Abstract
Heart failure (HF) with preserved ejection fraction (HFPEF) is an increasingly prevalent clinical syndrome with many unresolved issues regarding diagnosis, pathophysiology, and treatment. The major pathophysiological mechanisms underlying HFPEF are known to be fibrosis and reduced ventricular compliance, and hypertension (HTN) is perhaps the most significant risk factor for the development of left ventricular diastolic dysfunction (LVDD). Inflammation is one of the earliest events in cardiac stress situations such as pressure and/or volume overload and involves elevated levels of endothelial adhesion molecules as well as increased production and release of inflammatory cytokines and chemokines in the tissue. The latter promotes the infiltration of activated inflammatory cells, particularly monocytes, into the cardiac tissue. Increased monocyte infiltration is seen in the early and late stages of HTN and HFPEF. Once inside the tissue, monocytes differentiate into macrophages and promote cardiac inflammation, tissue injury, and myocardial fibrosis. This review focuses on inflammation as the initial and primary trigger of ventricular remodelling in HTN and LVDD, affecting progression to HFPEF. The link between inflammation and b-type natriuretic peptide (BNP), a clinical marker of cardiac pressure overload which is positively associated with cardiac dysfunction and HF, is also described. Finally, current and prospective therapeutic approaches for HFPEF based on modification of the inflammatory response are reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Heart failure with preserved ejection fraction
Heart failure (HF) is a complex clinical syndrome with increasing prevalence, high hospitalisation, and mortality rates, but poor diagnostic and treatment options [1]. HF is classified according to the degree of structural abnormality (American College of Cardiology (ACC)/American Heart Association (AHA) guidelines [2]) and by symptoms relating to functional capacity (New York Heart Association (NYHA) classification [1]), and is based upon ejection fraction (EF) which distinguishes between HF with reduced left ventricular (LV) ejection fraction (LVEF) (HFREF) and HF with preserved LVEF (HFPEF). HFREF defines HF with an underlying abnormality in systolic function leading to reduced expulsion of blood from the ventricle with every cardiac cycle (LVEF < 40 %). On the contrary, HFPEF is diagnosed based on signs and symptoms of HF, normal or only mildly abnormal LV systolic function (LVEF > 50 % in a non-dilated LV), and evidence of diastolic dysfunction defined by prolonged LV relaxation time, increased LV diastolic stiffness, slow LV filling, and elevated LV end-diastolic pressures [3, 4].
HFPEF contributes to approximately 50 % of the total HF population, and prevalence as well as incidence of HFPEF is continuing to rise due to increased awareness, increased comorbidities, and little advancement in therapy [5–8]. One year readmission and morbidity rates are identical to those of HFREF patients, and annual mortality is between 5 and 8 % [9]. However, as opposed to HFREF for which prognosis and survival have improved due to successful evidence-based therapies for HF, survival rates for HFPEF have remained unchanged during the last two decades [5].
In order to tackle HFPEF, major effort has been directed towards prevention and early diagnosis of the disease. Preventing disease progression at the earliest possible stage will substantially reduce the economic burden of multidrug treatment and recurrent hospitalisation, and will considerably improve patient well-being as well as the overall clinical outcome. To achieve this, an in-depth understanding of the pathophysiological events associated with early stage (pre-) HFPEF is a must. Indeed, a growing concern and a major limitation of progress in the field of HFPEF diagnosis, prognosis, and treatment is the lack of sufficient understanding of the mechanisms driving LV remodelling in HFPEF [10]. In addition, the complexity of measuring diastolic function parameters and attributing correct LV functional classification in animal models of diastolic dysfunction and HFPEF has made it difficult to identify pertinent disease mechanisms that are relevant to patients with LV diastolic dysfunction (LVDD) and HFPEF [11].
From a pathophysiological point of view, it is considered that one of the most significant risk factors and a leading cause of LVDD and HFPEF is chronic pressure overload (i.e. hypertension (HTN)), with approximately 60–80 % of the patients diagnosed with HFPEF presenting with HTN [5, 8, 12, 13]. Increases in blood pressure are directly associated with an increased risk of HF in the long term as demonstrated by the Framingham study [14]. Other molecular and cellular mechanisms promoting LVDD symptoms including slow LV relaxation and elevated diastolic LV stiffness include changes in the extracellular matrix (ECM) and cardiomyocytes, posttranslational changes in the sarcomeric protein titin including phosphorylation and isoform switch, increased nitrosative and oxidative stress, reduced bioavailability for nitric oxide, and reduced myocardial cyclic guanosine monophosphate (cGMP) and protein kinase G (PKG) signalling, and these have been comprehensively reviewed in a recent paper by van Heerebeek et al. [15].
Whilst we appreciate the multivariate nature of symptoms and causes of HFPEF, for the purpose of this review, we will focus specifically on the most established pathophysiological sequence of events causing HFPEF, namely the natural progression from chronic pressure overload and LV diastolic dysfunction to HFPEF.
Pathophysiology of HFPEF
During the progression of hypertensive heart disease, a constellation of structural and functional cardiac abnormalities including LV hypertrophy (LVH), myocardial fibrosis, ischaemia, myocyte impairment, endothelial dysfunction, and increased arterial stiffness result from the underlying chronic HTN [6, 13]. A signature event in the early response to pressure overload is the accumulation of activated inflammatory cells (especially monocytes) in the heart, which, via the release of inflammatory mediators such as monocyte chemotactic protein-1(MCP1) and tumour necrosis factor alpha (TNFα), and fibrogenic activators such as transforming growth factor beta (TGFβ), maintain and augment further pro-inflammatory and pro-fibrotic processes [16–18]. This leads to structural and mechanical remodelling of the heart muscle mainly due to excessive interstitial deposition of ECM proteins such as collagens. In parallel with these inflammatory and fibrotic changes, the myocardium responds to mechanical strain via an adaptive hypertrophic increase in cardiomyocyte diameter, myofibrillar density, and myocyte passive tension, which are reported in chronic HTN patients who subsequently develop diastolic dysfunction [19, 20]. Echocardiographic evidence of concentric LVH is found in the majority of HFPEF patients [2, 20]. In the hypertrophied heart, substantial ventricular distortion is prevented by the activation of compensatory mechanisms like interstitial myocardial fibrosis. The latter aims to ensure that the heart would not distort and that the force generated by the hypertrophied myocytes is distributed throughout the entire ventricle as efficiently as possible. However, the prolonged exposure of the heart to these “compensatory forces” ultimately overloads the heart causing severe myocardial stiffness and impaired cardiomyocyte contractility in patients with HTN. The significance of inflammation and myocardial fibrosis, two processes known to have particularly important and intriguing roles in the process of hypertensive cardiac remodelling in LVDD and HFPEF, are described in detail below.
LVDD in HTN with or without LVH occurs as a result of changes in myocardial structure determined by cellular and extracellular matrix dyssynchrony. These cause alterations in the active relaxation of the ventricle and/or changes in diastolic compliance resulting in delayed LV relaxation, reduced distensibility, and increased chamber stiffness [4, 21]. Currently, the gold standard for assessing diastolic function and diagnosing LVDD is two-dimensional transthoracic Doppler echocardiography [3, 4, 22].
Patients with LVDD can often be asymptomatic for months or years without developing symptoms of HFPEF. The borderline between LVDD and HFPEF is thin and defined primarily by the manifestation of symptoms for congestive HF, most common being breathlessness (dyspnoea) due to pulmonary congestion and lung oedema [3, 23]. Dyspnoea and fatigue on exertion develop as a result of reduced LV filling, low cardiac output as the disease progresses, and cardiac inflammation and remodelling intensifies with a further up-regulation of pro-inflammatory mediators, including MCP1 and intercellular adhesion molecule-1 (ICAM1); increases in fibrillar collagen deposition, collagen cross-linking and fibrosis; increases in the transition of cardiac fibroblasts to myofibroblasts; reductions in matrix metalloproteinases (MMP) expression or activity; and increases in tissue inhibitors of metalloproteinases (TIMP)—all causing gradual destruction of the normal cardiac interstitium [16, 24–30]. Along with these changes, and also as a result of them, inadequate activation of various cytokines and neurohormonal factors, including the renin–angiotensin–aldosterone system (RAAS), catecholamines, and endothelin [24, 31]; increased oxidative and nitrotyrosine content; impaired nitric oxide bioavailability; and impaired myocardial cGMP and PKG signalling [15] contribute to the early and late myocardial remodelling in LV diastolic dysfunction and HFPEF. All of these processes are intertwined forming intricate self-regulating networks. This makes it hard to describe the exact natural history of HFPEF, a topic of constantly increasing interest among clinicians and researchers. A schematic of the major pathophysiological events believed to be associated with pressure-overload-induced LVDD and HFPEF is presented in Fig. 1.
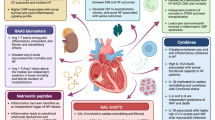
The significance of inflammation and the role of monocyte/macrophage—fibroblast interaction in hypertensive cardiac remodelling and HFPEF pathophysiology. Chronic pressure overload, i.e. hypertension (HTN) regulates pro-inflammatory mechanisms and activates circulating monocytes to infiltrate into the tissue, differentiate into macrophages, and cause inflammation. The infiltration is aided by gradients of chemoattractants like MCP1 secreted by endothelial cells and tissue macrophages; the expression of adhesion molecules on endothelial cells (ICAM, VCAM) and circulating monocytes (selectins, intergrins); and the expression signature of matrix-modulating enzymes and their inhibitors (i.e. MMP, TIMP). In the tissue-differentiated, TGFβ-producing macrophages activate fibroblasts to proliferate and produce collagen which subsequently results in the development of myocardial fibrosis and remodelling. Hypertensive inflammatory and fibrotic changes subsequently cause structural and hemodynamic alterations in the heart leading to LV diastolic dysfunction either directly or by causing LV hypertrophy, a result of high LV wall tension and compensatory increase in sarcomere size. In addition, pressure overload can directly cause LV hypertrophy. In either case, the resulting myocardial remodelling, along with an altered LV pressure–volume balance, an impaired cardiac contractility, delayed relaxation, increased myocardial stiffness, and reduced compliance cause LVDD. With time, further decompensation begins to project to other systems causing dyspnoea, fatigue, pulmonary congestion, and oedema. Patients with LVDD who present with symptoms of congestion are classified as HFPEF patients
With accumulating evidence demonstrating an injurious and vital impact of early cardiac and vascular remodelling in the development of HFPEF, novel, more effective therapeutic strategies for HFPEF should involve reversing or slowing down cardiac inflammation, ECM remodelling, and fibrosis in addition to treating HFPEF comorbidities (i.e. atrial fibrillation, hypertension, diabetes mellitus, and coronary artery disease (CAD)), which is advised in the 2012 ESC guidelines for the management of HFPEF [1].
Inflammation: the trigger of hypertensive HFPEF: the evidence
Inflammation in hypertensive cardiac remodelling
Initial evidence showing a role for inflammation in hypertensive cardiac remodelling and heart disease came from experimental and clinical observations of immune–inflammatory activation in patients with established chronic HF [32]. Since then, multiple studies have shown high levels of pro-inflammatory cytokines such as TNFα, interleukin (IL) 1, IL6, IL8, and MCP1 in the peripheral circulation and heart of HF patients [33–39]. Main sources for these cytokines are neutrophil granulocytes, monocytes, macrophages, and T cells, as well as platelets, endothelial cells, and vascular smooth muscle cells. These cytokines have repeatedly and consistently been shown to bring important prognostic information, which has led to the conclusion that these inflammatory mediators are critically implicated in the mechanisms of progression of HF, and, as recently described, specifically in HFPEF [40]. Accumulated evidence from such studies supports the existence of a repetitive and progressive state of immune–inflammatory activation that is strongly associated with the progression of ventricular diastolic dysfunction, a distinguishing feature in the pathogenesis of HFPEF, and is characterised by an intense release and activation of cytokines, complement, adhesion molecules, and autoantibodies in the circulation [33, 34].
In addition to the role of inflammation in late ventricular remodelling and HF stabilization, early vascular inflammation has been shown to be the most common precursor of comorbidities prevalent in HFPEF such as HTN, atherosclerosis, and CAD [29], and asymptomatic individuals with even slight evidence of low-grade vascular inflammation have been shown to have an elevated risk for subsequent major cardiovascular events [18, 41].
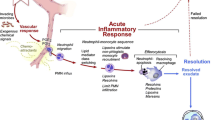
It is now well understood that in chronic vascular inflammatory disorders, such as atherosclerosis, CAD, and HTN, the earliest and most significant inflammatory event to initiate atherosclerotic vascular lesion formation is the recruitment of circulating inflammatory cells (primarily monocytes) that attach to the endothelium and transmigrate into the vascular lesion. Transmigration is made possible due to the interactions between specific cell surface molecules on monocytes (selectins, integrins, complement receptors) and endothelial adhesion molecules (ICAM1, vascular cell adhesion molecule-1(VCAM1)), and is directed by chemokine gradients (MCP1, IL8) secreted within the injured tissue and the activated vessel wall [42] (fig. 1). Once inside the vessel wall (or inside the myocardium), monocytes differentiate into macrophages, which play a key role in orchestrating responses to injury and promoting wound repair. Macrophage activation and function is controlled by complex cell–cell interactions and a milieu of secreted pro- and anti-inflammatory cytokines, fibrotic mediators, chemokines, and growth factors released in the tissue and the periphery. These complex interactions within the injured tissue define the specificity of macrophage activation and the phenotype of macrophages—pro-inflammatory type 1 (M1) or anti-inflammatory, pro-remodelling type 2 (M2), which dictate the function of these cells in the tissue [43, 44]. In a normal “healthy wound” situation, macrophages are called to the tissue where they deal with the injury and promote healing before they clear the tissue and/or undergo apoptosis. However, in a state of disease, exaggerated injury (e.g. sustained hypertension) or uncontrolled inflammation lead to the sustained presence of activated macrophages, which damage the tissue and potentially promote aberrant tissue remodelling. This suggests that modulation of monocyte and macrophage activation and function may be an efficacious strategy to prevent tissue damage and improve cardiovascular function.
Inflammation in animal models of hypertensive cardiac remodelling
Initial evidence from animal studies aimed at investigating the significance of inflammation in the aetiology and progression of HTN has confirmed the significance of this process for hypertensive myocardial remodelling and has also indicated that there exists an intricate cause–effect relationship between inflammatory and fibrotic processes in the hypertensive heart. In rat models of hypertension (spontaneously hypertensive rat (SHR) and renovascular hypertensive rat), distinctive macrophage and fibroblast accumulation have been detected in perivascular regions of the pressure-overloaded heart [45, 46]. In addition, altered ICAM1 expression, observed in the endothelium of SHR, suggested that pressure overload directly regulates the abnormal inflammatory responses associated with this disease [47]. In a rat model of pressure overload caused by suprarenal abdominal aortic constriction (AC), the rapid increase in arterial pressure triggered a series of inflammatory and fibrotic changes in the intramyocardial arterial wall [16]. In this model, the earliest event detected was up-regulation of MCP1 and ICAM1 (1-day post-insult) in the intramyocardial arteries. This was followed by the accumulation of perivascular macrophages, which co-localised to sites of cytokine expression, and also resulted in enhanced TGFβ expression, fibroblast proliferation, and conversion of fibroblasts to myofibroblasts (day 3). Later in the time course of events, reactive fibrosis, LVH, and myocyte hypertrophy developed (after day 7), and LVDD was diagnosed at day 28. Similarly, rats with experimental myocardial infarction which were treated with an angiotensin receptor blocker had attenuated LV remodelling due to reduced MCP1 expression and macrophage infiltration resulting in diminished myocardial fibrosis [48]. These models show clear evidence of a link between inflammation and fibrosis in this disease setting and establish inflammation as the initial event that triggers the onset of fibrosis in the perivascular space before expansion to the interstitium.
The induction of MCP1 to prime circulating monocytes to invade the vessel wall and thus initiate inflammation can be driven by stretch, but also by oxidative stress and activation of RAAS [49, 50], and significant activation of RAAS has been shown in HFPEF rather than in HFREF patients [51]. Angiotensin II has been shown to induce MCP1 expression in macrophages and up-regulate TGFβ in cardiac myocytes and fibroblasts in animal models of atherosclerosis [52] and pressure overload [17], suggesting that the activation of RAAS may precede the onset of inflammation and fibrosis in hypertensive heart failure. Inhibition of MCP1 with an anti-MCP1 monoclonal neutralising antibody in a rat model of developing diastolic dysfunction was shown to abolish macrophage infiltration and TGFβ induction, attenuate myocardial fibrosis, and improve diastolic function without affecting blood pressure, myocyte hypertrophy, or systolic function [16]. Similarly, MCP1 depletion in a mouse model of angiotensin II-infusion aimed at clarifying the early cellular mechanisms linking interstitial fibrosis with the onset of the tissue inflammatory response in cardiac failure showed that non-adaptive fibrosis resulting in HTN and hypertrophy requires induction of MCP1, which stimulates the differentiation of fibroblast precursor cells [53]. In addition, studies with MCP1 null mouse models of ischaemic cardiomyopathy and CCR2 (MCP1 receptor) knockout mice with HTN and LVH have shown markedly diminished interstitial fibrosis, low macrophage infiltration, and attenuated ventricular dysfunction [54, 55]. In one study, the reduction in cardiac fibrosis in angiotensin II-infused CCR2 knockout mice was attributed to impeded accumulation of bone marrow-derived fibroblast precursors in the heart [56]. Similar beneficial effects were noted also when ICAM1 function was blocked [27].
Further evidence supporting the role of leukocyte-induced inflammation in pressure overload and HF comes from a hypertensive animal model of oxidative and inflammatory stress. [57]. It was shown in deoxycorticosterone acetate and sodium chloride-treated rats (i.e. DOCA-salt hypertensive rats) that the intricate combination of inflammation and oxidative stress is a necessary basis for eliciting a chronic pathophysiological stress state—a prerequisite for initiating cardiovascular remodelling (expressed as HTN, hypertrophy, fibrosis, electrical conduction abnormalities, vascular hypertrophy, and dysfunction) and heart failure. In the same model, cardiac ECM remodelling was associated with an up-regulation of inflammatory mediators (nuclear factor kappa B (NFκB), VCAM, platelet-endothelial cell adhesion molecule (PECAM) 1), increased fibronectin, and MMP activity [58]. Finally, in two-month-old rats with hypertensive heart failure, the addition of rosuvastatin to the standard anti-hypertensive HF therapy (quinapril plus torasemide plus carvedilol) was able to improve cardiac remodelling associated with HFPEF, and these beneficial effects were due, at least in part, to decreased myocardial inflammation [59].
Taken together, these studies suggest that leukocyte-mediated perivascular inflammation is a key event in triggering early ECM changes, myocardial fibrosis, and LVDD in pressure overload. Therefore, targeting monocyte infiltration and macrophage function could be an effective new strategy to prevent hypertensive myocardial remodelling in HFPEF.
Inflammation in patients with hypertension-induced LVDD and HFPEF
The significance of inflammation in patients with diastolic LV dysfunction and HFPEF perhaps has been underestimated due to the complexity of diagnosing diastolic dysfunction in disease-relevant animal models and the overall lack of adequate translational models.
Existing data in patients with diagnosed HFPEF is scarce, but studies in patients with clinical conditions predisposing to and prevalent in HFPEF, such as HTN, CAD, LVDD, and metabolic syndrome, have helped to shed light on the processes involved.
In a study on cardiovascular damage in patients with metabolic syndrome, increased levels of inflammation (urinary albumin, C-reactive protein (CRP), TNFα, and TGFβ) were found to be independently associated with asymptomatic diastolic dysfunction [60]. In other studies, increased inflammation (CRP) [61], platelet activation, and endothelial dysfunction [62] were predictive of abnormal diastolic function in patients with stable CAD. Comorbidities such as HTN, CAD, obesity, and diabetes that are associated with the syndrome of HFPEF and the systemic inflammatory state (defined by high levels of peripheral IL6 and TNFα) induced by them has recently been shown to be predictive of incident HFPEF, but not incident HFREF [29]. High circulating IL6, TNFα, IL8, and MCP1 were also detected in a cross-sectional study of 275 stable hypertensive patients with and without HFPEF [30]. Two similar large cross-sectional studies, one in patients with acute dyspnoea and preserved LVEF [63] and one in HFPEF patients [64], identified the independent systemic inflammatory markers soluble ST2 (member of the IL1 receptor family) and PTX3 (pentraxin 3), respectively, to correlate with the presence of LVDD and HFPEF and to be strong predictors of mortality in these patients.
The importance of systemic inflammation, specifically in patients with HFPEF, has been highlighted in a recent study comparing physiologically distinct circulating biomarkers in HFPEF patients, HFREF patients, and community controls [40]. The authors provided important evidence of a distinguishing role for myocardial injury (high-sensitivity troponin T) with increased wall stress (N-terminal pro-BNP) in the pathophysiology of HFREF, and a role for systemic inflammation, defined by high levels of growth differentiation factor 15 (GDF15), as a crucial determinant specifically in the progression of HFPEF.
The systemic inflammatory state induced by HFPEF comorbidities starts to gradually affect the cardiac vascular endothelium resulting in increased expression of endothelial adhesion molecules including VCAM1 in the heart. This has been shown in left ventricular endomyocardial biopsy samples from HFPEF patients by Westermann et al. [28]. VCAM, among other endothelial adhesion molecules, is a marker of inflammatory endothelial activation and high expression leads to the activation and subendothelial migration of circulating leukocytes. HFPEF patients had high numbers of CD3, CD11, and CD45-positive leukocytes in the myocardium, increased inflammatory cell TGFβ expression, and increased levels of collagen I and III [28]. TGFβ is the best-known inducer of collagen production and stimulates the differentiation of fibroblasts into myofibroblasts thus altering the cardiac ECM homeostasis and predisposing to diastolic dysfunction. Based on these findings, the authors concluded that myocardial inflammation has an important role in HFPEF pathophysiology by promoting ECM changes and diastolic dysfunction. In addition, it has been demonstrated that activated myofibroblasts can themselves induce inflammation by producing cytokines and chemokines which stimulate inflammatory cell recruitment and activation [65]. This recurrent inflammatory boost may have detrimental effects on the heart by intensifying fibrosis and promoting diastolic dysfunction and subsequently HFPEF.
B-type natriuretic peptide (BNP) and cardiac inflammation: relevance to hypertension, diastolic dysfunction, and HFPEF
BNP is a circulating endocrine factor positively implicated in the regulation of blood pressure and is a useful prognostic and diagnostic marker for hypertension and HF regardless of ejection fraction [66–68]. BNP’s prognostic and diagnostic significance are partially related to its implication in the regulation of fibrotic and inflammatory pathways in the pressure-overloaded heart. BNP has potent autocrine/paracrine actions in the heart that are implemented following the interaction with its receptor, natriuretic peptide receptor A (NPRA), and the subsequent production of cGMP. NPRA is expressed in most cells of the heart including cardiomyocytes, and NPRA deletion in mice (Npr1−/−) has been shown to cause salt-resistant hypertension, cardiac hypertrophy, and fibrosis [69–71], whilst overexpression causes arterial hypotension [72]. Locally targeted overexpression of NPRA in cardiomyocytes attenuates pressure-induced LVH [73, 74]. On the other hand, myocyte-specific NPRA deletion in mice causes cardiac hypertrophy and impaired diastolic relaxation [75]. In the setting of deletion of the BNP gene (Nppb−/−), the knockout animals develop focal cardiac ventricular fibrotic lesions and increase ventricular expression of pro-fibrotic genes including angiotensin-converting enzyme (ACE), TGFβ3, and pro-α1-collagen [76]. The expression and function of BNP were more recently also shown in cardiac fibroblasts [77–79]. Data from in vivo studies with human cardiac fibroblasts support an important paracrine role for the peptide in the regulation of fibroblast proliferation and function in cardiac hypertrophy via opposing the actions of TGFβ, decreasing collagen synthesis, and increasing MMP activity [78–80]. Consistent with these data, our group has described protective effects of BNP in vitro in mechanically stretched fibroblasts, where treatment with the peptide attenuated the effects of TGFβ in inducing myofibroblast differentiation, indicated by a reduction in protein levels of α-smooth muscle actin [81]. In vivo studies, Npr1−/− mice had increased expression of fibrotic genes including MMP2, MMP9, TGFβ, TNFα, and total collagen [82]. Taken together, these studies establish BNP as a potential anti-fibrotic factor and a local regulator of ventricular remodelling in the heart.
The BNP/NPRA system has recently also been shown to regulate inflammatory networks in the diseased heart. Recent evidence from animal studies shows enhanced pro-inflammatory cytokine gene expression in Npr1−/− mice [83]. A three- to fivefold induction of TNFα, IL6, and TGFβ1 expression was demonstrated in the left ventricles of the knockout animals. In another study, knockout of NPRA in mice caused up-regulation of NFκB activity and TNFα expression supporting a role for BNP in counterbalancing these inflammatory mediators [82]. In line with this, cardiac-specific overexpression of TNFα and IL6 in mice proved to be sufficient to induce cardiac hypertrophy and LVDD [84, 85]. In vitro studies with neonatal rat ventricular cardiomyocytes have also shown increased synthesis and secretion of BNP following treatment of the cells with TNFα or IL1β [86]. Furthermore, the same study reported increased plasma BNP levels in the absence of hemodynamic changes in an in vivo mouse model of sepsis and supported a unique regulatory role for BNP (but not ANP) in the setting of an inflammatory process. In summary, it seems very likely that the BNP/NPRA/cGMP system has an important anti-inflammatory role in the heart.
Recent data have shown a substantial correlation between BNP levels and serum markers of inflammation in animal models as well as patients. BNP and IL6 gene levels were consistently elevated in cardiac hypertrophy complicated with diastolic LV dysfunction in spontaneously hypertensive rats, indicating active inflammatory processes in these hearts [87]. Additionally, a significant correlation between IL6, BNP, and LV end-diastolic dimension (LVEDD) values was found in patients with idiopathic LV dysfunction [88] and elevated BNP correlated with TNF and LVEDD in chronic HF patients [89]. Ahmad et al. [90] also identified an association between TNFα, IL6, NT-proBNP, and LV function recovery in patients with dilated cardiomyopathy. In addition, N-terminal proBNP levels correlated with CRP and systolic and diastolic blood pressure in chronic renal failure patients with or without known cardiomyopathy [91].
Along with the published data, our group also identified significant correlation between central BNP levels and levels of TNFα, IL6, and IL8 in hypertensive patients at risk of developing HFPEF [92]. Furthermore, peripheral BNP levels correlated with central levels of TNFα, IL6, IL8, and MCP1. This suggested that in asymptomatic HTN patients, a peripheral BNP measurement may be a useful marker of early, sub-clinical pathological processes including inflammation, ECM alterations, and cardiac remodelling—important pathophysiological determinants of HFPEF.
Whilst it is not known what promotes the release of inflammatory mediators in high-BNP patients, it is considered that BNP could be able to modulate the inflammatory response by affecting different immune cell functions, like leukocyte migration, and activation, or by interfering with the integrity of the vasculature, i.e. the endothelial and smooth muscle layers. In fact, a study published a few years ago showed that BNP can up-regulate the production of pro- and anti-inflammatory molecules like reactive oxygen and nitrogen species, leukotriene B4, and prostaglandin E2; increase IL10 levels; and affect cell motility of monocytic THP1 cells [93]. In another study, co-culture of peripheral blood mononuclear cells (PBMC) from cardiac transplant recipients with BNP caused a reduction in pro-inflammatory cytokines (TNFα, IL6, IL1α), whilst expression of anti-inflammatory and regulatory cytokines (IL4, IL5, IL13) was preserved [94]. Our group has also very recently shown evidence of a cardio-protective regulatory role of BNP in an in vitro inflammatory setting where BNP was able to directly oppose human monocyte migration to MCP1 [95]. The ability of BNP to block MCP1-induced chemotaxis was attenuated in monocytes from HTN and HFPEF patients suggesting that this potentially beneficial anti-inflammatory function of BNP is likely compromised in chronic pressure overload and HFPEF [95].
Even though most of these new data are relatively descriptive, when taken together, they suggest that there exists a circumstantial relationship between BNP, leukocyte (i.e. monocyte/macrophage) function, and inflammation—a concept which needs further exploration in the context of HFPEF and heart failure in general.
Current and prospective anti-inflammatory therapeutic approaches for HFPEF
The pathophysiological mechanisms underlying HFPEF are still not well understood, which is why it is not surprising that to date, there is no effective treatment for this complex syndrome. Current ESC/HFA and ACC/AHA guidelines recommend control of blood pressure, ischaemia, tachycardia, and targeting the two most important comorbidities, i.e. diabetes and LVH, to be the most effective approaches for HFPEF [1, 2]. However, the current classes of pharmacological agents used for reduction in blood pressure, congestion, and circulation volume, and reduction in ventricular remodelling, namely β-blockers, diuretics, aldosterone antagonists, ACE inhibitors, and angiotensin receptor blockers which have shown significant benefit for treatment of HFREF failed to show particular benefit in long-term outcome (mortality) or quality of life (exercise capacity, hospitalisation) in clinical trials with HFPEF patients [96–100]. This is also the reason why there is currently no specific evidence-based therapy for HFPEF. Data from the currently completed clinical endpoint trials in HFPEF using anti-hypertensive agents including the SWEDIC, SENIORS, V-HeFT II, DIG-PEF, PEP-CHF, CHARM-Preserved, and I-PRESERVE trials have been comprehensively reviewed by Paulus et al. [10] and Kindermann et al. [101]. Briefly, the beta-blocker carvedilol had no effect on primary endpoints (cardiovascular death and hospitalisations for HF) (SWEDIC), and treatment with nebivolol reduced primary outcomes by 14 % (regardless of EF), but had no significant effect on LA volume, EF, LA dimensions, and function (Seniors); treatment with the ACE inhibitor perindopril had no effect on mortality and HF hospitalisations (i.e. primary endpoint) (PEP-CHF); similarly, treatment with the digitalis glycoside digoxin also showed no effect on primary and secondary endpoints (DIG-PEF); finally, the use of the angiotensin II receptor antagonists candesartan (CHARM-Preserved) and irbesartan (I-PRESERVE) did not improve mortality in both trials, but a reduction in HF hospitalisations was reported in CHARM-Preserved. The negative results from these trials once again highlight the fact that controlling HTN alone in HFPEF is insufficient to treat the disease and that other medications, such as anti-inflammatories, should be used, in addition to anti-hypertensive drugs to address the multifactorial nature of HFPEF. Whilst anti-hypertensive drugs do not appear to have beneficial effects in HFPEF, the VALIDD trial (NCT00170924) showed that lowering blood pressure using a mixture of tailored anti-hypertensive agents with or without the angiotensin receptor blocker valsartan was able to improve diastolic function in LVDD patients by reducing blood pressure and increasing diastolic relaxation velocity irrespective of the type of anti-hypertensive agent used [100]. We therefore propose that therapeutic approaches intervening with the inflammatory pathways that are known to play a role in hypertensive heart disease should be utilised in addition to an anti-hypertensive treatment in at-risk patients or patients with LVDD and/or HFPEF to prevent the detrimental effects that inflammation has on matrix remodelling in the heart. This concept is supported by the recently completed Health ABC (Health, Aging, and Body Composition) study which showed strong association of inflammatory markers with HFPEF (as compared to HFREF) and pointed out the importance of identifying and targeting inflammation for improving risk stratification and reducing mortality in HFPEF [29]. Further support for the cause of targeting inflammation in HFPEF is provided by Collier et al. [30] who showed that increased pro-inflammatory cytokine levels predict future development of HFPEF in at-risk populations, i.e. hypertensive patients. The importance of inflammation in primary prevention of cardiovascular disease is also highlighted by the Jupiter trial which tested the effectiveness of rosuvastatin to reduce the rate of first major cardiovascular events (i.e. cardiovascular death, stroke, MI, hospitalisation for unstable angina, or arterial revascularization) in normoglycaemic individuals with high levels of the inflammatory mediator CRP [102]. The study found that only patients with high CRP (i.e. with evidence of systemic inflammation) benefited from the statin therapy, and by following treatment, these patients had reduced inflammation and significantly reduced incidence of major cardiovascular events [41], providing a further rationale for targeting inflammation in heart failure. Indeed, a range of broad-spectrum anti-inflammatory and immuno-modulatory approaches including anti-TNFα therapy (the RENEWAL programme), anti-oxidant therapy (A-HeFT trial), immuno-adsorption, immuno-modulation (ACCLAIM trial), and intravenous immunoglobulin therapy have been investigated and tested in clinical trials of chronic HF without significant overall benefit [103, 104]. These, however, were investigated in HFREF and not HFPEF patients. Whilst HFREF has an inflammatory component, it is unclear and unlikely that targeting inflammation in the late stages of systolic dysfunction would be of any benefit, a rationale which is supported by the negative results from these anti-inflammatory HFREF trials.
Ever since the failure of broad-spectrum anti-inflammatory therapies in HFREF, more specific treatment options have been explored in translational models of HF. Such anti-inflammatory strategies in hypertensive HFPEF-relevant animal models have shown promising results. These include targeting immunomodulatory and inflammatory cytokines and chemokines (MCP1 [16, 105], MCP3 [106], IL10 [107–109]), cytokine receptors (IL1 receptor [110, 111]), matrix-modulating enzymes (MMP) [112–119], pentraxins (PTX3) [120–122], and inflammatory signal transduction mediators (phosphatidylinositol 3-kinase gamma (PI3 Kγ) [123, 124]). Data and major conclusions from these experimental studies are summarised in Table 1.
Based on these experimental data that show a benefit of inflammatory modulation in hypertensive HFPEF-relevant animal models, it becomes evident that particular effort must be put into the precise identification of the inflammatory pathways which play a role in the immunopathogenesis of HFPEF in order to develop specific immune-modulating agents that could be used in clinical HFPEF trials. In fact, several small-scale clinical trials exploring the use of inflammatory modulators, which were shown to have beneficial effects in animal models of HFPEF, have been recently initiated. One such pilot study investigates the advantage of Anakinra, a non-glycosylated recombinant human IL1 receptor antagonist approved for the treatment of rheumatoid arthritis, in HFPEF patients (NCT01542502). The therapeutic agent is projected to have beneficial anti-inflammatory and anti-remodelling effects originating from antagonism of the pro-inflammatory cytokines IL-1α and IL-1β which are implicated in adverse ventricular remodelling, including pressure-overload-induced cardiac hypertrophy [125]. Another pilot study is currently investigating the effects of active vitamin D supplementation (paricalcitol) on left atrial volume index in HFPEF patients (NCT01630408). Paricalcitol has previously been shown to improve LV function by reducing LV mass, posterior wall thickness, and end-diastolic pressures, increasing fractional shortening, and regulating gene expression in high-salt diet fed Dahl salt-sensitive rats—a model of hypertensive HFPEF [126]. Many cardiovascular diseases including HTN, myocardial ischaemia, diabetic cardiomyopathy, and HF may arise from a low vitamin D status: low vitamin D is associated with poor prognosis in HF patients due to RAAS activation and increased inflammation [127]. However, it should be noted that the beneficial effects of paricalcitol on LV function may likely be due to direct anti-inflammatory effects and/or anti-hypertensive effects (e.g. antagonism of RAAS) which on their own may alleviate cardiac inflammation via separate signalling pathways. Sildenafil—a selective phosphodiesterase inhibitor—is another drug-targeting inflammation that holds therapeutic promise. Sildenafil was shown to rescue LV dysfunction, inflammation, and cardiac remodelling in angiotensin II-induced heart failure in mice providing rationale for its potential use as a new treatment strategy for adverse ventricular remodelling in severe hypertension and possibly HFPEF [128]. A recently completed (NCT01156636, [129]) and an ongoing (NCT01726049) clinical trial should elucidate the precise clinical benefits of this drug in HFPEF patients with pulmonary hypertension. However, besides promising results from several experimental and clinical trials, neutral results of the recently completed multi-centre, double-blind, placebo-controlled, randomised clinical trial RELAX (NCT00763867), which showed no significant improvement in exercise capacity or clinical status with administration of sildenafil (compared to placebo) in a large cohort of HFPEF patients, have decreased the overall enthusiasm in phosphodiesterase-5 inhibition for HFPEF therapy [130]. Certainly, more large randomised clinical trials in patients with HFPEF along with a better understanding of the ongoing inflammatory reactions in hypertension and diastolic LV dysfunction—the most prevalent factors in the pathophysiological progression of HFPEF, are needed to help decrease the socioeconomic burden of this increasingly prevalent and mortal syndrome.
References
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P (2012) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 14 (8):803–869
Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW (2009) 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 119(14):e391–e479
Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28(20):2539–2550
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22(2):107–133
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355(3):251–259
Hogg K, Swedberg K, McMurray J (2004) Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol 43(3):317–327
Paulus WJ, Tschope C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodelling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62(4):263–271
Liu Y, Haddad T, Dwivedi G (2013) Heart failure with preserved ejection fraction: current understanding and emerging concepts. Curr Opin Cardiol 28(2):187–196
Badano LP, Albanese MC, De Biaggio P, Rozbowsky P, Miani D, Fresco C, Fioretti PM (2004) Prevalence, clinical characteristics, quality of life, and prognosis of patients with congestive heart failure and isolated left ventricular diastolic dysfunction. J Am Soc Echocardiogr 17(3):253–261
Paulus WJ, van Ballegoij JJ (2010) Treatment of heart failure with normal ejection fraction: an inconvenient truth! J Am Coll Cardiol 55(6):526–537
Dubi S, Arbel Y (2010) Large animal models for diastolic dysfunction and diastolic heart failure-a review of the literature. Cardiovasc Pathol 19(3):147–152
McMurray JJ, Carson PE, Komajda M, McKelvie R, Zile MR, Ptaszynska A, Staiger C, Donovan JM, Massie BM (2008) Heart failure with preserved ejection fraction: clinical characteristics of 4133 patients enrolled in the I-PRESERVE trial. Eur J Heart Fail 10(2):149–156
Volpe M, McKelvie R, Drexler H (2010) Hypertension as an underlying factor in heart failure with preserved ejection fraction. J Clin Hypertens (Greenwich) 12(4):277–283
Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D (2002) Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 106(24):3068–3072
van Heerebeek L, Franssen CP, Hamdani N, Verheugt FW, Somsen GA, Paulus WJ (2012) Molecular and cellular basis for diastolic dysfunction. Curr Heart Fail Rep 9(4):293–302
Kuwahara F, Kai H, Tokuda K, Takeya M, Takeshita A, Egashira K, Imaizumi T (2004) Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension 43(4):739–745
Kai H, Kuwahara F, Tokuda K, Imaizumi T (2005) Diastolic dysfunction in hypertensive hearts: roles of perivascular inflammation and reactive myocardial fibrosis. Hypertens Res 28(6):483–490
Savoia C, Schiffrin EL (2007) Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci (Lond) 112(7):375–384
Kass DA, Bronzwaer JG, Paulus WJ (2004) What mechanisms underlie diastolic dysfunction in heart failure? Circ Res 94(12):1533–1542
van Heerebeek L, Borbely A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, Linke WA, Laarman GJ, Paulus WJ (2006) Myocardial structure and function differ in systolic and diastolic heart failure. Circulation 113(16):1966–1973
Zile MR, Brutsaert DL (2002) New concepts in diastolic dysfunction and diastolic heart failure: part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 105(11):1387–1393
Oh JK, Hatle L, Tajik AJ, Little WC (2006) Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol 47(3):500–506
Yturralde RF, Gaasch WH (2005) Diagnostic criteria for diastolic heart failure. Prog Cardiovasc Dis 47(5):314–319
Zile MR, Brutsaert DL (2002) New concepts in diastolic dysfunction and diastolic heart failure: part II: causal mechanisms and treatment. Circulation 105(12):1503–1508
Berk BC, Fujiwara K, Lehoux S (2007) ECM remodeling in hypertensive heart disease. J Clin Invest 117(3):568–575
Weber KT, Pick R, Jalil JE, Janicki JS, Carroll EP (1989) Patterns of myocardial fibrosis. J Mol Cell Cardiol 21(Suppl 5):121–131
Kuwahara F, Kai H, Tokuda K, Niiyama H, Tahara N, Kusaba K, Takemiya K, Jalalidin A, Koga M, Nagata T, Shibata R, Imaizumi T (2003) Roles of intercellular adhesion molecule-1 in hypertensive cardiac remodelling. Hypertension 41(3 Pt 2):819–823
Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschope C (2011) Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail 4(1):44–52
Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J (2010) Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol 55(19):2129–2137
Collier P, Watson CJ, Voon V, Phelan D, Jan A, Mak G, Martos R, Baugh JA, Ledwidge MT, McDonald KM (2011) Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail 13(10):1087–1095
Dostal DE, Baker KM (1992) Angiotensin II stimulation of left ventricular hypertrophy in adult rat heart. Mediation by the AT1 receptor. Am J Hypertens 5(5 Pt 1):276–280
Levine B, Kalman J, Mayer L, Fillit HM, Packer M (1990) Elevated circulating levels of tumour necrosis factor in severe chronic heart failure. N Engl J Med 323(4):236–241
Torre-Amione G (2005) Immune activation in chronic heart failure. Am J Cardiol 95(11A):3C–8C; discussion 38C–40C
Mann DL (2002) Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res 91(11):988–998
Adamopoulos S, Parissis JT, Kremastinos DT (2001) A glossary of circulating cytokines in chronic heart failure. Eur J Heart Fail 3(5):517–526
Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, Mann DL (1996) Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation 93(4):704–711
Hasper D, Hummel M, Kleber FX, Reindl I, Volk HD (1998) Systemic inflammation in patients with heart failure. Eur Heart J 19(5):761–765
Aukrust P, Ueland T, Lien E, Bendtzen K, Muller F, Andreassen AK, Nordoy I, Aass H, Espevik T, Simonsen S, Froland SS, Gullestad L (1999) Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 83(3):376–382
Aukrust P, Ueland T, Muller F, Andreassen AK, Nordoy I, Aas H, Kjekshus J, Simonsen S, Froland SS, Gullestad L (1998) Elevated circulating levels of C–C chemokines in patients with congestive heart failure. Circulation 97(12):1136–1143
Santhanakrishnan R, Chong JP, Ng TP, Ling LH, Sim D, Leong KT, Yeo PS, Ong HY, Jaufeerally F, Wong R, Chai P, Low AF, Richards AM, Lam CS (2012) Growth differentiation factor 15, ST2, high-sensitivity troponin T, and N-terminal pro brain natriuretic peptide in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail 14(12):1338–1347
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ (2008) Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359(21):2195–2207
Galkina E, Ley K (2009) Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol 27:165–197
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8(12):958–969
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122(3):787–795
Hinglais N, Heudes D, Nicoletti A, Mandet C, Laurent M, Bariety J, Michel JB (1994) Colocalization of myocardial fibrosis and inflammatory cells in rats. Lab Invest 70(2):286–294
Nicoletti A, Heudes D, Mandet C, Hinglais N, Bariety J, Michel JB (1996) Inflammatory cells and myocardial fibrosis: spatial and temporal distribution in renovascular hypertensive rats. Cardiovasc Res 32(6):1096–1107
Komatsu S, Panes J, Russell JM, Anderson DC, Muzykantov VR, Miyasaka M, Granger DN (1997) Effects of chronic arterial hypertension on constitutive and induced intercellular adhesion molecule-1 expression in vivo. Hypertension 29(2):683–689
Kohno T, Anzai T, Naito K, Sugano Y, Maekawa Y, Takahashi T, Yoshikawa T, Ogawa S (2008) Angiotensin-receptor blockade reduces border zone myocardial monocyte chemoattractant protein-1 expression and macrophage infiltration in post-infarction ventricular remodelling. Circ J 72(10):1685–1692
Nicoletti A, Michel JB (1999) Cardiac fibrosis and inflammation: interaction with hemodynamic and hormonal factors. Cardiovasc Res 41(3):532–543
Vaziri ND (2008) Causal link between oxidative stress, inflammation, and hypertension. Iran J Kidney Dis 2(1):1–10
Oghlakian GO, Sipahi I, Fang JC (2011) Treatment of heart failure with preserved ejection fraction: have we been pursuing the wrong paradigm? Mayo Clin Proc Mayo Clin 86(6):531–539
Hernandez-Presa M, Bustos C, Ortego M, Tunon J, Renedo G, Ruiz-Ortega M, Egido J (1997) Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-kappa B activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation 95(6):1532–1541
Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, Taffet GE, Entman ML (2010) Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol 49(3):499–507
Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, Kraemer D, Taffet G, Rollins BJ, Entman ML (2007) Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation 115(5):584–592
Ishibashi M, Hiasa K, Zhao Q, Inoue S, Ohtani K, Kitamoto S, Tsuchihashi M, Sugaya T, Charo IF, Kura S, Tsuzuki T, Ishibashi T, Takeshita A, Egashira K (2004) Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocytes in hypertension-induced vascular inflammation and remodeling. Circ Res 94(9):1203–1210
Xu J, Lin SC, Chen J, Miao Y, Taffet GE, Entman ML, Wang Y (2011) CCR2 mediates the uptake of bone marrow-derived fibroblast precursors in angiotensin II-induced cardiac fibrosis. Am J Physiol Heart Circ Physiol 301(2):H538–H547
Iyer A, Chan V, Brown L (2010) The DOCA-salt hypertensive rat as a model of cardiovascular oxidative and inflammatory stress. Curr Cardiol Rev 6(4):291–297
Ammarguellat FZ, Gannon PO, Amiri F, Schiffrin EL (2002) Fibrosis, matrix metalloproteinases, and inflammation in the heart of DOCA-salt hypertensive rats: role of ET(A) receptors. Hypertension 39(2 Pt 2):679–684
Gomez-Garre D, Gonzalez-Rubio ML, Munoz-Pacheco P, Caro-Vadillo A, Aragoncillo P, Fernandez-Cruz A (2010) Rosuvastatin added to standard heart failure therapy improves cardiac remodelling in heart failure rats with preserved ejection fraction. Eur J Heart Fail 12(9):903–912
Sciarretta S, Ferrucci A, Ciavarella GM, De Paolis P, Venturelli V, Tocci G, De Biase L, Rubattu S, Volpe M (2007) Markers of inflammation and fibrosis are related to cardiovascular damage in hypertensive patients with metabolic syndrome. Am J Hypertens 20(7):784–791
Williams ES, Shah SJ, Ali S, Na BY, Schiller NB, Whooley MA (2008) C-reactive protein, diastolic dysfunction, and risk of heart failure in patients with coronary disease: heart and soul study. Eur J Heart Fail 10(1):63–69
Lee KW, Blann AD, Lip GY (2005) Impaired tissue Doppler diastolic function in patients with coronary artery disease: relationship to endothelial damage/dysfunction and platelet activation. Am Heart J 150(4):756–766
Shah KB, Kop WJ, Christenson RH, Diercks DB, Henderson S, Hanson K, Li SY, deFilippi CR (2011) Prognostic utility of ST2 in patients with acute dyspnea and preserved left ventricular ejection fraction. Clin Chem 57(6):874–882
Matsubara J, Sugiyama S, Nozaki T, Sugamura K, Konishi M, Ohba K, Matsuzawa Y, Akiyama E, Yamamoto E, Sakamoto K, Nagayoshi Y, Kaikita K, Sumida H, Kim-Mitsuyama S, Ogawa H (2011) Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. J Am Coll Cardiol 57(7):861–869
Souders CA, Bowers SL, Baudino TA (2009) Cardiac fibroblast: the renaissance cell. Circ Res 105(12):1164–1176
Maisel A, Mueller C, Adams K Jr, Anker SD, Aspromonte N, Cleland JG, Cohen-Solal A, Dahlstrom U, DeMaria A, Di Somma S, Filippatos GS, Fonarow GC, Jourdain P, Komajda M, Liu PP, McDonagh T, McDonald K, Mebazaa A, Nieminen MS, Peacock WF, Tubaro M, Valle R, Vanderhyden M, Yancy CW, Zannad F, Braunwald E (2008) State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail 10(9):824–839
de Lemos JA, McGuire DK, Drazner MH (2003) B-type natriuretic peptide in cardiovascular disease. Lancet 362(9380):316–322
van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, Paulus WJ, Voors AA, Hillege HL (2013) B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol 61(14):1498–1506
Kuhn M, Holtwick R, Baba HA, Perriard JC, Schmitz W, Ehler E (2002) Progressive cardiac hypertrophy and dysfunction in atrial natriuretic peptide receptor (GC-A) deficient mice. Heart 87(4):368–374
Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers DL, Beuve A (1995) Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature 378(6552):65–68
Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, Pandey KN, Milgram SL, Smithies O, Maeda N (1997) Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci USA 94(26):14730–14735
Oliver PM, John SW, Purdy KE, Kim R, Maeda N, Goy MF, Smithies O (1998) Natriuretic peptide receptor 1 expression influences blood pressures of mice in a dose-dependent manner. Proc Natl Acad Sci USA 95(5):2547–2551
Zahabi A, Picard S, Fortin N, Reudelhuber TL, Deschepper CF (2003) Expression of constitutively active guanylate cyclase in cardiomyocytes inhibits the hypertrophic effects of isoproterenol and aortic constriction on mouse hearts. J Biol Chem 278(48):47694–47699
Kishimoto I, Rossi K, Garbers DL (2001) A genetic model provides evidence that the receptor for atrial natriuretic peptide (guanylyl cyclase-A) inhibits cardiac ventricular myocyte hypertrophy. Proc Natl Acad Sci USA 98(5):2703–2706
Holtwick R, van Eickels M, Skryabin BV, Baba HA, Bubikat A, Begrow F, Schneider MD, Garbers DL, Kuhn M (2003) Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest 111(9):1399–1407
Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, Itoh H, Saito Y, Tanaka I, Otani H, Katsuki M (2000) Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci USA 97(8):4239–4244
Cao L, Gardner DG (1995) Natriuretic peptides inhibit DNA synthesis in cardiac fibroblasts. Hypertension 25(2):227–234
Huntley BK, Sandberg SM, Noser JA, Cataliotti A, Redfield MM, Matsuda Y, Burnett JC Jr (2006) BNP-induced activation of cGMP in human cardiac fibroblasts: interactions with fibronectin and natriuretic peptide receptors. J Cell Physiol 209(3):943–949
Tsuruda T, Boerrigter G, Huntley BK, Noser JA, Cataliotti A, Costello-Boerrigter LC, Chen HH, Burnett JC Jr (2002) Brain natriuretic Peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res 91(12):1127–1134
Kapoun AM, Liang F, O’Young G, Damm DL, Quon D, White RT, Munson K, Lam A, Schreiner GF, Protter AA (2004) B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-beta in primary human cardiac fibroblasts: fibrosis, myofibroblast conversion, proliferation, and inflammation. Circ Res 94(4):453–461
Watson CJ, Phelan D, Xu M, Collier P, Neary R, Smolenski A, Ledwidge M, McDonald K, Baugh J (2012) Mechanical stretch up-regulates the B-type natriuretic peptide system in human cardiac fibroblasts: a possible defense against transforming growth factor-beta mediated fibrosis. Fibrogenesis Tissue Repair 5(1):9
Vellaichamy E, Khurana ML, Fink J, Pandey KN (2005) Involvement of the NF-kappa B/matrix metalloproteinase pathway in cardiac fibrosis of mice lacking guanylyl cyclase/natriuretic peptide receptor A. J Biol Chem 280(19):19230–19242
Vellaichamy E, Kaur K, Pandey KN (2007) Enhanced activation of pro-inflammatory cytokines in mice lacking natriuretic peptide receptor-A. Peptides 28(4):893–899
Hirota H, Yoshida K, Kishimoto T, Taga T (1995) Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc Natl Acad Sci USA 92(11):4862–4866
Vanderheyden M, Paulus WJ, Voss M, Knuefermann P, Sivasubramanian N, Mann D, Baumgarten G (2005) Myocardial cytokine gene expression is higher in aortic stenosis than in idiopathic dilated cardiomyopathy. Heart 91(7):926–931
de Bold AJ (2009) Cardiac natriuretic peptides gene expression and secretion in inflammation. J Investig Med 57(1):29–32
Haugen E, Chen J, Wikstrom J, Gronros J, Gan LM, Fu LX (2007) Parallel gene expressions of IL-6 and BNP during cardiac hypertrophy complicated with diastolic dysfunction in spontaneously hypertensive rats. Int J Cardiol 115(1):24–28
Giannessi D, Colotti C, Maltinti M, Del Ry S, Prontera C, Turchi S, Labbate A, Neglia D (2007) Circulating heat shock proteins and inflammatory markers in patients with idiopathic left ventricular dysfunction: their relationships with myocardial and microvascular impairment. Cell Stress Chaperones 12(3):265–274
Vaz Perez A, Doehner W, von Haehling S, Schmidt H, Zimmermann AV, Volk HD, Anker SD, Rauchhaus M (2010) The relationship between tumour necrosis factor-alpha, brain natriuretic peptide and atrial natriuretic peptide in patients with chronic heart failure. Int J Cardiol 141(1):39–43
Ahmad S, Otaal PS, Rai TS, Bahl A, Saikia UN, Manoj RK, Thungapathra M, Talwar KK, Khullar M (2009) Circulating proinflammatory cytokines and N-terminal pro-brain natriuretic peptide significantly decrease with recovery of left ventricular function in patients with dilated cardiomyopathy. Mol Cell Biochem 324(1–2):139–145
Ortega O, Gallar P, Munoz M, Rodriguez I, Carreno A, Ortiz M, Molina A, Oliet A, Lozano L, Vigil A (2004) Association between C-reactive protein levels and N-terminal pro-B-type natriuretic peptide in pre-dialysis patients. Nephron Clin Pract 97(4):c125–c130
Phelan D, Watson C, Martos R, Collier P, Patle A, Donnelly S, Ledwidge M, Baugh J, McDonald K (2012) Modest elevation in BNP in asymptomatic hypertensive patients reflects sub-clinical cardiac remodelling, inflammation and extracellular matrix changes. PLoS ONE 7(11):e49259
Chiurchiu V, Izzi V, D’Aquilio F, Carotenuto F, Di Nardo P, Baldini PM (2008) Brain Natriuretic Peptide (BNP) regulates the production of inflammatory mediators in human THP-1 macrophages. Regul Pept 148(1–3):26–32
Shaw SM, Critchley WR, Puchalka CM, Williams SG, Yonan N, Fildes JE (2012) Brain natriuretic peptide induces CD8 + T cell death via a caspase 3 associated pathway–implications following heart transplantation. Transpl Immunol 26(2–3):119–122
Glezeva N, Collier P, Voon V, Ledwidge M, McDonald K, Watson C, Baugh J (2013) Attenuation of monocyte chemotaxis-a novel anti-inflammatory mechanism of action for the cardio-protective hormone b-type natriuretic peptide. Journal of cardiovascular translational research 6(4):545–557
Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J (2003) Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 362(9386):777–781
Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A (2008) Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 359(23):2456–2467
Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J (2006) The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 27(19):2338–2345
Conraads VM, Metra M, Kamp O, De Keulenaer GW, Pieske B, Zamorano J, Vardas PE, Bohm M, Dei Cas L (2012) Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail 14(2):219–225
Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, Lacourciere Y, Hippler SE, Fields H, Naqvi TZ, Mulvagh SL, Arnold JM, Thomas JD, Zile MR, Aurigemma GP (2007) Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet 369(9579):2079–2087
Kindermann M, Reil JC, Pieske B, van Veldhuisen DJ, Bohm M (2008) Heart failure with normal left ventricular ejection fraction: what is the evidence? Trends Cardiovasc Med 18(8):280–292
Ridker PM (2003) Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation 108(19):2292–2297
Gong KZ, Song G, Spiers JP, Kelso EJ, Zhang ZG (2007) Activation of immune and inflammatory systems in chronic heart failure: novel therapeutic approaches. Int J Clin Pract 61(4):611–621
Heymans S, Hirsch E, Anker SD, Aukrust P, Balligand JL, Cohen-Tervaert JW, Drexler H, Filippatos G, Felix SB, Gullestad L, Hilfiker-Kleiner D, Janssens S, Latini R, Neubauer G, Paulus WJ, Pieske B, Ponikowski P, Schroen B, Schultheiss HP, Tschope C, Van Bilsen M, Zannad F, McMurray J, Shah AM (2009) Inflammation as a therapeutic target in heart failure? A scientific statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 11(2):119–129
Hayashidani S, Tsutsui H, Shiomi T, Ikeuchi M, Matsusaka H, Suematsu N, Wen J, Egashira K, Takeshita A (2003) Anti-monocyte chemoattractant protein-1 gene therapy attenuates left ventricular remodelling and failure after experimental myocardial infarction. Circulation 108(17):2134–2140
Westermann D, Savvatis K, Lindner D, Zietsch C, Becher PM, Hammer E, Heimesaat MM, Bereswill S, Volker U, Escher F, Riad A, Plendl J, Klingel K, Poller W, Schultheiss HP, Tschope C (2011) Reduced degradation of the chemokine MCP-3 by matrix metalloproteinase-2 exacerbates myocardial inflammation in experimental viral cardiomyopathy. Circulation 124(19):2082–2093
Nishio R, Matsumori A, Shioi T, Ishida H, Sasayama S (1999) Treatment of experimental viral myocarditis with interleukin-10. Circulation 100(10):1102–1108
Stumpf C, Lehner C, Yilmaz A, Daniel WG, Garlichs CD (2003) Decrease of serum levels of the anti-inflammatory cytokine interleukin-10 in patients with advanced chronic heart failure. Clin Sci (Lond) 105(1):45–50
Bolger AP, Sharma R, von Haehling S, Doehner W, Oliver B, Rauchhaus M, Coats AJ, Adcock IM, Anker SD (2002) Effect of interleukin-10 on the production of tumor necrosis factor-alpha by peripheral blood mononuclear cells from patients with chronic heart failure. Am J Cardiol 90(4):384–389
Suzuki K, Murtuza B, Smolenski RT, Sammut IA, Suzuki N, Kaneda Y, Yacoub MH (2001) Overexpression of interleukin-1 receptor antagonist provides cardioprotection against ischemia-reperfusion injury associated with reduction in apoptosis. Circulation 104(12 Suppl 1):I303–I308
Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GG, Van Tassell BW, Robati R, Roach LM, Arena RA, Roberts CS, Varma A, Gelwix CC, Salloum FN, Hastillo A, Dinarello CA, Vetrovec GW (2010) Interleukin-1 blockade with Anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am J Cardiol 105(10):1371–1377 e1371
Basalyga DM, Simionescu DT, Xiong W, Baxter BT, Starcher BC, Vyavahare NR (2004) Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation 110(22):3480–3487
Heymans S, Lupu F, Terclavers S, Vanwetswinkel B, Herbert JM, Baker A, Collen D, Carmeliet P, Moons L (2005) Loss or inhibition of uPA or MMP-9 attenuates LV remodelling and dysfunction after acute pressure overload in mice. Am J Pathol 166(1):15–25
Martos R, Baugh J, Ledwidge M, O’Loughlin C, Murphy NF, Conlon C, Patle A, Donnelly SC, McDonald K (2009) Diagnosis of heart failure with preserved ejection fraction: improved accuracy with the use of markers of collagen turnover. Eur J Heart Fail 11(2):191–197
Spinale FG (2007) Myocardial matrix remodelling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 87(4):1285–1342
Cox MJ, Hawkins UA, Hoit BD, Tyagi SC (2004) Attenuation of oxidative stress and remodeling by cardiac inhibitor of metalloproteinase protein transfer. Circulation 109(17):2123–2128
Li YY, Kadokami T, Wang P, McTiernan CF, Feldman AM (2002) MMP inhibition modulates TNF-alpha transgenic mouse phenotype early in the development of heart failure. Am J Physiol Heart Circ Physiol 282(3):H983–H989
Lindeman JH, Abdul-Hussien H, van Bockel JH, Wolterbeek R, Kleemann R (2009) Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: doxycycline selectively depletes aortic wall neutrophils and cytotoxic T cells. Circulation 119(16):2209–2216
Gu Y, Lee HM, Sorsa T, Simon SR, Golub LM (2010) Doxycycline [corrected] inhibits mononuclear cell-mediated connective tissue breakdown. FEMS Immunol Med Microbiol 58(2):218–225
Savchenko A, Imamura M, Ohashi R, Jiang S, Kawasaki T, Hasegawa G, Emura I, Iwanari H, Sagara M, Tanaka T, Hamakubo T, Kodama T, Naito M (2008) Expression of pentraxin 3 (PTX3) in human atherosclerotic lesions. J Pathol 215(1):48–55
Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, Vago L, Pasqualini F, Signorini S, Soldateschi D, Tarli L, Schweiger C, Fresco C, Cecere R, Tognoni G, Mantovani A (2004) Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation 110(16):2349–2354
Salio M, Chimenti S, De Angelis N, Molla F, Maina V, Nebuloni M, Pasqualini F, Latini R, Garlanda C, Mantovani A (2008) Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation 117(8):1055–1064
Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, Silengo L, Altruda F, Wetzker R, Wymann MP, Lembo G, Hirsch E (2004) PI3 K gamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell 118(3):375–387
Doukas J, Wrasidlo W, Noronha G, Dneprovskaia E, Fine R, Weis S, Hood J, Demaria A, Soll R, Cheresh D (2006) Phosphoinositide 3-kinase gamma/delta inhibition limits infarct size after myocardial ischemia/reperfusion injury. Proc Natl Acad Sci USA 103(52):19866–19871
Bujak M, Frangogiannis NG (2009) The role of IL-1 in the pathogenesis of heart disease. Arch Immunol Ther Exp 57(3):165–176
Bodyak N, Ayus JC, Achinger S, Shivalingappa V, Ke Q, Chen YS, Rigor DL, Stillman I, Tamez H, Kroeger PE, Wu-Wong RR, Karumanchi SA, Thadhani R, Kang PM (2007) Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci USA 104(43):16810–16815
Liu LC, Voors AA, van Veldhuisen DJ, van der Veer E, Belonje AM, Szymanski MK, Sillje HH, van Gilst WH, Jaarsma T, de Boer RA (2011) Vitamin D status and outcomes in heart failure patients. Eur J Heart Fail 13(6):619–625
Westermann D, Becher PM, Lindner D, Savvatis K, Xia Y, Frohlich M, Hoffmann S, Schultheiss HP, Tschope C (2012) Selective PDE5A inhibition with sildenafil rescues left ventricular dysfunction, inflammatory immune response and cardiac remodeling in angiotensin II-induced heart failure in vivo. Basic Res Cardiol 107(6):308
Guazzi M, Vicenzi M, Arena R, Guazzi MD (2011) Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 124(2):164–174
Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E (2013) Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 309(12):1268–1277
Conflict of interest
The authors Dr. Nadezhda Glezeva and Dr. John A. Baugh have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Glezeva, N., Baugh, J.A. Role of inflammation in the pathogenesis of heart failure with preserved ejection fraction and its potential as a therapeutic target. Heart Fail Rev 19, 681–694 (2014). https://doi.org/10.1007/s10741-013-9405-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-013-9405-8