data, we also included in our search studies published in languages other than English. Most importantly, our study included only patients with isolated OSA, without other comorbid conditions that can lead to PH, thereby increasing the robustness of the estimate for this patient population.
In conclusion, this meta-analysis provides evidence in support of the efficacy of CPAP therapy for patients with isolated OSA and PH. Further studies, particularly randomized controlled trials, are needed to clearly elucidate the role of this treatment modality. As pulmonary hypertension is an independent predictor of mortality in patients with OSA, reduction in PA pressure may improve survival.
References
Colish J, Walker JR, Elmayergi N, Almutairi S, Alharbi F, Lytwyn M, Francis A, Bohonis S, Zeglinski M, Kirkpatrick I, Sharma S, Jassal DS (2012) Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest 141:674–681
Basner RC (2014) Cardiovascular morbidity and obstructive sleep apnea. N Engl J Med 370:2339–2341
McLaughlin VV, Archer SL, Badesch DB, Writing Committee Members, ACCF Task Force Members (2009) ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 2009(119):2250–2294
Minai OA, Ricaurte B, Kaw R, Hammel J, Mansour M, McCarthy K, Golish JA, Stoller JK (2009) Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol 104:1300–1306
Golbin JM, Somers VK, Caples SM (2008) Obstructive sleep apnea, cardiovascular disease, and pulmonary hypertension. Proc Am Thorac Soc 5:200–206
Alchanatis M, Tourkohoriti G, Kakouros S, Kosmas E, Podaras S, Jordanoglou JB (2001) Daytime pulmonary hypertension in patients with obstructive sleep apnea: the effect of continuous positive airway pressure on pulmonary hemodynamics. Respiration 68:566–572
Dark DS (1996) Sleep apnea and pulmonary hypertension. Chest 109:300–301
Chaouat A, Weitzenblum E, Krieger J, Oswald M, Kessler R (1996) Pulmonary hemodynamics in the obstructive sleep apnea syndrome. Results in 220 consecutive patients. Chest 109:380–386
Sajkov D, Wang T, Saunders NA, Bune AJ, Neill AM, Mcevoy RD (1999) Daytime pulmonary hemodynamics in patients with obstructive sleep apnea without lung disease. Am J Respir Crit Care Med 159:1518–1526
Tilkian AG, Guilleminault C, Schroeder JS, Lehrman KL, Simmons FB, Dement WC (1976) Hemodynamics in sleep-induced apnea. Studies during wakefulness and sleep. Ann Intern Med 85:714–719
Podszus T, Bauer W, Mayer J, Penzel T, Peter JH, von Wichert P (1986) Sleep apnea and pulmonary hypertension. Klin Wochenschr 64:131–134
Guyatt GH, Oxman AD, Vist G, Kunz R et al (2011) GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol 64:407–415
Laks L, Lehrhaft B, Grunstein RR, Sullivan CE (1995) Pulmonary hypertension in obstructive sleep apnoea. Eur Respir J 8:537–541
Laks L, Lehrhaft B, Grunstein RR, Sullivan CE (1997) Pulmonary artery pressure response to hypoxia in sleep apnea. Am J Respir Crit Care Med 155:193–198
Chaouat A, Weitzenblum E, Kessler R, Oswald M, Sforza E, Liegeon MN (1997) Five-year effects of nasal continuous positive airway pressure in obstructive sleep apnoea syndrome. Eur Respir J 10:2578–2582
Niijima M, Kimura H, Edo H, Shinozaki T, Kang J, Masuyama S, Tatsumi K, Kuriyama T (1999) Manifestation of pulmonary hypertension during REM sleep in obstructive sleep apnea syndrome. Am J Respir Crit Care Med 159:1766–1772
Bady E, Achkar A, Pascal S, Orvoen-Frija E, Laaban JP (2000) Pulmonary arterial hypertension in patients with sleep apnoea syndrome. Thorax 55:934–939
Sajkov D, Wang T, Saunders NA, Bune AJ, McEvoy RD (2002) Continuous positive airway pressure treatment improves pulmonary hemodynamics in patients with obstructive sleep apnea. Am J Respir Crit Care Med 165:152–158
Arias MA, Garcia-Rio F, Alonso-Fernandez A, Martinez I, Villamor J (2006) Pulmonary hypertension in obstructive sleep apnoea: effects of continuous positive airway pressure: a randomized, controlled cross-over study. Eur Heart J 27:1106–1113
Krieger J, Sforza E, Apprill M, Lampert E, Weitzenblum E, Ratomaharo J (1989) Pulmonary hypertension, hypoxemia, and hypercapnia in obstructive sleep apnea patients. Chest 96:729–737
Sajkov D, Cowie RJ, Thornton AT, Espinoza HA, McEvoy RD (1994) Pulmonary hypertension and hypoxemia in obstructive sleep apnea syndrome. Am J Respir Crit Care Med 149:416–422
Sun X, Luo J, Xiao Y (2014) Continuous positive airway pressure is associated with a decrease in pulmonary artery pressure in patients with obstructive sleep apnoea: a meta-analysis. Respirology 19:670–674
Atwood CW Jr, McCrory D, Garcia JG, Abman SH, Ahearn GS (2004) American College of Chest P. Pulmonary artery hypertension and sleep-disordered breathing: ACCP evidence-based clinical practice guidelines. Chest 126:72S–77S
Fletcher EC, Schaaf JW, Miller J, Fletcher JG (1987) Long-term cardiopulmonary sequelae in patients with sleep apnea and chronic lung disease. Am Rev Respir Dis 135:525–533
Sforza E, Laks L, Grunstein RR, Krieger J, Sullivan CE (1998) Time course of pulmonary artery pressure during sleep in sleep apnoea syndrome: role of recurrent apnoeas. Eur Respir J 11:440–446
Faller DV (1999) Endothelial cell responses to hypoxic stress. Clin Exp Pharmacol Physiol 26:74–84
Ahmed T, Oliver W Jr, Wanner A (1983) Variability of hypoxic pulmonary vasoconstriction in sheep. Role of prostaglandins. Am Rev Respir Dis 127:59–62
Foster GE, Poulin MJ, Hanly PJ (2007) Intermittent hypoxia and vascular function: implications for obstructive sleep apnoea. Exp Physiol 92:51–65
Kourembanas S, McQuillan LP, Leung GK, Faller DV (1993) Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. J Clin Invest 92:99–104
Hannan RL, Kourembanas S, Flanders KC, Rogelj SJ, Roberts AB, Faller DV, Klagsbrun M (1988) Endothelial cells synthesize basic fibroblast growth factor and transforming growth factor beta. Growth Factors 1:7–17
Perkett EA, Badesch DB, Roessler MK, Stenmark KR, Meyrick B (1992) Insulin-like growth factor I and pulmonary hypertension induced by continuous air embolization in sheep. Am J Respir Cell Mol Biol 6:82–87
Shweiki D, Neeman M, Itin A, Keshet E (1995) Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: implications for tumor angiogenesis. Proc Natl Acad Sci USA 92:768–772
Kholdani C, Fares WH, Mohsenin V (2015) Pulmonary hypertension in obstructive sleep apnea: is it clinically significant? A critical analysis of the association and pathophysiology. Pulm Circ 5(2):220–227
Ignarro LJ, Byrns RE, Buga GM, Wood KS (1987) Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res 61:866–879
Garg UC, Hassid A (1989) Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest 83:1774–1777
Nakaki T, Nakayama M, Kato R (1990) Inhibition by nitric oxide and nitric oxide-producing vasodilators of DNA synthesis in vascular smooth muscle cells. Eur J Pharmacol 189:347–353
Phelan MW, Faller DV (1996) Hypoxia decreases constitutive nitric oxide synthase transcript and protein in cultured endothelial cells. J Cell Physiol 167:469
Kolar F, Ostadal B (1991) Right ventricular function in rats with hypoxic pulmonary hypertension. Pflugers Arch 419:121–126
Berkowitz R, Alhaj E, Manchikalapudi RB, Satya K, Dadfarmay S, Zakir R (2010) Determinants of right ventricular failure in patients admitted with acute left heart failure. Congest Heart Fail 16:243–248
Duchna HW, Myslinski W, Dichmann M, Rasche K, Schultze-Werninghaus G, Orth M (2006) Cardiac structure and function in patients with obstructive sleep apnea syndrome and co-prevalent arterial hypertension. Influence of CPAP therapy. Med Klin (Munich) 101(1):1–8
Abou Shehata ME, El-Desoky ME, Maaty AE, Abd-ElMaksoud AM, Suliman LA (2013) Pulmonary hypertension in obstructive sleep apnea hypopnea syndrome. Egypt J Chest Dis Tubercu 62:459–465
Marvisi M, Vento MG, Balzarini L, Mancini C, Marvisi C (2015) Continuous positive airways pressure and uvulopalatopharyngoplasty improves pulmonary hypertension in patients with obstructive sleep apnoea. Lung 193:269–274
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Ashtyani has served as an expert witness regarding pulmonary hypertension. All other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Imran, T.F., Ghazipura, M., Liu, S. et al. Effect of continuous positive airway pressure treatment on pulmonary artery pressure in patients with isolated obstructive sleep apnea: a meta-analysis. Heart Fail Rev 21, 591–598 (2016). https://doi.org/10.1007/s10741-016-9548-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-016-9548-5