Abstract
The aetiopathogenesis of acute and chronic myocarditis is rather complex as a great variety of infectious agents can induce cardiac inflammation. Moreover, many systemic and autoimmune diseases such as sarcoidosis, giant cell myocarditis and systemic lupus erythematodes, drugs and toxins have been described as non-infectious causes of inflammatory heart disorders. Myocarditis may cause sudden death and lead to dilated cardiomyopathy. The correct and timely diagnosis of myocarditis is still a difficult clinical challenge, since the clinical spectrum of myocarditis is broad and comprises (amongst others) even those patients with no symptoms or those presenting with acute cardiogenic shock. Although endomyocardial biopsy still represents the gold standard for the diagnosis of myocarditis, new non-invasive imaging techniques such as cardiovascular magnetic resonance (CMR) imaging promise the non-invasive diagnosis of myocarditis. Considering the hallmarks of acute and chronic myocarditis (accumulation of inflammatory cells; swelling, necrosis and/or apoptosis of cardiomyocytes; increase in extracellular space and water content; myocardial remodelling with fibrotic tissue replacement), an imaging modality such as CMR that enables non-invasive detection of changes in myocardial tissue composition is highly valuable and welcome. This review will focus on the ‘clinical role’ of CMR in the diagnosis of acute and chronic myocarditis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Today, cardiovascular diseases represent the number one cause of morbidity and mortality in western societies. Amongst these cardiovascular diseases, myocarditis is an inflammatory heart disease that may be caused by different pathogens and triggers [1, 2] and is gaining increasing importance since it may cause sudden death (in particular in young athletes) and lead to dilated cardiomyopathy [3, 4]. Although endomyocardial biopsy (EMB) still represents the gold standard for the diagnosis of myocarditis [5–7], new non-invasive imaging techniques such as cardiovascular magnetic resonance (CMR) imaging promise the non-invasive diagnosis of myocarditis [2, 8]. Therefore, this review will focus on the ‘clinical role’ of CMR in the diagnosis of acute and chronic myocarditis.
Pathophysiology of myocarditis: experiences from animal models
The aetiopathogenesis of acute and chronic myocarditis is rather complex as a great variety of infectious agents comprising bacteria, protozoa, fungi and, most importantly, viruses can induce cardiac inflammation [1, 2]. In addition, many systemic and autoimmune diseases such as sarcoidosis, giant cell myocarditis and systemic lupus erythematodes, drugs and toxins have been described as non-infectious causes of inflammatory heart disorders [9].
To date, the deepest insights into the pathogenesis of myocarditis were gained by using coxsackievirus B3 (CVB3) infected mice [10–12] as they perfectly match the different outcome of enteroviral myocarditis in humans. Only certain mouse strains with a defined genetic background suffer from an extensive cardiac infection and severe cardiac injury and inflammation [11, 13]. Coxsackieviruses of group B which enter preferably cardiomyocytes via specific receptors (CAR for CVB1-6) induce severe cytopathic damage due to virus replication in the first 2 weeks post-infection [14]. As a consequence of myocyte injury, the virus-specific innate immunity as well as the humoral and cellular immune response evolves. Resistant animals (C57BL/6, Sv/129 mice) are able to eliminate the infectious agent within 2 weeks post–infection; thus, the inflammation completely resolves. However, in susceptible mouse strains (e.g. A/J, ABY/SnJ, ASW/SnJ, ACA/SnJ, SWR/J, Balb/c) viral RNA and consequently, inflammation persist in the heart over several weeks or months [11]. There is evidence that dependent on the individual genetic predisposition in these susceptible animals the ongoing infection and inflammation can trigger autoimmune reactions in the heart most likely as a result of myocyte necrosis and subsequent liberation of self-antigens previously hidden to the immune system [15]. It is assumed that the release of large quantities of cardiomyocyte proteins passes over the threshold required for the induction of an autoimmune response, resulting in the activation of self-reactive T cells [16–18]. Several studies in mice and rats suggest molecular mimicry as one of the pathogenetic mechanisms which might be relevant in the perpetuation of a chronic inflammatory heart disease [19, 20].
In the MRL mouse strain, cardiac myosin mimicking streptococcal M protein peptide NT4 was found to induce tolerance and to prevent CVB3-induced myocarditis, suggesting T cell mimicry between coxsackievirus and streptococcal M protein [19]. In addition, immunization with peptides derived from Borrelia burgdorferi, Treponema pallidum and Chlamydia trachomatis mimicking the alpha-myosin heavy chain epitope M7A-alpha was found to be associated with the activation of autoaggressive T and B lymphocytes, the production of autoantibodies and histopathological changes in the heart muscle [21]. Goser et al. [22] reported that mice immunized against cardiac troponin I develop a myocardial inflammation with upregulation of inflammatory chemokines followed by cardiac fibrosis. On the other hand, Luppi et al. [23] argued against the idea of molecular mimicry against a specific heart antigen, hypothesizing that CVB3 might encode a superantigen (or upregulate an endogenous superantigen-like molecule) that is relevant in pathogenesis of myocarditis as a limited TCR Vß gene family usage in the presence of variable CDR3 regions was observed in patients.
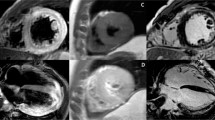
However, as to whether CVB3 and cardiac antigens induce the same pathology in the heart is still unclear. Visualizing the patterns of inflammation, there is evidence that lytic viruses such as CVB3 induce an acute and chronic myocarditis consisting mainly of macrophages and T lymphocytes in both ventricles (Fig. 1a–c), whereas the autoimmune models of myocarditis using proteins or peptides from myocytes in presence of adjuvans-releasing antigens from Mycobacterium tuberculosis (e.g. CFA) or Bordetella pertussis reveal pericarditis (Fig. 1d) rather than myocarditis.
Acute (a, b) and chronic (c, d) murine myocarditis. CVB3 myocarditis (a–c): high numbers of lytically infected myocytes as detected by radioactive in situ hybridization (black grains) are found in both ventricles (a). Virus replication results in myocyte necrosis (yellow stars) and invasion of immune cells (b, HE). At later stages of viral myocarditis in susceptible mice, fibrosis (blue fibrils) evolves in presence of ongoing inflammation (c, Masson Trichrome). In contrast to viral myocarditis, autoimmune myocarditis (here induced by myosin) often reveals patterns of pericarditis (arrow) suggesting different pathogenic mechanisms (d, HE)
Taken together, the pathogenesis of myocarditis comprises three interleaved phases: First, pathogens such as viruses or bacteria infect myocardial cells and replication leads to myocardial damage. Subsequently, an immunological response by the host immune system is triggered and myocardial inflammation often further increases cardiac injury. Finally, chronic myocardial inflammation may lead to myocardial fibrosis, ventricular remodelling and dysfunction.
Essential principles and advantages of CMR
The principles of magnetic resonance imaging (MRI) were discovered in the first half of the twentieth century: The physical principles were first described by Rabi in 1939, and the first MRI machine was constructed by Block in 1941 and subsequently used to analyse the structure of materials [24]. The first ‘diagnostic’ human scan was performed by Damadian in 1977. Today, MRI scanners are commonly available even in tertiary care centres and represent a ‘sine qua non’ diagnostic tool for numerous diseases. In particular, cardiovascular MRI (CMR) has gained worldwide acceptance in the diagnosis and therapy surveillance of numerous cardiac diseases due to a couple of advantages [25, 26]: (1) CMR enables a highly accurate evaluation of structural (anatomic) as well as functional cardiac parameters at the same time; (2) CMR is free of some major limitations that are unavoidable in other cardiac imaging modalities such as a poor acoustic window in echocardiography or radiation burden in computed tomography; (3) CMR enables a non-invasive characterization of myocardial tissue which so far could only be performed ex vivo by pathologists.
In particular, the correct and timely diagnosis of myocarditis (acute and chronic) is still a difficult clinical challenge, since the clinical spectrum of myocarditis is broad and comprises (amongst others) even those patients with no symptoms or those presenting with acute cardiogenic shock [2, 6, 8, 27]. Considering the hallmarks of acute and chronic myocarditis (accumulation of inflammatory cells; swelling, necrosis and/or apoptosis of cardiomyocytes; increase in extracellular space and water content; myocardial remodelling with fibrotic tissue replacement) [2, 27, 28], an imaging modality such as CMR that enables non-invasive detection of changes in myocardial tissue composition is highly valuable and welcome. In this context, CMR offers various imaging sequences that target different hallmarks of acute and/or chronic myocarditis [8]: (1) Cine-CMR enables the accurate detection of regional as well as global functional abnormalities that may come along with myocarditis; (2) Contrast imaging after intravenous administration of Gadolinium-based compounds enables both a) detection of early capillary leakage (increased vascular permeability) based on T1-weighted early gadolinium enhancement (EGE) imaging and b) accurate diagnosis of myocardial necrosis and/or apoptosis based on T1-weighted late gadolinium enhancement (LGE) imaging; (3) T2-weighted oedema imaging allows detection of myocardial oedema and tissue hyperaemia.
Actually, these aforementioned CMR techniques have been either developed based on animal studies focusing on acute as well as chronic myocardial infarction or adopted from non-cardiac MRI applications. For example, T1-weighted LGE imaging was first introduced in the 1990s and successfully applied to rabbits and dogs with man-made myocardial infarction by the group of Judd and Kim from the John Hopkins Hospital [29, 30]. They could demonstrate (amongst others) that >90 % of the myocardium showing hyperenhancement after intravenous administration of Gadolinium-based contrast compounds is non-viable due to acute myocardial infarction with cardiomyocyte necrosis [31]. Subsequently, this technique was applied to patients with myocardial infarction and further optimized. Simonetti et al. [32] developed an improved CMR sequence (segmented inversion recovery turboFLASH) that substantially improved differentiation between injured and normal regions by showing a ~500 % signal intensity increase in infarcted myocardial areas compared to non-infarcted normal areas. After proving diagnostic success in patients with myocardial infarction, this technique was also applied to patients with clinically suspected or biopsy-proven myocarditis [33]. Hence, convincing pre-clinical data demonstrating a successful or meaningful use of the respective CMR technique in appropriate animal models of myocarditis such as CVB3 models—with convincing correlation between in vivo acquired CMR images and ex vivo performed histopathological workup—are scarce, and the limited available data were only collected in the last years (after successful implementation of the respective CMR technique in patients) [34, 35].
Diagnosis of myocarditis based on T1-weighted late gadolinium enhancement (LGE) imaging
Late gadolinium enhancement (LGE) CMR allows direct visualization of myocyte necrosis and fibrosis, which can differentiate the pattern of injury in myocarditis from ischaemic myocardial infarction [8, 26, 36]. This method is now so well established in clinical practice that it has entered clinical consensus statements [8].
Background: the use of late gadolinium enhancement (LGE) in myocarditis
Friedrich et al. [37] first described the use of gadolinium-enhanced CMR in patients with myocarditis, using a T1-weighted spin-echo sequence with early gadolinium enhancement (EGE), demonstrating that myocarditis evolves from a focal to a disseminated process during the first 2 weeks. Subsequently, newer techniques using segmented inversion recovery gradient-echo sequences and late gadolinium enhancement (LGE) achieved an improvement in contrast (of up to 500 % between diseased and normal myocardium), allowing detection of even small areas of myocardial injury [32, 38]. Rieker et al. [33] were the first to apply such a CMR sequence to patients with biopsy-proven myocarditis in 2002. Mahrholdt et al. [38] then used LGE imaging to study severely diseased patients with clinically suspected myocarditis. The results showed that (1) LGE is a frequent finding (88 %) associated with active myocarditis on histopathology (Fig. 2); (2) the LGE pattern in myocarditis is often patchy, usually involving the epicardial quartile of the myocardial wall with one or several foci, frequently localized to the lateral free wall (Figs. 2, 3) and (3) areas of LGE decreased over time (Fig. 3).
Cine- and LGE-CMR images (in short- and long-axis views) of a patient with acute myocarditis with corresponding histological findings in biopsy samples taken from the left ventricular free wall. Ongoing inflammation with predominance of macrophages was documented based on Masson Trichrome and anti-CD68+ stainings
Summary of systematic studies using late gadolinium enhancement (LGE) in patients with myocarditis
There have since been a number of studies demonstrating the usefulness of LGE in the diagnosis of myocarditis [38–42], with somewhat differing values regarding the specificity and the sensitivity of this technique [8].
Previous studies have shown consistently that LGE is predominantly localized either in the septal wall (like an intramural rim) and/or in the subepicardial layers of the left ventricular free wall (mostly patchy distribution) [43]. Serial studies demonstrated a decrease in the extent of LGE often associated with an improvement in left ventricular systolic function only in those patients with a subepicardial LGE pattern in the left ventricular free wall [43]. In contrast, an intramural septal LGE pattern does not regress over time, and patients seem to have a worse outcome [43–45]. Interestingly, subendocardial or transmural LGE patterns are also observed, as described by Yilmaz et al. [42]. Seventy-one patients with clinically suspected myocarditis underwent coronary angiography and intracoronary acetylcholine testing for evaluation of coronary vasospasm, EMB and CMR. Coronary vasospasm was shown to be the main reason for the chest pain often encountered in patients with myocarditis especially in those patients with biopsy-proven parvovirus B19 (PVB19) infection. Coronary vasospasm may also explain subendocardial or transmural LGE in some patients with myocarditis.
The diagnostic performance of LGE imaging in myocarditis has been tested in a number of studies (Table 1). Abdel-Aty et al. [39] examined 25 patients with clinically suspected myocarditis compared to controls using CMR, including (a) T2-weighted triple inversion recovery for the edema ratio (ER), (b) early gadolinium enhancement (EGE) imaging for global relative enhancement (gRE) and (c) LGE. Although LGE alone was highly specific (specificity 100 %, sensitivity 44 % and diagnostic accuracy 71 %), the overall diagnostic accuracy of CMR improved to 85 % by using a combination of the three parameters. More recently, Rottgen et al. [41] performed EMB validation for this CMR protocol in 131 patients with suspected acute myocarditis, and found that gRE and LGE, but not ER, significantly correlated with the presence of myocarditis on histology. Again, LGE alone had a high specificity (88 %), but low sensitivity (31 %), whereas a combination of the three parameters improved diagnostic accuracy and specificity.
Moreover, LGE imaging is also useful in chronic myocarditis. De Cobelli et al. [46] found that LGE imaging identified areas of inflammation in up to 70 % of patients with biopsy-proven chronic myocarditis. Gutberlet et al. [47] studied 83 patients with suspected chronic myocarditis using LGE, ER and gRE with EMB immunohistologic correlation. LGE had a relatively high specificity (80 %), but much lower sensitivity (27 %) and diagnostic accuracy (49 %). A combination approach improved diagnostic accuracy.
Limitations of using late gadolinium enhancement (LGE) alone in the diagnosis of myocarditis
A number of factors may contribute to the variability in sensitivity and diagnostic accuracy of using LGE imaging alone in the tissue characterization of myocarditis, including study design, selection of patients, method of validation, timing of CMR in relation to the myocarditic process and technical considerations.
There may be a window for optimal LGE imaging in myocarditis. LGE is due to myocyte necrosis and fibrosis [8]. In the early stages of myocarditis, increased signal intensity on LGE imaging is mainly due to the accumulation of gadolinium within myocytes through acutely injured membranes [8, 28], but severe extracellular oedema could also sufficiently increase the volume of distribution of gadolinium to cause visually detectable changes in signal intensity [28, 39]. However, focal myocarditis, especially early in disease, may not always be large enough for necrotic myocytes to be visualized with the pixel size used, and repeat CMR 1–2 weeks later may be required [8]. Diffuse or borderline myocarditis may not lead to observable LGE. In the later stages, previously damaged tissues are replaced by fibrosis with increased interstitial space, leading to an increased volume of distribution for gadolinium and increased signal intensity on LGE imaging (Fig. 4). However, LGE can decrease or even disappear over time [43, 48]. Mahrholdt et al. [43] found that 95 % of those with active myocarditis by EMB demonstrated LGE, whereas only 40 % of those with healing myocarditis showed LGE. Thus, LGE may show variable sensitivity to the underlying disease process depending on the time of imaging in relation to the stage of disease [49], which can affect its diagnostic performance.
Exemplary comparison of ‘active’ versus ‘borderline myocarditis.’ The upper and lower rows display histological and CMR findings in active myocarditis and borderline myocarditis, respectively (lymphocytes, necrosis and LGE are indicted by arrowheads). In the patient with borderline myocarditis, CMR was not able to diagnose myocarditis due to low extent of inflammation. With permission from Baccouche et al. [57]
Finally, parameters for imaging such as the slice thickness prescribed and threshold of what is considered pathological enhancement compared to a presumably normal area of myocardium, which seem to vary amongst studies (Table 1), also affect the reported diagnostic performance of LGE.
Summary
Presence of LGE is one useful criterion in the diagnosis of myocarditis. The pattern of LGE allows differentiation of myocarditis from myocardial infarction and is strongly associated with histopathological evidence of myocarditis. LGE has a relatively high specificity in the diagnosis of myocarditis, but low sensitivity, although its diagnostic performance is influenced by a number of factors, especially the timing of LGE imaging to the stage of the myocarditic process. LGE imaging alone is not sufficient to absolutely rule in or rule out myocarditis. Using a combination of different imaging sequences which are sensitive to the different processes in myocarditis, such as oedema, hyperemia and myocyte necrosis/fibrosis, may provide better diagnostic accuracy [8].
Diagnosis of myocarditis based on T2-weighted oedema imaging and T1-weighted early gadolinium enhancement (EGE) imaging
While LGE imaging can distinguish patterns of myocyte necrosis and fibrosis in myocarditis from ischaemic injury, it cannot be used as a marker for other processes in myocarditis, such as edema, capillary leakage and hyperemia [8]. T2-weighted and early gadolinium enhancement (EGE) imaging are well-validated techniques to detect oedema and hyperemia, respectively, and are two of the three Lake Louise Consensus Criteria, in addition to LGE, recommended for diagnosing myocarditis [8].
The use of T2-weighted CMR in patients with myocarditis
T2-weighted CMR is sensitive to myocardial oedema. This is due to the prolongation of the transverse component of proton relaxation time, T2, when protons are bound to water molecules, increasing signal intensity (SI) on T2-weighted images [50]. Gagliardi et al. [51] first described T2-weighted CMR findings in children with myocarditis using a T2-weighted spin-echo sequence at 0.2 Tesla (T). Advances in the 1990s led to the development of the faster, breath-hold short tau triple inversion recovery (STIR) turbo spin-echo sequence for fat and blood suppression at 1.5 T [52], with improved image quality. A comparison of the diagnostic accuracy of T2-weighted STIR imaging in myocarditis trials are listed in Table 2.
T2-weighted STIR imaging has been validated to have a good diagnostic performance by Abdel-Aty et al. [39] in patients with suspected myocarditis. It allowed the detection of global oedema by comparing myocardial T2 signal intensity (SI) to that of skeletal muscle to calculate the edema ratio (ER). At an ER of 1.9, the sensitivity, specificity and diagnostic accuracy was 84, 74 and 79 %, respectively. The pattern of edema in myocarditis is subepicardial, transmural or global, not always associated with LGE, and LGE areas are smaller than focal areas with increased T2 (Fig. 5). This may explain why the diagnostic accuracy of CMR improved to 85 % when a combination of T2-weighted STIR, LGE and EGE, rather than any single modality alone, is used.
T2-weighted and late gadolinium enhancement (LGE) images in a patient with acute myocarditis. Note the predilection for the lateral wall and predominantly subepicardial and mid-wall distribution of increased T2 signal intensity and LGE. STIR short tau inversion recovery, TSE turbo spin echo, SSFP steady state free precession
As mentioned above, a recently published study found that findings on oedema imaging did not significantly correlate with myocarditis on immunohistology [41]. The sensitivity, specificity and diagnostic accuracy for ER were low at 58, 57 and 58 %, respectively. However, given that there is no reliable histological technique to display the presence and extent of cellular edema [50], it is difficult to validate T2-weighted edema imaging against immunohistology for myocarditis, which is based on the findings of inflammatory infiltrates and myocyte necrosis: clinical validation with carefully selected cohorts may improve the in vivo detection of oedema on CMR.
In chronic myocarditis, T2-weighted STIR imaging is less sensitive in detecting oedema. T2-weighted STIR detected changes in outpatients with clinically suspected acute myocarditis with a sensitivity, specificity and diagnostic accuracy of 74, 93 and 81 %, respectively [49], but in only 35 % of patients with EMB-proven chronic myocarditis and none in those with ‘borderline’ myocarditis [46]. Gutberlet et al. [47] studied 83 patients clinically suspected of chronic myocarditis and found that only 52 % demonstrated an elevated ER, 74 % of which demonstrated immunohistologic findings of myocarditis, yielding a sensitivity, specificity and diagnostic accuracy of 67, 69 and 68 %, respectively.
Limitations of T2-weighted CMR in the diagnosis of myocarditis
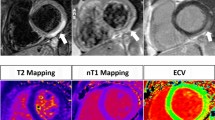
The signal-to-noise ratio (SNR) of T2-weighted images, especially that acquired by T2-weighted STIR, is strongly dependent on imaging parameters [8]. The use of the body coil results in lower SNR. Poor dark blood preparation can result in bright subendocardial SI, especially at the apex. Skeletal muscle inflammation, demonstrated in patients with acute myocarditis [53], can lead to false negative results in detecting global myocardial edema [8, 53]. Image quality is susceptible to artefacts due to motion, breathing and arrhythmia [54]. These factors decrease the sensitivity of this technique, especially in detecting small, focal, borderline or low-grade chronic myocarditis. Newer SSFP-based T2-weighted sequences [54, 55] (Fig. 1) and T2-mapping techniques [56] have been developed which may circumvent some of the technical issues, but have not been systematically validated in patients with myocarditis.
The use of early gadolinium enhancement (EGE) in the diagnosis of myocarditis
As part of the inflammatory response, regional vasodilation and hyperemia increases blood volume in injured tissue, causing an increase in the uptake of gadolinium-based contrast agents in the early vascular phase of gadolinium kinetics [37]. Gadolinium then quickly distributes into the interstitial space. Consequently, early gadolinium enhancement (EGE) reflects an overall increase in gadolinium distribution in the early washout period into the intravascular and interstitial space as a result of increased blood flow, cell damage and extravasation of fluid in areas of inflammation [8, 37].
Friedrich et al. [37] first used EGE in patients with myocarditis to demonstrate that myocarditis evolves from a focal to a disseminated process during the first 2 weeks. Global relative enhancement (gRE) of the myocardium was derived by measuring the signal intensity of pre- and post-gadolinium contrast T1-weighted images and relating to that of skeletal muscle. Laissy et al. [53] reaffirmed, using EGE and dynamic serial post-contrast perfusion imaging, that myocarditis starts as a focal process, and that there is evidence of skeletal muscle inflammation in myocarditis. An increased early gadolinium enhancement ratio (gRE) is defined by either a signal intensity enhancement ratio between myocardium and skeletal muscle of ≥4.0 or, in the case of skeletal muscle myositis (increase in skeletal muscle SI of ≥20 %), an absolute myocardial enhancement of ≥45 % [8].
Abdel-Aty et al. [39] compared gRE, ER and LGE in the diagnosis of clinically suspected myocarditis, and, using receiver operator characteristic analysis, established that gRE with a cut-off value of 4.0 had a sensitivity, specificity and diagnostic accuracy of 80, 68 and 74 %, respectively. T2-based ER did not correlate to gRE, possibly due to edema causing capillary compression, hindering abnormal contrast enhancement, and partially explaining why the CMR diagnostic accuracy improves with using a combination of the three parameters.
When validated against immunohistology for myocarditis, EGE demonstrated a relatively high specificity, both in acute myocarditis (specificity 74 %, sensitivity 49 %, accuracy 57 %) [41] and in chronic myocarditis (specificity 86 %, sensitivity 62 %, accuracy 72 %) [47], although both studies concluded that a combination of CMR parameters improve overall specificity and diagnostic accuracy. A comparison of the diagnostic accuracy of EGE in myocarditis trials are listed in Table 3.
Limitations of using EGE in the diagnosis of myocarditis
The main limitation of EGE relates to the quality of images acquired using current fast spin-echo sequences, which is susceptible to irregular heart rates and breathing movements [8] and can adversely affect the diagnostic performance of EGE especially in detecting small areas of myocarditis. The time required to obtain pre- and post-contrast EGE images is also relatively long compared to other CMR sequences.
Summary
T2-weighted and EGE-CMR are useful additional tools in the diagnosis of myocarditis, reflecting the presence of oedema in the former, and a combination of hyperemia, myocytes damage and extravasation of fluid in the latter; when present, these can demonstrate active disease. Like the predominantly non-ischaemic pattern of LGE in myocarditis, T2 and EGE findings also tend to be subepicardial when focal or transmural and global when disseminated. Both tend to be more specific when present, rather than sensitive, especially in detecting small areas of injury or low-grade disease. As with the use of LGE as a diagnostic criterion for myocarditis, the use of either T2-STIR oedema ratio (ER) or early gadolinium enhancement ratio (gRE) alone is insufficient to rule in or rule out myocarditis. A combination of these three parameters can improve the diagnostic accuracy of myocarditis on CMR.
Cardiovascular magnetic resonance (CMR) versus endomyocardial biopsy (EMB): superiority, inferiority or synergy?
Despite impressive technical progress in non-invasive CMR imaging over the last years and successful clinical implementation of multi-parametric CMR imaging in suspected myocarditis, there are still some major drawbacks that have to be addressed:
The currently available and clinically established techniques regarding myocardial tissue characterization (such as T1-weighted LGE imaging, T2-weighted oedema imaging or T1-weighted EGE imaging) allow the non-invasive diagnosis of myocarditis with a high specificity, but suboptimal sensitivity (as compared to histopathology and immunohistology as the gold standard). Considering typical values of in-plane resolution for the respective techniques (typically ~1.4 × 1.4 mm in case of T1-weighted LGE or T2-weighted oedema imaging) [38, 39] in contrast to the histological extent of myocardial areas with ‘patchy’ inflammation (typically only a few hundred microns), the still limited resolution capacity of non-invasive CMR represents an important disadvantage compared to the diagnostic capability of invasive endomyocardial biopsy (EMB). Hence, theoretically the presence of myocarditis is visualized by CMR only if at least one ‘confluent’ hot spot of myocardial inflammation approaches the aforementioned value of in-plane resolution. Obviously, such a ‘confluent’ hot spot of myocardial inflammation (that is required for successful CMR detection) represents a rather advanced and severe phase of inflammation. Therefore, the diagnostic sensitivity of CMR is particularly limited in case of mild/subtle myocardial inflammation. This important issue was highlighted by Baccouche et al. [57] who demonstrated that the diagnosis of myocarditis was more frequently made by CMR in patients with active myocarditis compared to those with borderline myocarditis, since patients with active myocarditis showed more segments with the finding of LGE in accordance with higher serum values of cardiac enzymes as markers of the severity of myocardial damage compared to subjects with borderline myocarditis. This indicates that a higher extent of myocardial damage will be associated with a greater diagnostic accuracy of (at least) LGE-CMR.
Another drawback of diagnosing myocarditis by using CMR is that potential therapeutic options in case of myocarditis can only be implemented if the exact cause of myocarditis – such as viral, bacterial, giant cell or eosinophilic - is known. Based on a huge armamentarium of sophisticated diagnostic techniques (comprising histology, immunohistochemistry and molecular pathology) [6, 7], a pathologist is able to provide more detailed information about the nature of the inflammatory process compared to CMR. A comprehensive histopathological workup of EMBs was shown to be highly sensitive for the detection of myocardial inflammation (even in the absence of focal lymphocytic infiltration) and has been suggested to identify patients responding to immunomodulatory therapy in preliminary studies [58, 59]. Previously, Why et al. prospectively evaluated 120 patients with clinical suspicion of myocarditis and/or dilated cardiomyopathy and divided them into two groups on the basis of the presence or absence of enteroviral genome in the biopsy samples [60]. Mortality and progression to cardiac transplantation during the follow-up was greater in the enterovirus positive group than in the enterovirus negative group. Furthermore, the detection of enterovirus RNA in the myocardium was shown by multivariate regression analysis to be an independent predictor of clinical outcome. Accordingly, Frustaci et al. recently published the results of their TIMIC study [61] and showed that immunosuppressive therapy (with cortisone and azathioprin) for six months improved left ventricular systolic function only in those patients with inflammatory cardiomyopathy who did not have virus genome persistence in their EMBs. However, viral persistence (without inflammation) cannot be assessed by CMR. Therefore, invasive sampling of EMBs still constitutes the gold standard in the diagnosis of myocarditis and is the only way to directly detect inflammatory infiltrates in the myocardium.
However, just as CMR, EMB also has method-specific limitations [57]. Although workup of EMBs still constitutes the gold standard in the diagnosis of myocarditis, this method is limited by a) the ‘sampling error’ resulting in ‘false’ negative results due to missing the area of inflammation (due to the patchy distribution of myocarditis) when taking biopsies [62], b) the invasiveness of this procedure with a low but non-negligible risk of complications [63, 64] and c) the inability to perform serial biopsies or even no biopsy in patients with preserved left ventricular function as guidelines do not support this in such patients. In contrast, non-invasive imaging strategies such as CMR allow a) the diagnosis of myocarditis non-invasively without the risk of complications, b) to assess the entire myocardium based on different axes and views and c) to repeat the procedure at any time and follow up changes in the extent and degree of myocardial inflammation (to a certain limited level). Hence, considering those aforementioned advantages and limitations of these two methods, the question which method to use first in case of clinically suspected myocarditis inevitably arises from a clinical point of view.
Recently, Baccouche et al. [57] evaluated the diagnostic performance of both CMR and endomyocardial biopsy in the same patients with clinically suspected myocarditis. They found that biopsy was superior (at least) to LGE-based CMR imaging in diagnosing myocarditis, because of its capabilities based on immunohistochemistry and nested-PCR enabling the detection of minor forms such as borderline myocarditis or virus genome presence. However, there was a high diagnostic conformity between CMR-based and biopsy-based results. Hence, it may be reasonable to initially perform non-invasive CMR (without the risk of complication) in patients with clinically suspected myocarditis. If the initial CMR study establishes the diagnosis of myocarditis, additional invasive biopsy is unlikely to change this diagnosis. In contrast, if the initial CMR study is non-conclusive, but a diagnosis needed for instance in a patient with persistent symptoms, invasive biopsy can still be employed as a second step, since biopsy allows to capture more mild/subtle forms of myocarditis, which cannot be detected with CMR. Theoretically, such an algorithm will avoid biopsies in a substantial number of patients and therefore minimize the risk of complications associated with biopsy. However, such a stepwise approach is obviously not a perfect alternative because CMR information is less detailed and—in contrast to biopsy—does not allow to evaluate the exact degree of inflammation, the presence of special forms of myocarditis (such as giant cell or eosinophilic myocarditis which require specific therapies), or the presence and type of virus.
Moreover, the study of Baccouche et al. demonstrated that the combined application of CMR and EMB yielded a considerable diagnostic synergy: The combined approach was superior to each single technique regarding the final diagnostic yield and could overcome some of the well-known limitations of CMR and EMB as individually applied techniques. In addition, recent data by Kindermann et al. [65] suggest that the presence of inflammation in biopsies has prognostic implications, and previous CMR data indicate that the presence and distribution of LGE may also have prognostic implications [43–45]. Therefore, a combined approach (comprising CMR and EMB) could also be superior for future risk stratification and implementation of specific therapies. However, an exact algorithm, when and in which patient and in which procedure should be preferentially used, is unfortunately still not available. Whether it is necessary to obtain detailed histopathological information using biopsies in addition to a preceding conclusive CMR study has to be decided in consideration of the individual circumstances.
LGE-targeted biopsy to increase the sensitivity of EMB: illusion or reality?
Theoretically, guidance of biopsy by CMR (to the area of LGE) might be helpful to improve the accuracy and safety of EMB procedure by minimizing the sampling error caused by the patchy distribution of inflammatory areas in case of myocarditis. However, one important caveat has to be remembered: the area of LGE may be small and consequently cannot exactly be reached by the bioptome due to the limited steerability of the bioptome. Appropriate data addressing this important issue are quite scarce. Based on the results of an earlier study [38], it was hypothesized—in line with the aforementioned conception—that the diagnostic yield of biopsy might be increased if EMBs were obtained from the ventricle demonstrating LGE. However, data of this previous study were based on 32 patients only and did not comprise biventricular biopsy in every patient of this study group. Therefore, this clinically important issue was addressed in more detail by Yilmaz et al. [64] in a larger study group: Data from 116 patients who had undergone biventricular EMBs (with histopathological diagnosis of myocarditis) in addition to a CMR study (showing LGE) were available in this recent study. The distribution of LGE (in the septal or LV free wall) in relation to the EMB results was precisely analysed. Surprisingly, there were no substantial differences in the number of diagnostic LV-EMBs, RV-EMBs or biventricular (LV and RV) EMBs when related to the site of LGE disproving the initial hypothesis of LGE-targeted biopsy in order to increase the sensitivity of EMB.
As discussed in detail previously [64], LGE is a non-specific sign of myocardial damage and only indicates an increase in extracellular space (that may be due to acute necrosis as well as chronic fibrosis). Hence, the missing increase in the diagnostic yield of biopsies obtained from the ventricle showing LGE in the study of Yilmaz et al. may be due to the potential detection of LGE in patients with already healed myocarditis (without any inflammatory infiltrates left). Theoretically, histopathological EMB workup that focuses on the detection of inflammatory cells and/or antigens may result in non-pathological or non-diagnostic findings in such patients with already healed myocarditis, while CMR may be indicative of myocarditis due to the presence of LGE. Consequently, a LGE-guided biopsy in such patients would not increase the diagnostic yield, but decrease the specificity of the CMR procedure for the diagnosis of myocarditis when the EMB procedure is considered as the gold standard. Unfortunately, there are no data yet relating the diagnostic yield of EMB to tailoring the procedure to focal increases in the T2 signal which may be more indicative of the focal nature of the acute inflammatory process.
Quo vadis: future perspectives and challenges
Considering the diagnostic value of CMR in the workup of patients with clinically suspected myocarditis, the benchmark regarding its diagnostic yield and success will be set by current and future capabilities of histopathological EMB workup. For example, Heidecker et al. [66] have shown recently that transcriptomic biomarkers from even a ‘single’ EMB yield clinically relevant and accurate molecular signatures and thereby essentially improve the clinical detection of patients with inflammatory diseases of the heart. They identified a transcriptome-based biomarker containing 62 genes that distinguished myocarditis with 100 % sensitivity (95 % confidence interval 46–100) and 100 % specificity (95 % confidence interval 66–100). Hence, apart from the hitherto established huge armamentarium of sophisticated diagnostic EMB-techniques (comprising histology, immunohistochemistry and molecular pathology) that already allow detailed diagnoses, newer techniques such as the analysis of transcriptomic biomarkers promise a further increase in the sensitivity as well as specificity of EMBs.
Overall, non-invasive CMR has to overcome two major drawbacks as compared to the diagnostic capabilities of histopathological EMB workup: (1) The sensitivity of current CMR techniques regarding the diagnosis of myocarditis has to be improved. (2) CMR techniques need to be developed that enable more detailed information regarding the ‘composition’ and ‘origin’ of myocarditis. Addressing the first point, newly developed quantitative mapping techniques promise to increase both sensitivity and specificity of CMR-based diagnoses. For example, Giri et al. [56] have recently shown that quantitative T2 mapping addresses the well-known problems associated with T2-weighted imaging of the heart and offers the potential for increased accuracy in the detection of myocardial edema. Moreover, T1 mapping promises to overcome the limitation of needing large areas of necrosis to get a sufficient T1 contrast for LGE imaging [67]. Regarding the second point, predominating forms of myocarditis in humans are caused by viral pathogens and are characterized by macrophage-rich inflammation. Hence, macrophages infiltrating the myocardium or other ‘molecular targets’ overexpressed in myocardial inflammation constitute interesting targets for ‘molecular’ CMR imaging since such approaches have already yielded promising results in cardiac applications [68, 69].
References
Cooper LT Jr (2009) Myocarditis. N Engl J Med 360:1526–1538
Yilmaz A, Klingel K, Kandolf R et al (2009) Imaging in inflammatory heart disease: from the past to current clinical practice. Hellenic J Cardiol. 50:449–460
D’Ambrosio A, Patti G, Manzoli A et al (2001) The fate of acute myocarditis between spontaneous improvement and evolution to dilated cardiomyopathy: a review. Heart 85:499–504
Fabre A, Sheppard MN (2006) Sudden adult death syndrome and other non-ischaemic causes of sudden cardiac death. Heart 92:316–320
Aretz HT (1986) Diagnosis of myocarditis by endomyocardial biopsy. Med Clin North Am 70:1215–1226
Cooper LT, Baughman KL, Feldman AM et al (2007) The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. Eur Heart J 28:3076–3093
Cunningham KS, Veinot JP, Butany J (2006) An approach to endomyocardial biopsy interpretation. J Clin Pathol 59:121–129
Friedrich MG, Sechtem U, Schulz-Menger J et al (2009) Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol 53:1475–1487
Schultheiss HP, Kuhl U, Cooper LT (2011) The management of myocarditis. Eur Heart J 32(21):2616–25
Esfandiarei M, McManus BM (2008) Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol 3:127–155
Klingel K, Hohenadl C, Canu A et al (1992) Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proc Natl Acad Sci USA 89:314–318
Yajima T, Knowlton KU (2009) Viral myocarditis: from the perspective of the virus. Circulation 119:2615–2624
Klingel K, Rieger P, Mall G et al (1998) Visualization of enteroviral replication in myocardial tissue by ultrastructural in situ hybridization: identification of target cells and cytopathic effects. Lab Invest 78:1227–1237
Bergelson JM, Cunningham JA, Droguett G et al (1997) Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320–1323
Rose NR (2009) Myocarditis: infection versus autoimmunity. J Clin Immunol 29:730–737
Caforio AL, Mahon NJ, Tona F et al (2002) Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail 4:411–417
Caforio AL, Daliento L, Angelini A et al (2005) Autoimmune myocarditis and dilated cardiomyopathy: focus on cardiac autoantibodies. Lupus 14:652–655
Malkiel S, Kuan AP, Diamond B (1996) Autoimmunity in heart disease: mechanisms and genetic susceptibility. Mol Med Today. 2:336–342
Cunningham MW (2004) T cell mimicry in inflammatory heart disease. Mol Immunol 40:1121–1127
Li Y, Heuser JS, Cunningham LC et al (2006) Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J Immunol 177:8234–8240
Bachmaier K, Le J, Penninger JM (2000) “Catching heart disease”: antigenic mimicry and bacterial infections. Nat Med 6:841–842
Goser S, Andrassy M, Buss SJ et al (2006) Cardiac troponin I but not cardiac troponin T induces severe autoimmune inflammation in the myocardium. Circulation 114:1693–1702
Luppi P, Rudert WA, Zanone MM et al (1998) Idiopathic dilated cardiomyopathy: a superantigen-driven autoimmune disease. Circulation 98:777–785
Prasad A (2007) The (amorphous) anatomy of an invention: the case of magnetic resonance imaging (MRI). Soc Stud Sci 37:533–560
Cannan C, Friedrich MG (2010) Cardiac magnetic resonance imaging: current status and future directions. Expert Rev Cardiovasc Ther 8:1175–1189
Vohringer M, Mahrholdt H, Yilmaz A et al (2007) Significance of late gadolinium enhancement in cardiovascular magnetic resonance imaging (CMR). Herz 32:129–137
Pauschinger M, Noutsias M, Lassner D et al (2006) Inflammation, ECG changes and pericardial effusion: whom to biopsy in suspected myocarditis? Clin Res Cardiol 95:569–583
Liu PP, Mason JW (2001) Advances in the understanding of myocarditis. Circulation 104:1076–1082
Kim RJ, Chen EL, Lima JA et al (1996) Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation 94:3318–3326
Kim RJ, Fieno DS, Parrish TB et al (1999) Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 100:1992–2002
Judd RM, Lugo-Olivieri CH, Arai M et al (1995) Physiological basis of myocardial contrast enhancement in fast magnetic resonance images of 2-day-old reperfused canine infarcts. Circulation 92:1902–1910
Simonetti OP, Kim RJ, Fieno DS et al (2001) An improved MR imaging technique for the visualization of myocardial infarction. Radiology 218:215–223
Rieker O, Mohrs O, Oberholzer K et al (2002) Cardiac MRI in suspected myocarditis. Rofo 174:1530–1536
Korkusuz H, Esters P, Naguib N et al (2009) Acute myocarditis in a rat model: late gadolinium enhancement with histopathological correlation. Eur Radiol 19:2672–2678
Korkusuz H, Esters P, Huebner F et al (2010) Accuracy of cardiovascular magnetic resonance in myocarditis: comparison of MR and histological findings in an animal model. J Cardiovasc Magn Reson 12:49
Mahrholdt H, Wagner A, Judd RM et al (2005) Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J 26:1461–1474
Friedrich MG, Strohm O, Schulz-Menger J et al (1998) Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation 97:1802–1809
Mahrholdt H, Goedecke C, Wagner A et al (2004) Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation 109:1250–1258
Abdel-Aty H, Boye P, Zagrosek A et al (2005) Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol 45:1815–1822
Laissy JP, Hyafil F, Feldman LJ et al (2005) Differentiating acute myocardial infarction from myocarditis: diagnostic value of early- and delayed-perfusion cardiac MR imaging. Radiology 237:75–82
Rottgen R, Christiani R, Freyhardt P et al (2011) Magnetic resonance imaging findings in acute myocarditis and correlation with immunohistological parameters. Eur Radiol 21:1259–1266
Yilmaz A, Mahrholdt H, Athanasiadis A et al (2008) Coronary vasospasm as the underlying cause for chest pain in patients with PVB19 myocarditis. Heart 94:1456–1463
Mahrholdt H, Wagner A, Deluigi CC et al (2006) Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 114:1581–1590
Assomull RG, Lyne JC, Keenan N et al (2007) The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J 28:1242–1249
Wu KC, Weiss RG, Thiemann DR et al (2008) Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol 51:2414–2421
De CF, Pieroni M, Esposito A et al (2006) Delayed gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J Am Coll Cardiol 47:1649–1654
Gutberlet M, Spors B, Thoma T et al (2008) Suspected chronic myocarditis at cardiac MR: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology 246:401–409
Mavrogeni S, Spargias C, Bratis C et al (2011) Myocarditis as a precipitating factor for heart failure: evaluation and 1-year follow-up using cardiovascular magnetic resonance and endomyocardial biopsy. Eur J Heart Fail 13:830–837
Jeserich M, Konstantinides S, Pavlik G et al (2009) Non-invasive imaging in the diagnosis of acute viral myocarditis. Clin Res Cardiol 98:753–763
Friedrich MG (2010) Myocardial edema—a new clinical entity? Nat Rev Cardiol 7:292–296
Gagliardi MG, Bevilacqua M, Di RP et al (1991) Usefulness of magnetic resonance imaging for diagnosis of acute myocarditis in infants and children, and comparison with endomyocardial biopsy. Am J Cardiol 68:1089–1091
Simonetti OP, Finn JP, White RD et al (1996) “Black blood” T2-weighted inversion-recovery MR imaging of the heart. Radiology 199:49–57
Laissy JP, Messin B, Varenne O et al (2002) MRI of acute myocarditis: a comprehensive approach based on various imaging sequences. Chest 122:1638–1648
Kellman P, Aletras AH, Mancini C et al (2007) T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med 57:891–897
Aletras AH, Kellman P, Derbyshire JA et al (2008) ACUT2E TSE-SSFP: a hybrid method for T2-weighted imaging of edema in the heart. Magn Reson Med 59:229–235
Giri S, Chung YC, Merchant A et al (2009) T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson 11:56
Baccouche H, Mahrholdt H, Meinhardt G et al (2009) Diagnostic synergy of non-invasive cardiovascular magnetic resonance and invasive endomyocardial biopsy in troponin-positive patients without coronary artery disease. Eur Heart J 30(23):2869–79
Frustaci A, Chimenti C, Calabrese F et al (2003) Immunosuppressive therapy for active lymphocytic myocarditis: virological and immunologic profile of responders versus nonresponders. Circulation 107:857–863
Wojnicz R, Nowalany-Kozielska E, Wojciechowska C et al (2001) Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: 2-year follow-up results. Circulation 104:39–45
Why HJ, Meany BT, Richardson PJ et al (1994) Clinical and prognostic significance of detection of enteroviral RNA in the myocardium of patients with myocarditis or dilated cardiomyopathy. Circulation 89:2582–2589
Frustaci A, Russo MA, Chimenti C (2009) Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J 30(16):1995–2002
Hauck AJ, Kearney DL, Edwards WD (1989) Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc 64:1235–1245
Holzmann M, Nicko A, Kuhl U et al (2008) Complication rate of right ventricular endomyocardial biopsy via the femoral approach: a retrospective and prospective study analyzing 3048 diagnostic procedures over an 11-year period. Circulation 118:1722–1728
Yilmaz A, Kindermann I, Kindermann M et al (2010) Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation 122:900–909
Kindermann I, Kindermann M, Kandolf R et al (2008) Predictors of outcome in patients with suspected myocarditis. Circulation 118:639–648
Heidecker B, Kittleson MM, Kasper EK et al (2011) Transcriptomic biomarkers for the accurate diagnosis of myocarditis. Circulation 123:1174–1184
Flett AS, Hayward MP, Ashworth MT et al (2010) Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation 122:138–144
Nahrendorf M, Jaffer FA, Kelly KA et al (2006) Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation 114:1504–1511
Sosnovik DE, Nahrendorf M, Deliolanis N et al (2007) Fluorescence tomography and magnetic resonance imaging of myocardial macrophage infiltration in infarcted myocardium in vivo. Circulation 115:1384–1391
Acknowledgments
A.Y. is financially supported by a grant from the Robert-Bosch-Foundation (grant-ID I1).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yilmaz, A., Ferreira, V., Klingel, K. et al. Role of cardiovascular magnetic resonance imaging (CMR) in the diagnosis of acute and chronic myocarditis. Heart Fail Rev 18, 747–760 (2013). https://doi.org/10.1007/s10741-012-9356-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-012-9356-5