Abstract
Echocardiography is one of the most important clinical tools in the diagnosis and management of various pericardial diseases, including constrictive pericarditis, effusive constrictive pericarditis, pericardial effusion, tamponade, absence of the pericardium and cysts or tumors. During recent years, remarkable progress has been made in echocardiography: cardiac tissue Doppler analysis (TDI), strain and strain rate imaging by speckle tracking imaging (STE) and three-dimensional (3D) echocardiography. The assessment of early diastolic annulus velocity and annulus reversus by TDI improves the differentiation of constriction from restrictive myocardial disease, which can be further facilitated by STE as a complementary tool. 3D echocardiography may be useful for the more precise assessment of pericardial diseases, such as pericardial effusion or pericardial masses as it provides incremental value to 2D echocardiography by detecting anatomic structures with higher accuracy. Applications of these newer echocardiographic techniques in the assessment of pericardial diseases are discussed in this chapter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
TDI and STE characterize the mechanics of myocardial contraction and relaxation (deformation imaging) and find applications in many cardiac pathologies. Tissue Doppler velocity estimation is based on the same principles as pulsed-wave and color Doppler echocardiography for blood flow. To distinguish between signals originating from moving tissue and blood flow, a high-pass filter is used to image blood velocities and a low-pass filter is used to display tissue velocities. TDI can be applied to the assessment of both regional and global left ventricular (LV) function. Measuring velocities of myocardial segments gives information about regional ventricular contractility, while the measurement of mitral annular velocities provides information on overall longitudinal LV systolic and diastolic function.
STE is an alternative and innovative way to obtain myocardial tissue motion information [1]. Two-dimensional (2D) strain imaging techniques measure Langrangian strain and strain rate (SR) by tracking echocardiographic B-mode speckles (natural acoustic markers or acoustic backscatter generated by ultrasound interactions (reflection, scattering and interference) within the myocardium (generally made up of 20–40 pixels)). The speckle pattern created by the interference remains reasonably stable serving as a digital fingerprint for the given myocardial area. The geometric shift of each speckle represents myocardial motion and can be tracked from frame to frame, thus allowing for the calculation of strain and SR.
The development of real-time 3D echocardiography with matrix transducer technology and analyzing software made a more reliable analysis of LV function feasible [2]. 3D echocardiography improves the accuracy of determining LV volumes and mass compared with 2D echo because geometric assumptions are eliminated [3]. As a result, these measurements correlate well with those of direct MRI measurements. The major proven advantage of 3D echocardiography is the improvement in the accuracy of the echocardiographic evaluation of cardiac anatomy and the more realistic and comprehensive view of intracardiac structures [4]. With 3D, the full-volume data set cropped in any desired plane, the entire views of inter-atrial and inter-ventricular septum and their relations with neighboring structures can be displayed.
Tissue Doppler imaging (TDI) in pericardial diseases
CP often masquerades as other cardiovascular or non-cardiovascular disease and often poses diagnostic and therapeutic dilemmas for physicians. The correct diagnosis and appropriate therapy are frequently delayed. Lately, application of TDI facilitated the diagnosis of CP by echocardiography along with 2-D and Doppler echocardiography. Since the mechano-elastic properties of the myocardium are preserved in CP, the longitudinal mitral annular velocities remain normal or can be exaggerated as lateral expansion in CP is limited. Garcia et al. [5] were the first to report that measurement of longitudinal axis expansion by TDI using the lateral mitral annulus provided a clinically useful distinction between CP and restrictive cardiomyopathy (RCM) (Fig. 1). Rajagopalan et al. [6] showed that a peak early diastolic lateral annulus velocity (e′ or E′) velocity ≥8 cm/s could discriminate between the entities CP and RCM with high sensitivity (89 %) and specificity (100 %). Studies by Ha et al. and by Sohn et al. [7, 8] recommended that e′ velocity can provide a helpful diagnostic indicator and should be measured routinely in the evaluation of heart failure or suspected CP. Ha et al. [7] recommended the same 8 cm/s cut-off value for CP diagnosis where e′ velocity is equal or greater than 8 cm/s, with 95 % sensitivity and 96 % specificity. Ha et al. [9] also evaluated the role of TDI in the diagnosis of CP in patients without diagnostic respiratory variation of transmitral early diastolic filling velocity. It was confirmed that e′ velocity was well-preserved independent of any respiratory variation in mitral inflow velocities. Other studies suggested that e′ should be used with caution if CP is combined with myocardial diseases, extensive annular calcification or segmental non-uniform myocardial velocities [10–12]. It has been shown by Choi et al. [13] that the addition of extra parameters to the e′ velocities such as measurement of s′ velocities and the time difference between onset of mitral inflow and onset of e′ increases sensitivity and provides additional information to e′ for the differentiation of CP from RCM.
Several investigators have shown that E/e′ ratio correlates well with LV filling pressure [14, 15]. E/e′ >15 identifies increased LV filling pressure while E/e′ <8 describes normal filling pressure. Ha and his associates introduced the concept of “annulus paradoxus,” which describes the paradoxical behavior of the mitral annulus in CP [16] (Fig. 2). He found that an inverse relationship exists between E/e′ and LV filling pressure, which can be explained by the fact that in CP the medial mitral annulus has an exaggerated longitudinal motion leading to an increase in e′, despite high filling pressures.
Early diastolic mitral inflow (E) and annular velocities in 2 separate patients: on the right: 54-year-old man with CP. PCWP was 18 mm Hg and E/E′ was 17. A: late filling velocity; A′, late diastolic annular velocity on the left: 56-year-old man with constrictive pericarditis (CP). Note that E is ≈ 100 cm/s and early diastolic annulus velocity (E′) is 20 cm/s. Pulmonary capillary wedge pressure (PCWP) was 31 mm Hg, and E/E′ was 5. From Ha [16]
In normal subjects, the mitral lateral annulus e′ velocity is higher than the medial annulus e′ velocity [17]. Reuss et al. [18] identified the reversal of the normal relationship of mitral lateral e′ and medial e′ velocities in CP, where mitral lateral e′ velocity is lower than medial e′ velocity; therefore, lateral/medial e′ ratio is inverted, which was termed “annulus reversus” (Fig. 3). This finding is caused by the tethering of the adjacent fibrotic and scarred pericardium, which influences the lateral mitral annulus in patients with CP. In a patient with preserved mitral e′ velocities (>8 cm/s) and a low E/e′ ratio (<8) with high LV filling pressure, the recognition of annulus reversus should alert to the diagnosis of constrictive pericarditis.
Doppler representation of annulus reversus: The normal relationship (top panel) between medial mitral early diastolic annulus velocity (e′) and lateral mitral e′ is reversed in constrictive pericarditis (middle panel) but not in restrictive cardiomyopathy (bottom panel). s′: systolic annulus velocity, a′: late diastolic annulus velocity From Reuss [18]
In a recent study by Choi, it was shown that besides the lateral/medial e′ ratio, the tricuspid lateral e′/septal e′ ratio is a useful diagnostic parameter for CP, differentiating it from RCM. Moreover, reduced lateral e′ was inversely correlated with the pericardial thickness on their respective side.
In general, TDI offers incremental diagnostic information to M-mode, 2D echo and transmitral flow Doppler for detecting constrictive physiology, with a reported sensitivity and specificity of 88.8 and 94.8 %, respectively [19].
Kim et al. [20] examined the medial annular velocities in patients with CP after pericardiectomy in 16 patients and found that e′ decreased significantly after pericardiectomy.
Our group confirmed this finding and showed that all mitral and tricuspid annular velocities decrease after pericardiectomy with normalization of the mitral lateral/medial e′ velocity ratio [21]. In some patients, low annular velocities unmasked by pericardiectomy may reflect underlying myocardial damage or atrophy secondary to long-standing encasement and penetration of the myocardium by calcium spurs, persistent inflammation and, since the pericardium might be firmly adherent to the myocardium, additional injury at the time of surgery. We have also found that all mitral and tricuspid annular velocities (e′, a′ and s′) are higher in primary CP than those in secondary CP group, an observation that can be explained by concomitant myocardial disease due to radiation or ischemic heart disease [21].
More recently, Lu and colleagues studied the motion of pericardium, the out-layer of myocardium and the inner layer of myocardium using TDI. In normal subjects, the motions of the outer layer myocardium and the inner layer myocardium were identical. However, in the CP patients, the motion of outer layer myocardium was significantly reduced approaching that of pericardium while the motion of inner layer myocardium was better preserved than that of outer layer myocardium. These findings may further help to establish the diagnosis of CP [22].
Speckle tracking echocardiography (STE) in pericardial diseases
By utilizing STE, in a study of 26 CP and 19 RCM patients, Sengupta confirmed the two distinct patterns of diastolic restoration mechanism in the two different patient pool [23]. CP patients had markedly abnormal circumferential deformation, torsion and untwisting velocity along with relative sparing of the longitudinal mechanics. By contrast, patients with restriction had abnormal longitudinal mechanics (reduced longitudinal strain, particularly at the LV base) with relative sparing of LV rotation, thus specifically differentiating the abnormal diastolic restoration mechanics of the left ventricle seen in CP and RCM (Fig. 4).
Left ventricular (LV) longitudinal velocity and untwisting velocity in constrictive pericarditis (CP) and restrictive cardiomyopathy (RCM): Color M-mode display of apical untwisting velocity (rotational rate of the LV apex [RotR]) obtained from speckle tracking of the LV apex in short-axis view shows markedly attenuated early diastolic rate of untwisting in CP (a, arrows), whereas longitudinal early diastolic velocities (VL) from the LV base in apical 4-chamber view (b, arrows) are normal. In contrast, patients with RCM show a normal early diastolic rate of untwisting (c, arrows) and reduced longitudinal early diastolic velocities from the LV base (d, arrows) From Sengupta [23]
Longitudinal strain assessment by speckle tracking echocardiography in constrictive pericarditis: The longitudinal lateral strain values are lower than the medial strain values due to the lateral constraint of the pericardium. ML mid-lateral segment of the left ventricle (LV), BL basal lateral segment of the LV, MS mid-septal segment of the LV, BS basal septal segment of the LV
Furthermore, a comparison of the LV mechanics with pericardial thickness, as measured by CT, in patients with CP has shown a significant correlation between decreased circumferential strain and degree of pericardial thickening at the apex [24].
Our group has investigated the usefulness of the ratio of medial/lateral strain measurement in the differential diagnosis of CP from RCM by assessing STE longitudinal strain [25]. We found that the longitudinal strain of the lateral wall was lower than that of the septal wall in CP group (Fig. 5), while the reverse was true in control and cardiac amyloidosis. Therefore, the medial/lateral ratio of longitudinal strain in CP group was higher than that in normal group or amyloid group. The differential longitudinal strain assessment by STE can be valuable in differentiating CP from restrictive diseases; however, further studies with larger population are required to confirm these findings.
Perimyocarditis is common in clinical practice. Up to 15 % of patients with acute pericarditis have significant myocardial involvement as assessed by markers of myocardial lesion [26]. Echocardiography is essential for the diagnosis of LV dysfunction and follow-up [26]. By assessing three-layers strain and twist angle with speckle tracking echocardiography, longitudinal and circumferential strain in three myocardial layers were found to be decreased in patients with acute perimyocarditis (n = 38) compared with normals (n = 20), except for the apical epicardial strain [27]. The LV twist angle was decreased in perimyocarditis versus normal, mostly due to lower apical rotation. In perimyocarditis with normal LV systolic function, the longitudinal strain was decreased in basal and mid-ventricular segments in 3 myocardial layers. Circumferential strain was not significantly different. Perimyocarditis process starts from the epicardial layer, which contains fibers with oblique orientation that determine longitudinal shortening. This may explain reduced longitudinal strain and preserved circumferential strain in patients with perimyocarditis and apparently normal systolic LV function.
Congenital absence of the pericardium is a rare malformation that hardly manifests clinically and is usually detected by echocardiography or other cardiac imaging modalities such CT or cardiac MRI as an incidental finding or discovered during cardiac surgery or at autopsy [28, 29]. As reported in a case study, strain and torsion measured by speckle tracking imaging with the use of vector velocity imaging technique were abnormal, and global longitudinal strain and torsion were reduced in a patient with congenital absence of the pericardium [30]. Although it is suggestive by echocardiography findings, confirmative diagnosis can be achieved by either cardiac CT or cardiac MRI, which provide direct visualization of pericardium and the surrounding tissue and pericardial tissue characterization.
3D echocardiography in pericardial diseases
3D transoesophageal echocardiography (TEE) can be useful in demonstrating the extent of pericardial thickening in CP. 3D real-time echocardiography provides additional information throughout the diagnosis relative to the extent of constriction over 2D echocardiography, as it can visualize the parietal and visceral pericardium en face in a manner not possible with 2D transthoracic echocardiography (TTE) [31]. This allows the examination of each layer separately for the presence of pathology and/or fibrin deposits and determination of the exact location and the extent of such pathological findings. Moreover, it allows us to follow the pericardium not only over the LV, but also the right ventricle (RV) and both atria. In CP, 3DTTE is typically able to determine the full extent of the constrictive process by localizing the calcifications and thus determining how much each ventricular wall is involved. However, the pericardial anatomy and calcification are better assessed by computed tomography if they are needed for clinical decision in patients with CP.
Echocardiography has become the standard imaging modality for assessing pericardial effusion. By 3D echo, the full extent of pericardial effusion can be visualized and its location is determined with a greater detail and accuracy when compared to 2D TTE (Fig. 6). 3D provides additional information regarding the size, thickness and the extent of the strands within the pericardial effusion and the relationship to cardiac structures [32]. 3D revealed the strands to be an extensive network of intrapericardial adhesions extending from the visceral pericardium to the thickened parietal pericardium. 3D also allows for the full assessment of the tethering of ventricular walls by adhesions in patients with pericarditis besides the detection of pericardial effusion. Superior imaging with 3D TEE over 2D TEE had been also reported [33].
3D echocardiogram showing a pericardial effusion (EFF); “Virtual” electronic dissection reveals the layered or stratified nature of the visceral pericardium (arrows). LV left ventricle, PP parietal pericardium From Zagol [35]
In addition to echocardiography techniques, cardiac MRI and cardiac CT play an important role in loculated pericardial effusion or intrapericardial clot, since there is no window restriction, which is a big limitation for TTE, especially for postoperative patient or for COPD or obesity patient. Furthermore, cardiac MRI with and without Gadolinium contrast delay enhancement can evaluate tissue characterization for pericardium, myocardium, pericardial fluid and surrounding tissue and organ, which are very helpful for searching the etiology of pericardial effusion [34].
3D TTE may be superior to 2D TTE in uncovering mass lesions involving the pericardium, such as tuberculous granulomas, metastatic disease, hematomas (Fig. 7) and pericardial cysts. Evaluation of pericardial hematomas, their location, size and extent can be assessed more comprehensively when compared to 2D TTE, and the characteristics of the interior of the hematoma can be examined to determine whether it is entirely fluid or contains clot components.
Live/real-time three-dimensional transthoracic echocardiography in a 17-year-old male with a bullet injury and subsequent development of pericardial hematoma; The red dots outline a loculated component of a very large pericardial hematoma. RV right ventricle, LV left ventricle from Hernandez [31]
Effusive CP is a clinical syndrome characterized by both pericardial effusion and pericardial constriction, where constrictive hemodynamics persists after PE is removed.
The extent and distribution of pericardial thickening, both parietal and visceral can be well-demonstrated by 3D echocardiography. Electronic “dissection” of the 3D echo image can demonstrate that the visceral pericardium is stratified into two or more layers, though it appears to be homogenous on the 2D echo [35] (Fig. 6). However, all these case reports need to be confirmed in studies with larger sample size and compare the 3D echo result with cardiac MRI finding.
Ventricular septal bounce in early diastole, a plethoric inferior vena cava and exaggerated respiratory fluctuation in velocities across all valves can be also noted by 3D echo.
Overall, 3D echocardiography has an incremental value in improving anatomic definition of cardiac structures allowing a more accurate diagnosis.
Transient constriction
CP is traditionally presumed to be irreversible. However, a Spanish group of investigators and our center have described reversible CP, in which constrictive physiology and hemodynamics resolve without pericardiectomy [36–40]. Although the reasons for this resolution are not well established, it has been postulated that inflammation and edema lead to pericardial thickening, poor compliance and constriction. If pericardial inflammation gets treated with an anti-inflammatory agent before it becomes scarred, constrictive hemodynamics may resolve without recurrence. Constriction resolves approximately at an average time of 3 months. (Fig. 8). Recently, we systemically studied the clinical, imaging feature and inflammatory markers of reversible constriction. Compared with the persistent group, the reversible patients had more idiopathic disease and more collagen vascular disease as the etiology while fewer had prior radiation or cardiac surgery. However, there were no significant differences regarding other clinical features, echocardiographic features, Doppler and tissue Doppler features between the reversible and irreversible groups. These patients usually have a small to moderate amount of pericardial effusion, and as the pericardial effusion resolves, the pericardium remains inflamed, thickened and non-compliant, resulting in constrictive hemodynamics. These patients present with dyspnea, peripheral edema, increased jugular venous pressure and occasionally ascites, as in patients with constrictive pericarditis. Relatively new onset symptoms should make clinicians consider the possibility of the transient form of CP. The TTE findings of transient CP are similar to those of chronic CP: abnormal ventricular septal motion, respiratory variation in mitral E velocity and normal or increased mitral annular early diastolic e′ velocity measured by TDI.
Transient constrictive pericarditis: a Echocardiographic findings in constrictive pericarditis. Parasternal long-axis view of a 39-year-old man who presented 1 month after a motor vehicle accident with dyspnea, showing markedly increased pericardial thickness (arrows). b Follow-up echocardiogram performed 1 month later, showing near-normal pericardial thickness (arrows). Ao aorta, LA left trium, LV left ventricle, RV right ventricle From Haley [39]
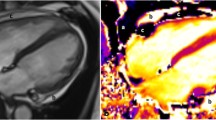
By using late Gadolinium enhancement (LGE) cardiac MRI, we found that the maximal pericardial thickness measured on LGE was significantly greater in the reversible than in the persistent CP group. Presence or absence of LGE in pericardium was documented and was rated qualitatively as none (no apparently visible LGE), mild (faint LGE in pericardium that has signal intensity less than the signal of the ventricular blood pool), moderate (obvious enhancement that is visually similar to the ventricular blood pool) or severe (obvious significant LGE in pericardium that has signal intensity visually greater than ventricular blood pool). A LGE pericardial thickness of 3 mm or moderate–severe pericardial LGE on qualification had a reasonable sensitivity and specificity to predict reversible CP. Furthermore, reversible CP patients had more baseline systemic inflammation as evidenced by higher baseline C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) levels than the persistent patients. There is also a positive correlation between white blood cell count or ESR and the pericardial LGE thickness (R = 0.55, P = 0.01 and R = 0.43, P = 0.08, respectively). In the reversible group, anti-inflammatory therapy was associated with a significant reduction in CRP, ESR and white blood cell count, and pericardial thickness determined by cardiac MRI LGE, steady-state free precession and fast-spin echo imaging with clinical and echocardiographic evidence of resolution of constrictive hemodynamics. We suggest that patients with CP and evidence of pericardial LGE thickness detected by cardiac MRI and systemic inflammation as evidenced by elevated CRP and ESR should be treated with anti-inflammatory medical therapy or even with steroids in more severe cases before consideration of pericardiectomy [36].
Conclusion
TDI, with the assessment of early diastolic annulus velocity and annulus reversus, improves the differentiation of constriction from restrictive myocardial disease. STE as a complementary tool can further facilitate such differential diagnosis. 3D echocardiography adds incremental value to 2D echocardiography by detecting anatomic structures with higher accuracy and may be useful for the more precise assessment of pericardial diseases, such as pericardial effusion or pericardial masses. 3D can identify the best site for pericardiocentesis and provides more precise information regarding pericardial effusion. In challenging or non-diagnostic cases, cardiac MRI and cardiac CT studies will be helpful. A subset of patients with CP, namely transient CP patients, can be managed with an effective medical therapy with a good chance of resolution before pericardiectomy would be considered.
References
Oh JK, Seward JB, Tajik AJ (2006) Tissue Doppler imaging, strain imaging, and dyssynchrony assessment. In: The echo manual, 3rd edn. Lippincott Williams and Wilkins (a Wolters Kluwer business), pp 80–86
Soliman OI, Krenning BJ, Geleijnse ML, Nemes A, van Geuns RJ, Baks T, Anwar AM, Galema TW, Vletter WB, ten Cate FJ (2007) A comparison between QLAB and TomTec full volume reconstruction for real time three-dimensional echocardiographic quantification of left ventricular volumes. Echocardiography 24:967–974
Qi X, Cogar B, Hsiung MC, Nanda NC, Miller AP, Yelamanchili P, Baysan O, Wu YS, Lan GY, Ko JS, Cheng CH, Lin CC, Huang CM, Yin WH, Young MS (2007) Live/real time three-dimensional transthoracic echocardiographic assessment of left ventricular volumes, ejection fraction, and mass compared with magnetic resonance imaging. Echocardiography 24:166–173
Stefanidis AS, Margos PN, Kotsakis AA, Papasteriadis EG (2009) Three-dimensional echocardiographic documentation of pacemaker lead perforation presenting as acute pericarditis. Hellenic J Cardiol 50:335–337
Garcia MJ, Rodriguez L, Ares M, Griffin BP, Thomas JD, Klein AL (1996) Differentiation of constrictive pericarditis from restrictive cardiomyopathy: assessment of left ventricular diastolic velocities in longitudinal axis by Doppler tissue imaging. J Am Coll Cardiol 27:108–114
Rajagopalan N, Garcia MJ, Rodriguez L, Murray RD, Apperson-Hansen C, Stugaard M, Thomas JD, Klein AL (2001) Comparison of new Doppler echocardiographic methods to differentiate constrictive pericardial heart disease and restrictive cardiomyopathy. Am J Cardiol 87:86–94
Ha JW, Ommen SR, Tajik AJ, Barnes ME, Ammash NM, Gertz MA, Seward JB, Oh JK (2004) Differentiation of constrictive pericarditis from restrictive cardiomyopathy using mitral annular velocity by tissue Doppler echocardiography. Am J Cardiol 94:316–319
Sohn DW, Kim YJ, Kim HS, Kim KB, Park YB, Choi YS (2004) Unique features of early diastolic mitral annulus velocity in constrictive pericarditis. J Am Soc Echocardiogr 17:222–226
Ha JW, Oh JK, Ommen SR, Ling LH, Tajik AJ (2002) Diagnostic value of mitral annular velocity for constrictive pericarditis in the absence of respiratory variation in mitral inflow velocity. J Am Soc Echocardiogr 15:1468–1471
Sengupta PP, Mohan JC, Mehta V, Arora R, Pandian NG, Khandheria BK (2005) Accuracy and pitfalls of early diastolic motion of the mitral annulus for diagnosing constrictive pericarditis by tissue Doppler imaging. Am J Cardiol 93:886–890
Arnold MF, Voigt JU, Kukulski T, Wranne B, Sutherland GR, Hatle L (2001) Does atrioventricular ring motion always distinguish constriction from restriction? A Doppler myocardial imaging study. J Am Soc Echocardiogr 14:391–395
Butz T, Langer C, Scholtz W, Jategaonkar S, Bogunovic N, Horstkotte D, Faber L (2008) Severe calcification of the lateral mitral annulus in constrictive pericarditis: a potential pitfall for the use of echocardiographic tissue Doppler imaging. Eur J Echocardiogr 9:403–405
Choi EY, Ha JW, Kim JM, Ahn JA, Seo HS, Lee JH, Rim SJ, Chung N (2007) Incremental value of combining systolic mitral annular velocity and time difference between mitral inflow and diastolic mitral annular velocity to early diastolic annular velocity for differentiating constrictive pericarditis from restrictive cardiomyopathy. J Am Soc Echocardiogr 20:738–743
Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA (1997) Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 30:1527–1533
Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ (2000) Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 102:1788–1794
Ha JW, Oh JK, Ling LH, Nishimura RA, Seward JB, Tajik AJ (2001) Annulus paradoxus: transmitral flow velocity to mitral annular velocity ratio is inversely proportional to pulmonary capillary wedge pressure in patients with constrictive pericarditis. Circulation 104:976–978
Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R (2010) Normative reference values for the tissue Doppler imaging parameters of left ventricular function: a population-based study. Eur J Echocardiogr 11:51–56
Reuss CS, Wilansky SM, Lester SJ, Lusk JL, Grill DE, Oh JK, Tajik AJ (2009) Using mitral ‘annulus reversus’ to diagnose constrictive pericarditis. Eur J Echocardiogr 10:372–375
Choi JH, Choi JO, Ryu DR, Lee SC, Park SW, Choe YH, Oh JK (2011) Mitral and tricuspid annular velocities in constrictive pericarditis and restrictive cardiomyopathy: correlation with pericardial thickness on computed tomography. JACC Cardiovasc Imaging 4:567–575
Kim JS, Ha JW, Im E, Park S, Choi EY, Cho YH, Kim JM, Rim SJ, Yoon YN, Chang BC (2009) Effects of pericardiectomy on early diastolic mitral annular velocity in patients with constrictive pericarditis. Int J Cardiol 133:18–22
Veress G, Ling LH, Kim KH, Dal-Bianco JP, Schaff HV, Espinosa RE, Melduni RM, Tajik JA, Sundt TM 3rd, Oh JK (2011) Mitral and tricuspid annular velocities before and after pericardiectomy in patients with constrictive pericarditis. Circ Cardiovasc Imaging 4:399–407
Lu XF, Wang XF, Cheng TO, Xie MX, Lu Q (2009) Diagnosis of constrictive pericarditis by quantitative tissue Doppler imaging. Int J Cardiol 137:22–28
Sengupta PP, Krishnamoorthy VK, Abhayaratna WP, Korinek J, Belohlavek M, Sundt TM 3rd, Chandrasekaran K, Mookadam F, Seward JB, Tajik AJ, Khandheria BK (2008) Disparate patterns of left ventricular mechanics differentiate constrictive pericarditis from restrictive cardiomyopathy. JACC Cardiovasc Imaging 1:29–38
Sengupta P, Eleid MF, Sundt TM, Chandrasekaran K, Tajik AJ, Khandheria BK (2008) Regional variability of pericardial thickness influences left ventricular diastolic recoil mechanics in constrictive pericarditis. J Am Soc Echocardiogr 21:518
Veress G, Kim KH, Masaki M, Espinosa RE, Oh JK (2010) Differential diagnosis of constrictive pericarditis from restrictive myocardial disease by speckle tracking echocardiography. J Am Coll Cardiol 55:10A
Imazio M (2011) Pericarditis: pathophysiology, diagnosis, and management. Curr Infect Dis Rep 13:308–316
Leitman M, Bachner-Hinenzon N, Adam D, Fuchs T, Theodorovich N, Peleg E, Krakover R, Moravsky G, Uriel N, Vered Z (2011) Speckle tracking imaging in acute inflammatory pericardial diseases. Echocardiography 28:548–555
Karia DH, Xing YQ, Kuvin JT, Nesser HJ, Pandian NG (2002) Recent role of imaging in the diagnosis of pericardial disease. Curr Cardiol Rep 4:33–40
Connolly HM, Click RL, Schattenberg TT, Seward JB, Tajik AJ (1995) Congenital absence of the pericardium: echocardiography as a diagnostic tool. J Am Soc Echocardiogr 8:87–92
Topilsky Y, Tabatabaei N, Freeman WK, Saleh HK, Villarraga HR, Mulvagh SL (2010) Images in cardiovascular medicine. Pendulum heart in congenital absence of the pericardium. Circulation 121:1272–1274
Hernandez CM, Singh P, Hage FG, Nanda NC, Hsiung MC, Wei J, Chang CY, Lee KC, Sue SH, Yin WH, Aly NA, Deshmukh O, Biswas M, Gupta I, Sanam K, Sen U (2009) Live/real time three-dimensional transthoracic echocardiographic assessment of pericardial disease. Echocardiography 26:1250–1263
D’Cruz IA, Minderman D, Dockery BK (2009) Three-dimensional imaging of intrapericardial adhesions within a large pericardial effusion. Can J Cardiol 25:366
Scohy TV, Maat AP, McGhie J, ten Cate FJ, Bogers AJ (2009) Three-dimensional transesophageal echocardiography: diagnosing the extent of pericarditis constrictiva and intraoperative surgical support. J Card Surg 24:305–308
Misselt AJ, Harris SR, Glockner J, Feng D, Syed IS, Araoz PA (2008) MR imaging of the pericardium. Magn Reson Imaging Clin N Am 16:185–199
Zagol B, Minderman D, Munir A, D’Cruz I (2007) Effusive constrictive pericarditis: 2D, 3D echocardiography and MRI imaging. Echocardiography 24:1110–1114
Feng D, Glockner J, Kim K, Martinez M, Syed IS, Araoz P, Breen J, Espinosa RE, Sundt T, Schaff HV, Oh JK (2011) Cardiac magnetic resonance imaging pericardial late gadolinium enhancement and elevated inflammatory markers can predict the reversibility of constrictive pericarditis after antiinflammatory medical therapy: a pilot study. Circulation 124:1830–1837
Sagrista-Sauleda J, Permanyer-Miralda G, Candell-Riera J, Angel J, Soler–Soler J (1987) Transient cardiac constriction: an unrecognized pattern of evolution in effusive acute idiopathic pericarditis. Am J Cardiol 59:961–966
Oh JK, Hatle LK, Mulvagh SL, Tajik AJ (1993) Transient constrictive pericarditis: diagnosis by two-dimensional Doppler echocardiography. Mayo Clin Proc 68:1158–1164
Haley JH, Tajik AJ, Danielson GK, Schaff HV, Mulvagh SL, Oh JK (2004) Transient constrictive pericarditis: causes and natural history. J Am Coll Cardiol 43:271–275
Akyuz S, Yaylak B, Ergelen M, Uyarel H (2010) Transient constrictive pericarditis: an elusive diagnosis. Future Cardiol 6:785–790
Conflict of interest
Drs. Veress, Feng, and Oh have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Veress, G., Feng, D. & Oh, J.K. Echocardiography in pericardial diseases: new developments. Heart Fail Rev 18, 267–275 (2013). https://doi.org/10.1007/s10741-012-9325-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-012-9325-z