Abstract
Oxidative stress is considered to play an important role in the pathogenesis of diabetes-induced cardiovascular disease (CVD), which is invariably associated with abnormal blood lipid profile, insulin resistance and metabolic syndrome. Stress, smoking, high saturated fat intake as well as low fruit and vegetable intakes have been shown to increase oxidative stress and hyperlipidemia, which increase the predisposition of diabetic subjects to atherosclerosis, stroke and coronary heart disease. The oxidation of low-density lipoprotein by oxidative stress is essential for the development of atherosclerosis, and the reduction in oxidative stress as well as blood glucose and cholesterol is considered critical for the prevention of diabetes-induced CVD. Although epidemiological studies have demonstrated that vitamin C and vitamin E decrease the incidence of coronary heart disease, different clinical trials have failed to support the beneficial effect of these antioxidants. Nonetheless, it has been suggested that natural forms of these vitamins may be more efficacious than synthetic vitamins, and this may explain the inconsistencies in results. Antioxidants, N-acetyl-l-cysteine and resveratrol, have also been shown to attenuate the diabetes-induced cardiovascular complications. It has been indicated that the antioxidant therapy may be effective in a prevention strategy rather than as a treatment for CVD. The evidence presented here supports the view that cardiovascular complications in diabetes may be induced by oxidative stress and appropriate antioxidant therapy may be promising for attenuating the progression of diabetes-induced CVD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
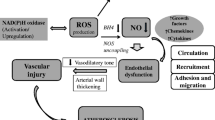
It is now well known that cardiovascular complications such as cardiac dysfunction, atherosclerosis, microangiopathy, ischemia/reperfusion (I/R) injury, cardiomyopathy and heart failure are associated with chronic diabetes [1–3]. Although insulin insufficiency or insulin resistance, elevated level of glucose, abnormal lipid profile and metabolic syndrome are considered to cause diabetes-associated cardiovascular abnormalities, the exact mechanisms for the development of cardiac dysfunction in diabetes subjects are poorly understood. In view of the involvement of oxidative stress in the pathogenesis of heart disease [4], it has been suggested that oxidative stress plays a critical role in inducing cardiomyopathy and heart failure in chronic diabetes [5, 6]. It should be mentioned that the occurrence of oxidative stress in the cell is a result of an imbalance between the production of reactive oxygen species (ROS) and the removal of ROS by antioxidants and endogenous antioxidant defense system [7]. ROS includes superoxide anion (\( ^{ \cdot } {\text{O}}_{2}^{ - } \)) and hydroxyl anion (\( ^{ \cdot } {\text{OH}} \)), whereas hydrogen peroxide (H2O2) is a potent oxidant molecule. The excessive production of ROS and oxidants is attributed to stressful situations, cigarette smoking, high blood cholesterol, iron supplement, metabolic syndrome and some therapeutic agents. Furthermore, oxidative stress is known to induce damage to cell membrane, cause DNA fragmentation and increase cell death [8]. It is pointed out that the paradigm of redox signaling whereby some oxidants are considered to function as intracellular signaling molecules has now been established [9, 10]. Such signaling can contribute to signaling for either cell death or survival. Not all oxidant molecules have a role in signal transduction as this seems to depend on the cell type and animal species. Furthermore, it has become evident that low concentrations of oxidants or exposure for a transient period stimulate signal transduction for cardiomyocyte function and gene expression for cell survival, while high concentrations of oxidants as well as exposure for a prolonged period of time produce oxidative stress and subsequent harmful outcomes [11, 12]. It is also noteworthy that the oxidation of low-density lipoprotein (LDL) to oxidized LDL (ox-LDL) by ROS has also been reported to contribute to the processes involved in lipid deposition and inflammatory response in blood vessels [13]. These oxidative stress-mediated changes, as seen in animals on high-fat diet, are involved in the pathogenesis of heart attack, atherosclerosis and cardiomyopathy (Fig. 1). In fact, several biomarkers of oxidative stress are commonly seen in diabetes, angina, hypertension, hyperlipidemia and open-heart surgery.
Pre-clinical studies have revealed that different antioxidants exert beneficial effects in I/R injury to the heart and brain, improve diabetes-induced cardiovascular complications and prevent the development of atherosclerosis [14, 15]. In view of the role of atherosclerosis as well as endothelial and vascular abnormalities in the diabetes-induced cardiovascular complications [16], this review will not only focus on the antioxidant therapy of diabetes-induced cardiac dysfunction, but will also discuss the effectiveness of antioxidants in the treatment for atherosclerosis. Since hypoperfusion and subsequent I/R of the myocardium play a significant role in the development of diabetes-induced cardiomyopathy [2, 17], this article will describe the actions of antioxidants on I/R-induced injury. Because anticancer drug-induced cardiomyopathy is also considered to involve oxidative stress [18], the use of antioxidants in doxorubicin-induced cardiomyopathy will be discussed for the purpose of comparison with diabetes-induced cardiomyopathy. Despite the evidence from experimental studies and clinical trials showing inconsistencies in beneficial effects of antioxidant therapy for CVD [6, 19], this review is intended to summarize the preclinical evidence and the results of clinical trials to emphasize a perspective of the potential value of antioxidants for prevention as well as management of diabetes-induced cardiomyopathy and heart failure. It is hoped that the observations recorded in this article will provide the evidence regarding the role of oxidative stress as well as the mechanisms of the beneficial effects of antioxidants for cardiovascular complications in chronic diabetes.
Diabetes-induced cardiovascular complications and antioxidant therapy
An increase in ROS production and lipid peroxidation has been reported in both experimental models of diabetes and patients with diabetes. Tappia et al. [20] have reported that dietary supplementation of antioxidants for streptozotocin (STZ)-induced diabetes in rats attenuated myocardial cell necrosis, improved blood lipid profile and increased cardiac contractile function. In type I diabetic patients, red blood cell content of malondialdehyde (MDA), an oxidative stress biomarker, is elevated along with an increase in blood ketone concentration [21]. In an in vitro study, red blood cells incubated with ketones including acetoacetate, β-hydroxybutyrate and acetone were found to show enhanced production of hydroxyl radicals, increased membrane lipid peroxidation and reduced cellular glutathione (GSH) levels. These effects of ketones were markedly suppressed by incubation of red blood cells with vitamin E and N-acetyl-l-cysteine (NAC). In a randomized, crossover, double-blind clinical trial, plasma vascular cell adhesion molecule (VCAM)-1 concentration was reported to be higher in non-insulin-dependent diabetic subjects as compared to healthy individuals [22]; oral administration of NAC (1.2 g/day) decreased the plasma VCAM-1 concentration. Since VCAM-1 has been reported to contribute to deep vein thrombosis and atherosclerosis [23], it is possible that antioxidants may prevent diabetic vascular disease by inhibition of VCAM-1 production.
Recent studies [24, 25] have demonstrated that diabetes-induced vascular complications are related to the increased levels of extracellular advanced glycation end products (AGEs). LDL glycation is known to depress the rate of receptor-mediated LDL metabolism and cause vascular abnormalities. A major source of AGE is the consumption of heat-processed foods [26] because during prolonged exposure of food to high temperatures as in cooking, a significant amount of AGEs is formed. About 10 % of ingested AGEs are incorporated in tissues and LDL. AGEs have also been found to increase the production of ox-LDL and activate mitogen-activated protein kinase (MAPK) and VCAM-1. Interestingly, the elevated level of VCAM-1 and NF-κB and activation of MAPK in diabetic patients were depressed by NAC as well as other antioxidants [26].
In diabetic patients, a decrease in platelet antioxidant, glutathione level and an increase in oxidant concentrations have been reported [27]. The higher levels of oxidants lead to platelet hyperaggregability. It is noteworthy that platelet aggregation is a major cause of thrombosis, atherosclerosis and other types of CVD. Growth factors released from platelets such as lysophosphatidic acid and platelet-derived growth factor play a role in cardiac remodeling, production of ox-LDL and vascular abnormalities [28]. Gibson et al. [27] demonstrated that NAC at the concentrations of 10–100 μM, which are achievable blood concentrations following oral administration, depressed platelet aggregation and increased platelet glutathione concentration in patients with type 2 diabetes. These findings suggest that NAC may be of value for the prevention of diabetes-related thrombosis. Administration of vitamin E to rats with STZ-induced diabetes was also observed to attenuate the diabetes-induced cardiac dysfunction and subcellular defects [4].
Since endothelial cell dysfunction is known to occur in diabetes, antioxidant supplements have been shown to exert beneficial effects on endothelial function. A clinical trial conducted by Neri et al. [29] in 46 untreated type 2 diabetic patients with impaired glucose tolerance and 46 healthy volunteers demonstrated that VCAM-1, MDA, ox-LDL and von Willebrand factor (vWF) were markedly higher in diabetics after a moderate-fat meal as compared to the healthy individuals. A 15-day diet supplementation period with vitamin E (300 mg/day), vitamin C (250 mg/day) and NAC (600 mg/day) significantly reduced these changes. Since vWF is released from damaged endothelial cells, antioxidant therapy could be seen to suppress endothelial damage in type 2 diabetes with impaired glucose tolerance. In view of the fact that endothelial cell dysfunction is the primary step for atherosclerosis and thrombosis, the protective effects of antioxidants on endothelial cells can be seen to provide evidence for its clinical use in diabetes-induced cardiovascular complications. Different sites including endothelial dysfunction and platelet aggregation due to oxidative stress for the action of NAC during the development of CVD in diabetes are shown in Fig. 2.
Proposed sites of action of antioxidant, N-acetyl-l-cysteine (NAC), on diabetes-induced cardiovascular complications. NAC reduces endothelial dysfunction and platelet aggregation as well as ketone- and AGE-induced oxidative stress. AGE advanced glycation end product, VCAM vascular cell adhesion molecule, MAPK mitogen-activated protein kinase, LPA lysophosphatidic acid
Cardioprotective effects of antioxidants in I/R
The occurrence of transient spasm of both macrovasculature and microvasculature in the diabetic heart [2] has been suggested to cause I/R injury. Experimental studies have shown that antioxidants protect the heart from I/R injury and hypertrophic cardiomyopathy [30]; however, the results from clinical trials have been inconsistent. In 30 patients undergoing coronary artery bypass grafting surgery, blood MDA levels and troponin-I concentrations were found to be significantly elevated, with the peak levels at 12 h after the operation. These increases were attenuated by NAC (50 mg/kg body weight) treatment. Furthermore, NAC treatment improved the ejection fraction, left ventricular end-diastolic volume, left ventricular end-systolic volume and left ventricular end-systolic medial wall stress, except that the cardiac index was not significantly different between control and NAC-treated groups [31]. In addition, the time of stay in hospital was reported to be shorter in NAC-treated patients. This study suggested that NAC supplementation in cold-blood cardioplegia may protect the heart from I/R-induced injury during open-heart surgery. On the other hand, in another group of patients undergoing open-heart surgery, while the plasma levels of MDA and VCAM-1 were significantly higher in the group (without NAC) than in the NAC-treated group, no significant differences in troponin-I concentrations were observed between NAC-treated and untreated groups [32]. These investigators concluded that NAC supplementation may attenuate coronary endothelial activation and myocardial oxidative stress without affecting the cellular injury in coronary artery bypass graft surgery [32]. It should also be mentioned that Prabhu et al. [33] have reported that lower levels of plasma MDA levels were associated with an improvement in the postoperative ejection fraction following NAC treatment, whereas the plasma troponin-I levels in both groups were similar. Thus, NAC seems to reduce oxidative stress during cardiac surgery, but its protective effect on I/R injury remains inconclusive [34].
NAC has been reported to potentiate the efficacy of nitroglycerin (NTG) when these two drugs were used concomitantly [35]. The vasodilator effect of NTG is mediated through the activation of cyclic guanylate cyclase (cGMP), which may be sulfhydryl (SH) group dependent, whereas NAC, the donor of SH groups, may enhance the hemodynamic effect of NTG. However, NAC and NTG exerted no effect on platelet aggregation when given separately, but their combination resulted in an inhibition of platelet aggregation. This has been attributed to the fact that the combination use of NAC and NTG results in the formation of S-nitroso-NAC, which is considered to be a potent inhibitor of platelet aggregation. In addition, Horowitz et al. [35] reported that the combination use of NAC and NTG reduced the incidence of myocardial infarction (MI) in patients having unstable angina pectoris. In this study with 46 patients, 24 were given both NTG (5 μg/min) and NAC (10 g/day), while the other group of 22 was given NTG (5 μg/min) and placebo (5 % dextrose). While the frequency of chest pain episodes was not significantly different between these two groups, three patients in the NTG and NAC group had acute MI and 10 patients in NTG and placebo group showed acute MI. This indicated that the combination of NAC and NTG reduced the rate of MI. It is noteworthy that the major side effects of the combination therapy with NAC and NTG were symptomatic hypotension and dizziness. Taken together, the inconsistencies in these findings may be due to the difference in sample size and stage of the disease. Nevertheless, NAC appears to be of potential benefit against cardiac I/R injury and thus may prove to be of therapeutic value in preventing diabetes-induced cardiomyopathy and heart failure.
Prevention of atherosclerosis by antioxidants
Some experimental studies have provided evidence that different antioxidants decrease the size of atherosclerotic plaque [36, 37]. Epidemiological studies [38, 39] have revealed that some vitamins significantly reduced the morbidity and mortality due to CVD; however, this beneficial effect required a minimum of 2-year intervention period. In this regard, vitamin E was found to be a more potent antioxidant than vitamin C and β-carotene. Despite the positive results of epidemiological and experimental studies, most of the clinical trials for examining the use of antioxidants in the treatment for atherosclerosis and coronary heart disease have failed to show any beneficial effects. In a double-blind clinical trial [40], 423 postmenopausal women were divided into four groups: (a) 108 women were given hormone replacement therapy (HRT) placebo and vitamin placebo; (b) 103 women were given HRT and vitamin placebo; (c) 105 women were given HRT placebo, vitamin C and vitamin E; and (d) 107 women were given HRT, vitamin C and E. In this study, 400 IU of vitamin E and 500 mg of vitamin C or placebo were taken twice a day for four and a half years. There were no significant effects of vitamin E and vitamin C treatment on the plasma LDL and HDL levels as well as coronary lumen diameters. In fact, when treatment and placebo groups were compared, vitamin E and vitamin C groups had a higher cardiovascular death rate and more non-fatal MI. The lack of clinical benefit of vitamin E and vitamin C in this population may be attributed to a number of factors including inadequate monitoring of the oxidative stress biomarkers, insufficient dosage of the vitamins used and the stage of the disease when the trial started. In fact, antioxidants were more efficient when given before the pathological change has developed [41]. For example, the anti-atherogenic effects of antioxidant supplements were more evident when given in the hyperlipidemia stage than in the stage when the plaque is already formed. Furthermore, the combination use of different antioxidants was more effective than the use of the single antioxidant because vitamin E is oxidized in vivo, and the administration of vitamin E with vitamin C or NAC prevented the oxidation of vitamin E. Finally, these agents produce antioxidant effects through different mechanisms, and their combined use may exert additive or synergetic action [29]. Thus, it appears that antioxidants may produce beneficial effects on atherosclerosis in diabetic populations by attenuating progression of the disease and may not be of any benefit for regression of the abnormality.
Protection against drug-induced cardiotoxicity by antioxidants
In order to determine whether the beneficial effects of antioxidants are limited to improving heart function in diabetic cardiomyopathy, the effects of these agents have also been investigated in anthracycline-induced cardiomyopathy. It should be noted that anthracyclines including doxorubicin have been shown to induce cardiac dysfunction and increase the levels of ROS and H2O2 in cardiomyocytes [42, 43]; these highly reactive drugs cause cell membrane lipid peroxidation, intracellular calcium overload and apoptosis. Wattanapitayakul et al. [44] demonstrated that vitamin C and NAC protected H9C2 cells from death induced by doxorubicin under in vitro conditions. Chularojmontri et al. [45] have reported that doxorubicin significantly depressed SOD and catalase activities, while caspase-3 activity was increased twofold in H9C2 cells. Plant extracts of Curcuma longa L., Morus alba L., Phyllanthus emblica L. and Piper rostratum Roxbx. were shown to be more potent antioxidants than vitamin C and NAC. Vitamin C, NAC and Phyllanthus urinaria extract were reported to increase SOD and catalase activities in the absence or presence of doxorubicin. Phyllanthus urinaria extract was more potent than vitamin C and NAC in suppressing the caspase-3 activity in the presence of doxorubicin. However, a clinical study with vitamin C, vitamin E and NAC showed no beneficial cardiovascular effects in cancer patients receiving anthracyclines [46]. Thus, the results of antioxidant therapy in doxorubicin-induced cardiomyopathy are similar to those in diabetic cardiomyopathy and support the view that antioxidants prevent the progression of heart failure under these pathological conditions, but these interventions may not be of any benefit once the damage has been done by the oxidative stress.
Mechanisms of prevention of CVD by resveratrol
In order to discuss the mechanisms of action of antioxidants for preventing CVD in general and diabetic cardiomyopathy in particular, a novel antioxidant, resveratrol, was used for this purpose. Resveratrol is found in a variety of fruits including grapes, berries and plums as well as Polygonum Cuspidatum, a plant that has been used in Chinese folk medicine to remove blood stasis and promote blood circulation [47]. Resveratrol has gained attention because it is found in red wine associated with “French Paradox.” It is noteworthy that French people normally consume more saturated fat in their diet and still have lower incidence of coronary heart disease; this has been attributed to high consumption of red wine [48]. In animal studies, resveratrol has been found to attenuate inflammation, oxidative stress and neovascularization [49, 50]. Matos et al. [51] reported that resveratrol (2 mg/kg/day) can significantly reduce the size of atherosclerotic plaque; this resveratrol action was due to a reduction of VCAM-1, MCP-1 and IL-6. Resveratrol supplementation increased the lifespan, insulin sensitivity and the number of mitochondria in mice fed a high-calorie diet [52]. These beneficial actions of resveratrol were considered to be mediated by the activation of AMP-activated protein kinase, which is known to regulate fatty acid metabolism and insulin sensitivity. Zghonda et al. [53] showed that resveratrol and its dihydrodimer, ε-viniferin, inhibited proliferation and migration of vascular smooth muscle cells induced by platelet-derived growth factor, increased nitric oxide production and depressed the production of ROS. The actions of resveratrol and ε-viniferin were mediated through the activation of phosphatidylinositol 3-kinase-Akt and p38 MAP kinase pathways. By using primate Microcebus Murinus, Marchal [54] observed that resveratrol supplementation (200 mg/day/kg body weight) exerted similar effects as calorie restriction on aging-related metabolic syndrome. In this study, supplementation with resveratrol for a period of 21 months did not improve insulin sensitivity and glucose tolerance; however, resveratrol supplementation for a period of 33 months reduced glycemia and insulin resistance [54] in this experimental model of diabetes.
Treatment with resveratrol has been reported to restore cardiac contractile function as well as reduce thrombin-induced platelet aggregation and thromboxane B2 levels in type I diabetic rat model [55]. Since platelet aggregation has an important role in the development of stroke, heart attack and atherosclerosis [56], some investigators have found that high platelet aggregation in different experimental models of diabetes was inhibited by resveratrol in a dose-dependent manner [57, 58]. Stef et al. [59] have also reported that resveratrol is effective in inhibiting collagen- and epinephrine-induced platelet aggregation in aspirin resistance and high-risk cardiac patients. This effect of resveratrol can be seen to provide protection from heart attack and stroke. Resveratrol was observed to attenuate ox-LDL as well as reduce apoptosis of cerebrovascular endothelial cells [60]. Robich et al. [61] reported that resveratrol reduced total plasma cholesterol by about 30 % but increased blood flow and angiogenesis in the swine heart. A modified form of resveratrol, longevinex, was found to improve endothelial cell function in patients with metabolic syndrome [62]. In another double-blind clinical trial with 40 postinfarction patients [63], resveratrol was observed to increase red blood cell deformability and decrease platelet aggregation. An improvement in cardiac function, endothelial cell function and blood lipid profile was also seen by resveratrol treatment; however, lower bioavailability and a short half-life of resveratrol were reported to limit its clinical applicability [64]. Nonetheless, resveratrol was reported to suppress breast cancer cell growth by inhibiting estrogen receptors and increasing the expression of insulin-like growth factor [65]. An antitumor effect of resveratrol has also been reported by Jang et al. [66], who revealed that this agent caused a dose-dependent inhibition of tumor growth in both in vitro and in vivo models. Since inflammatory processes are involved in the development of cancer, the effects of resveratrol on the activity of cyclooxygenase were also investigated. It was observed that resveratrol produced effects similar to those of indomethacin, a classical anti-inflammatory drug [66], and thus the beneficial effect of resveratrol on CVD may be related to its anti-inflammatory action [13]. Resveratrol has also been shown to exert beneficial effects in obesity, hyperglycemia, diabetes, hypertension, atherosclerosis and other cardiovascular diseases [49, 51, 52, 54, 61–63, 66, 67], and these are summarized in Table 1.
Conclusions
From the foregoing discussion, it is evident that oxidative stress plays a critical role in the development of CVD in diabetes. Various sources of oxyradical generation for the development of oxidative stress in the diabetic heart are shown in Fig. 3. There is an increase in the risk of heart failure, atherosclerosis and coronary artery disease under conditions of an imbalance in the production of oxyradicals and scavenging of ROS as well as oxidant molecules (Fig. 4). Although vitamin C and vitamin E have been shown to exert positive effects in experimental CVD, their beneficial actions in clinical trials are controversial [68, 69]. For the normal healthy population with a balanced diet, it is very rare to have a deficiency of vitamin C and E, and thus supplements of synthetic antioxidants do not appear to be necessary. Fresh vegetables and fruit, red wine and resveratrol-containing beverage may be added to the diet for preventive purpose [67, 70–72]. For individuals with elevated blood glucose, abnormal blood lipid profile as well as diabetes, and other conditions with increased biomarkers of oxidative stress, antioxidant supplementation may prevent or slow the progression of CVD and diabetes-induced cardiomyopathy [73]. Recent clinical trials to emphasize this point are summarized in Table 2 [74–80].
Since it is difficult to identify the effective dose and determine the efficacy of antioxidants without knowing blood oxidant levels, different biomarkers of oxidative stress should be monitored during the intervention period. In patients with existing CVD, it is becoming evident that antioxidants may not be able to reverse the pathological changes. Nonetheless, it is pointed out that the combined use of vitamin C, vitamin E and NAC has been recommended for reducing side effects and to produce synergistic beneficial effects in CVD. Because resveratrol is an antioxidant and reduces blood cholesterol [49–54], it is expected that this agent would prevent and retard the development of atherosclerosis and coronary heart disease. In view of the inconsistent association between antioxidants and CVD, further work is required to determine whether long-term antioxidant supplements exert beneficial effects in individuals with unhealthy lifestyle habits. It is also evident that vitamin C, NAC and resveratrol are more effective than vitamin E for prevention and slowing the progression of diabetes-induced CVD. Accordingly, a natural supplement consisting of resveratrol, vitamin C and other antioxidants is suggested to have a great economic and health value in the modern world [74]. However, it is pointed out that there is a wealth of information on other plant-based medicines as antioxidants that cannot be covered in the present review and is reserved for a separate review article. In summary, this paper has attempted to review the role of antioxidants in the treatment for diabetes-induced cardiovascular complications. From the evidence provided, it can be suggested that antioxidants have the potential to serve as adjuncts to therapeutic approaches for attenuating the progression of diabetes-induced CVD.
References
Regan TJ (1983) Congestive heart failure in the diabetic. Annu Rev Med 34:161–168
Dhalla NS, Pierce GN, Innes GN, Beamish RE (1985) Pathogenesis of cardiac dysfunction in diabetes mellitus. Can J Cardiol 1:263–281
Schaffer SW (1991) Cardiomyopathy associated with non-insulin-dependent diabetes. Mol Cell Biochem 107:1–20
Dhalla NS, Lui X, Panagia V, Takeda N (1998) Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res 40:239–247
Dhalla NS, Rangi S, Zieroth S, Xu Y-J (2012) Alterations in sarcoplasmic reticulum and mitochondrial functions in diabetic cardiomyopathy. Exptl Clin Cardiol 17:115–120
Ceriello A, Motz E (2004) Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol 24:816–823
Chen AF, Chen DD, Daiber A, Faraci FM, Li H, Rembold CM, Laher I (2012) Free radical biology of the cardiovascular system. Clin Sci 123:73–91
Roberts C, Sindhu KK (2009) Oxidative stress and metabolic syndrome. Life Sci 84:705–712
Dhalla NS, Temsah RM, Netticadan T (2000) Role of oxidative stress in cardiovascular diseases. J Hypertens 18:655–673
Tappia PS, Dent MR, Dhalla NS (2006) Oxidative stress and redox regulation of phospholipase D in myocardial disease. Free Rad Bio Med 41:349–361
Das DK (2001) Redox regulation of cardiomyocyte survival and death. Antioxid Redox Signal 3:23–37
Herrlich P, Bohmer FD (2000) Redox regulation of signal transduction in mammalian cells. Biochem Pharmacol 59:35–41
Adameova A, Xu YJ, Duhamel TA, Tappia PS, Shan L, Dhalla NS (2009) Anti-atherosclerotic molecules targeting oxidative stress and inflammation. Curr Pharm Des 15:3094–3107
Makazan Z, Saini HK, Dhalla NS (2007) Role of oxidative stress in alterations of mitochondrial function in ischemic-reperfused hearts. Am J Physiol 292:1986–1994
Xu YJ, Zhang M, Ji L, Elimban V, Chen L, Dhalla NS (2012) Suppression of high lipid diet induced atherosclerosis by sarpogrelate. J Mol Cell Med 16:2394–2400
Kanter JE, Bornfeldt KE (2012) Inflammation and diabetes-accelerated atherosclerosis: myeloid cell mediators. Trend Endocrinol Metab. doi:10.1016/j.tem.2012.10.002
Bell DSH, Face MB (2003) Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diab Care 26:2433–2441
Hadi N, Yousif NG, Al-Amran FG, Huntei NK, Mohammad BI, Ali SJ (2012) Vitamin E and telmisartan attenuates doxorubicin induced cardiac injury in rat through down regulation of inflammatory response. BMC Cardiovasc Disord 12:63
Steinberg D (1993) Antioxidant vitamins and coronary heart disease. N Eng J Med 328:1487–1489
Tappia PS, Thliveris J, Xu YJ, Aroutiounova N, Dhalla NS (2011) Effects of amino acid supplementation on myocardial cell damage and cardiac function in diabetes. Exp Clin Cardiol 16:e17–e22
Jain SK, McVie R (1999) Hyperketonemia can increase lipid peroxidation and lower glutathione levels in human erythrocytes in vitro and type 1 diabetic patients. Diabetes 49:1850–1855
De Mattia G, Bravi MC, Laurenti O, Cassone-Faldetta M, De Luca O, Armiento A, Ferri C (1998) Reduction of oxidative stress by oral N-acetyl-l-cysteine treatment decreases plasma soluble vascular cell adhesion molecule-1 concentrations in non-obese, non-dyslipidaemic, normotensive, patients with non-insulin-dependent diabetes. Diabetologia 41:1392–1396
Gonzalez-Ordonez AJ, Fernandea-Carreira JM, Fernandez-Alvarez CR, Obaya RV, Macias-Robles MD, Gonzalez-Franco A, Garcia MAA (2003) The concentration of soluble vascular cell adhesion molecule-1 and lipids are independent associated with venous thromboembolism. Thrombosis 88:1035–1043
Wu CH, Huang SM, Yen GC (2011) Silymarin: a novel antioxidant with antiglycation and antiinflammatory properties in vitro and in vivo. Antioxid Redox Signal 14:353–366
Cai W, Torreggiani M, Zhu L, Chen X, He JC, Striker GE, Vlassara H (2009) AGER1 regulates endothelial cell NADPH oxidase-dependent oxidant stress via PKC-δ: implications for vascular disease. Am J Physiol 298:C624–C634
Cai W, He JC, Zhu L, Peppa M, Lu C, Uribarri J, Vlassara H (2004) High levels of dietary advanced glycation end products transform low-density lipoprotein into a potent redox-sensitive mitogen-activated protein kinase stimulant in diabetic patients. Circulation 110:285–291
Gibson KR, Winterburn TJ, Barrett F, Sharma S, MacRyry SM, Megson IL (2011) Therapeutic potential of N-acetylcysteine as an antiplatelet agent in patients with type-2 diabetes. Cardiovasc Diabetol 10:43–50
Xu YJ, Aziz OA, Bhugra P, Arneja AS, Mendia MR, Dhalla NS (2003) Potential role of lysophosphatidic acid in hypertension and atherosclerosis. Can J Cardiol 19:1525–1536
Neri S, Signorelli SS, Torrisi B, Pulvrenti D, Mauceri B, Abate G, Ignaccolo L, Bordonaro F, Cilio D, Calvagno S, Leotta C (2005) Effects of antioxidant supplementation on postprandial oxidative stress and endothelial dysfunction: a single-blind, 15-day clinical trial in patients with untreated type-2 diabetes, subjects with impaired glucose tolerance, and healthy controls. Clin Ther 27:1764–1773
Marian AJ (2009) Experimental therapies in hypertrophic cardiomyopathy. J Cardiovasc Transl Res 2:483–492
Koramaz I, Pulathan Z, Usta S, Karahan C, Alver A, Yaris E, Kalyoncu NI (2006) Cardioprotective effect of cold-blood cardioplegia enriched with N-acetylcysteine during coronary artery bypass grafting. Ann Thorac Surg 81:613–618
Rodrigues AJ, Evora PR, Bassetto S, Alves L Jr, Scorzoni FA, Origuela EA, Vicente WV (2009) Blood cardioplegia with N-acetylcysteine may reduce coronary endothelial activation and myocardial oxidative stress. Heart Surg Forum 12:E44–E48
Prabhu A, Sujatha DI, Kanagarajan N, Vijayalakshmi MA, Ninan B (2009) Effect of N-acetylcysteine in attenuating ischemic reperfusion injury in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. Ann Vasc Surg 23:645–651
Tossios P, Bloch W, Huebner A, Raji MR, Dodos F, Klass O, Suedkamp M, Kasper SM, Hellmich M, Mehlhorn U (2003) N-acetylcysteine prevents reactive oxygen species-mediated myocardial stress in patients undergoing cardiac surgery: results of a randomized, double-blind, placebo-controlled clinical trial. J Thorac Cardiovas Surg 126:1513–1520
Horowitz JD, Henry CA, Syrjanen ML, Louis WJ, Fish RD, Smith TW, Antman EM (1988) Combined use of nitroglycerin and N-acetylcysteine in the management of unstable angina pectoris. Circulation 77:787–794
Rosenblat M, Volkova N, Aviram M (2012) Pomegranate phytosterol (β-sitosterol) and polyphenolic antioxidant (punicalagin) addition to statin, significantly protected against macrophage foam cells formation. Atherosclerosis. doi:10.1016/j.atherosclerosis.2012.10.054
Sparrow CP, Doebber TW, Olszewski J, Wu MS, Ventre J, Stevens KA, Chao YS (1992) Low density lipoprotein is protected from oxidation and progression in rabbits by the antioxidant N, N′-diphenyl-phenylenediamine. J Clin Invest 89:1885–1891
Jha P, Flather M, Lonn E, Farkouh M, Yusuf S (1995) The antioxidant vitamins and cardiovascular disease: a critical review of epidemiologic and clinical trial data. Ann Intern Med 123:860–872
Steinberg D, Participants W (1992) Antioxidants in the prevention of human atherosclerosis. Circulation 85:2338–2344
Waters DD, Alderman EL, Hsia J, Howard BV, Cobb FR, Rogers WJ, Ouyang P, Thompson P, Tardif JC, Higgison L, Bittner V, Steffes M, Gordon DJ, Proschan M, Younes N, Verter JI (2002) Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women. JAMA 288:2432–2440
Steinhubal SR (2008) Why have antioxidants failed in clinical trials? Am J Cardiol 102(suppl):14D–19D
Dorr RT (1996) Cytoprotective agents for anthracyclines. Semin Oncol 23:23–24
Swain SM, Whaley FS, Ewer MS (2003) Congestive heart failure in patients treated with Doxorubicin. Cancer 97:2869–2879
Wattanapitayakul SK, Chularojmontri L, Herunsalee A, Charuchongkolwongse S, Niumsakul S, Bauer JA (2005) Screening of antioxidants from medicinal plants for cardioprotective effect against doxorubicin toxicity. Basic Clin Pharmacol Toxicol 96:80–87
Chularojmontri L, Wattanapitayakul SK, Herunsalee A, Charuchongkolwongse S, Niumsakul S, Srichairat S (2005) Antioxidative and cardioprotective effects of Phyllanthus urinaria L. on doxorubicin-induced cardiotoxicity. Biol Pharm Bull 28:1165–1171
van Dalen EC, Caron HN, Dickinson HO, Kremer LCM (2011) Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev 15:CD003917
Smoliga JM, Baur JA, Hausenblas HA (2011) Resveratrol and health—a comprehensive review of human clinical trials. Mol Nutr Food Res 55:1129–1141
Saini H, Dhami P, Xu YJ, Cheema S, Arneja A, Dhalla NS (2006) Modification of biochemical and cellular processes in the development of atherosclerosis by red wine. In: Cheema SK (ed) Biochemistry of atherosclerosis. Springer Science +Business Media, LLC., New York, pp 475–494
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Couteur DL, Shaw RJ, Navas P, Puigserver P, Ingram DK, Cabo RD, Sinclair DA (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444(7117):337–342
Ku CR, Lee HJ, Kim SK, Lee EY, Lee MK, Lee EJ (2012) Resveratrol prevents streptozotocin-induced diabetes by inhibiting the apoptosis of pancreatic ß-cell and the cleavage of poly(ADP-ribose) polymerase. Endocr J 59:103–109
Matos RS, Baroncini LA, Precoma LB, Winter G, Lambach PH, Caron EY, Kaiber F, Precoma DB (2012) Resveratrol causes antiatherogenic effects in an animal model of atherosclerosis. Arq Bras Cardiol 98:136–142
Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nature Rev Drug Discov 5:493–506
Zghonda N, Yoshida S, Araki M, Kusunoki M, Mliki A, Ghorbel A, Miyazaki H (2011) Greater effectiveness of ε-viniferin in red wine than its monomer resveratrol for inhibiting vascular smooth muscle cell proliferation and migration. Biosci Biotechnol Biochem 75:1259–1267
Marchal J, Blanc S, Epelbaum J, Aujard F, Pifferi F (2012) Effects of chronic restriction or dietary resveratrol supplementation on insulin sensitivity markers in a primate Microcebus Murinus. PLoS ONE 7:e34289
Turan B, Tuncay E, Vassort G (2012) Resveratrol and diabetic cardiac function: focus on recent in vitro and in vivo studies. J Bioenerg Biomemb 44:281–296
Duhamel TA, Xu YJ, Arneja AS, Dhalla NS (2007) Targeting platelets for prevention and treatment of cardiovascular disease. Expert Opin Ther Targ 11:1523–1533
Malinowska J, Olas B (2011) Response of blood platelets to resveratrol during a model of hyperhomocysteinemia. Platelets 22:277–283
Esker S, Banerjee A, Simone TM, Gallati CA, Mousa SA (2009) Resveratrol as a supplemental therapeutic in cardiovascular and metabolic syndromes: a critical review. Curr Nut Food Sci 5:1–8
Stef G, Csiszar A, Lerea K, Ungvari Z, Veress G (2006) Resveratrol inhibits aggregation of platelets from high-risk cardiac patients with aspirin resistance. J Cardiovasc Pharmacol 48:1–5
Chang HC, Chen TC, Tai YT, Chen TL, Chiu WT, Chen RM (2011) Resveratrol attenuates oxidized LDL-evoked lox-2 signaling and consequently protects against apoptotic insults to cerebrovascular endothelial cells. J Cereb Blood Flow Metab 31:842–854
Robich MP, Osipov RM, Nezafat R, Feng J, Clements RT, Bianchi C, Boodhwani M, Coady MA, Laham RJ, Sellke FW (2010) Myocardial protection, perioperative management, and vascular biology. Circulation 122:S142–S149
Fujitaka K, Otani H, Jo F, Jo H, Nomura E, Iwasaki M, Nishikawa M, Iwasaka T, Das DK (2011) Modified resveratrol longevinex improves endothelial function in adults with metabolic syndrome receiving standard treatment. Nutr Res 31:842–847
Magyar K, Halmosi R, Palfi A, Feher G, Czopf L, Fulop A, Battyany I, Sumegi B, Toth K, Szabados E (2012) Cardioprotection by resveratrol: a human clinical trial in patients with stable coronary artery disease. Clin Hemotheol Microcirc 50:179–187
Patel KR, Scott E, Brown VA, Gescher AJ, Steward WP, Brown K (2011) Clinical trials of resveratrol. Ann N Y Acad Sci 1215:161–169
Lu R, Serrero G (1999) Resveratrol, a natural product derived from grape inhibits the growth of human breast cancer cells. J Cell Physiol 179:297–304
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CWW, Fong HHS, Farnsworth NR, Kinghorn D, Mehta RG, Moon RC, Pezzuto JM (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Kaneko H, Anzai T, Morisawa M, Kohno T, Nagai T, Anzai A, Takahashi T, Shimoda M, Sasaki A, Maekawa Y, Yoshimura K, Aoki H, Tsubota K, Yoshikawa T, Okada Y, Ogawa S, Fukuda K (2011) Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization. Atherosclerosis 217:350–357
Diaz MN, Frei B, Vita JA, Keaney JF (1997) Antioxidants and atherosclerotic heart disease. New Eng J Med 337:408–416
Voloshyna I, Hussaini SM, Reiss AB (2012) Resveratrol in cholesterol metabolism and atherosclerosis. J Med Food 15:763–773
Chong ZZ, Shang YC, Wang S, Maiese K (2011) SIRT1: new avenues of discovery for disorders of oxidative stress. Expert Opin 16:167–178
Das S, Santani DD, Dhalla NS (2007) Experimental evidence for cardioprotective effects of red wine. Exp Clin Cardiol 12:5–10
Riccioni G, D’Orazio N, Salvatore C, Franceschelli S, Pesce M, Speranza L (2012) Carotenoids and vitamins C and E in the prevention of cardiovascular disease. Int J Vit Nutr Res 82:15–16
Will JC, Ford ES, Browman BA (1999) Serum vitamin C concentration and diabetes: finding from the third national health and nutrition examination surveys 1988–1994. Am J Nutr 70:49–52
Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Garcia-Conesa MT, Tomas-Babberan FA, Espin JC (2012) One –year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am J Cardiol 110:356–363
Bhatt JK, Thomas S, Nanjan MJ (2012) Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res 32:537–541
Dakhale GN, Chaudhari HV, Shrivastava M (2011) Supplementation of vitamin C reduced blood glucose and improves glycosylated hemoglobin in type 2 diabetes mellitus: a randomized, double-blind study. Adv Pharmacol Sci. doi:10.1155/2011/195271
Mazloom Z, Hejazi N, Dabbaghmanesh MH, Tabatabaei HR, Ahmadi A, Ansar H (2011) Effect of vitamin C supplementation on postprandial oxidative stress and lipid profile in type 2 diabetic patients. Pak L Biol Sci 14:900–904
Illison VK, Rondo PH, de Olivera AM, D’Abronzo FH, Campos KF (2011) The relationship between plasma α-tocopherol concentration and vitamin E intake in patients with type 2 diabetes mellitus. Int J Vit Nutr Res 81:12–20
Shab-Bidar S, Mazloum Z, Mousavi-Shirazifard Z (2012) Daily vitamin E supplement do not improve metabolic and glycemic control in type 2 diabetic patients: a double blinded randomized controlled trial. J Diabetes. doi:10.1111/j.1753-0407.2012
Treweeke AT, Winterburn TJ, Mackenzie I, Barrett F, Barr C, Rushworth GF, Dransfield I, Macrury SM, Megson IL (2012) N-acetylcysteine inhibits platelet-monocyte conjugation in patients with type 2 diabetes with depleted intraplatelet glutathione: a randomized controlled trial. Diabetologia. doi:10.1007/s00125-012-2685-z
Acknowledgments
Infrastructural support for this project was provided by St. Boniface Hospital Research Foundation. Dr. N. S. Neki was a visiting professor from Department of Internal Medicine, Government Medical College, Amritsar, India.
Conflict of interest
None of the authors have any conflict of interest with any granting agency.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, YJ., Tappia, P.S., Neki, N.S. et al. Prevention of diabetes-induced cardiovascular complications upon treatment with antioxidants. Heart Fail Rev 19, 113–121 (2014). https://doi.org/10.1007/s10741-013-9379-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-013-9379-6