Abstract
In this study we investigated the expression of HIF-1ɑ in dental pulps of the teeth with severe periodontitis. The expression of HIF-1ɑ in dental pulps of the teeth with severe periodontitis was detected by immunohistochemistry, immunofluorescence and real-time PCR. Bone marrow macrophages (BMMs) were cultured under hypoxia in vitro. HIF-1ɑ, osteoclast-specific factors (NFATc1, CTSK and c-fos) and RANKL-induced osteoclastogenesis were evaluated by immunofluorescence, TRAP staining and western blotting. High levels of HIF-1ɑ protein were detected in dental pulps of teeth with severe periodontitis, whereas few positive HIF-1ɑ expressions were detected in healthy dental pulps. Hypoxia occurred in the dental pulps in response to heavy periodontitis. Many HIF-1ɑ-positive infiltratory inflammatory cells were observed around blood vessels. Tooth internal resorption was found in some teeth with severe periodontitis. The HIF-1ɑ levels were upregulated in BMMs under hypoxia, which also promoted osteoclast formation and resorption by NFATc1, CTSK and c-fos. Teeth with severe periodontitis show hypoxic dental pulps and increased potential of osteoclastic differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypoxia (low oxygenation) may influence normal and different pathological processes, plays a significant in the regulation of various inflammatory processes. Hypoxia-inducible factor 1α(HIF-1α) is a key player in regulating cellular responses to hypoxia (Cramer et al. 2003; Semenza 2014). Under normal oxygen conditions, HIF-1α is hardly undetectable. When cells are in hypoxic conditions, the level of the HIF-1α protein obviously increases (Blouin et al. 2004).
Periodontal tissues and dental pulps have embryonic, anatomic, and functional inter-relationships that can lead to worsening of disease, resulting in combined periodontal-endodontic lesions. The diseased teeth coexist with periodontal lesions and pulpal diseases, the infection from pulpal-periapical diseases, or from periodontal, or both (Xia and Qi 2013). Pulpal and periodontal tissue lesions are closely related for their similarities in microbiology and cellular infiltrates (Sunitha et al. 2008). The severity of periodontitis is positively correlated with the change of pulp histology (Wan et al. 2015). Accumulating scientific evidence shows that hypoxia present in the microenvironment of periodontitis (Gölz et al. 2015; Yu et al. 2015). Hypoxia and HIF-1α get involved in the inflammatory responses to periodontitis (Takedachi et al. 2017). Up to now, there are still many controversies on the pathogenesis of combined periodontal-endodontic lesions. As part of a simplified procedure, we chose periodontal-resource lesions as our research goal by observing the effect of severe periodontitis on pulp tissues, which is helpful to elucidate the complex pathogenesis of combined periodontal-endodontic lesions. Macrophage is one of main infiltratory inflammatory cells in both dental pulpitis and periodontitis. Macrophages possess broad proinflammatory and destructive potential, considerably contribute to inflammation and differentiate into osteoclasts (Chen and Yan 2001; Liu et al. 2016). Whether hypoxia might occur in dental pulps of teeth with severe periodontitis is still unknown. In this study, histopathological observation and HIF-1ɑ expression were applied to evaluate hypoxic conditions in the dental pulps of teeth with severe periodontitis. We investigated the response of bone marrow macrophages to hypoxic culture and tested whether HIF-1ɑ regulates osteoclastogenesis in vitro.
Methods and materials
Samples
Eighteen Intact teeth extracted for chronic severe periodontitis were chosen. Severe periodontitis was diagnosed according to the criteria defined by the American Academy of Periodontology. Teeth with caries, restoration, microcracks, orthodontic movement and occlusal trauma were excluded. The participants had no serious systemic diseases and long-term medication history. The study was approved by Human Research Projects of the Ethics Committee of Jinan Stomatological Hospital. All patients provided voluntary informed consent to participate in the study. Six intact teeth with severe periodontitis were included for tissue staining. Equal healthy third molars as control. The teeth for tissue staining were fixed in 4% paraformaldehyde and decalcified with 15% EDTA for 8 months. Serial paraffin sections of 5 um in thickness were prepared. HE staining was applied for histological observation of dental pulp cells. Immunohistochemistry and immunofluorescence were used to observe the distribution of HIF-1α positive cells. The teeth for real-time PCR and western blotting study were not fixed, fresh pulp tissues were acquired from these teeth and then rapidly frozen in liquid nitrogen.
Cell culture
C57/BL6 male mice (3 weeks old) were chosen for primary cell culture. The study was carried out under the guidance of animal welfare and the “3R” principle and approved by Animal Research Projects of the Ethics Committee of Jinan Stomatological Hospital. Primary mouse bone marrow-derived macrophages (BMMs) were isolated from the femurs and tibias of these mice after inhalation anesthesia and were cultured in complete α-MEM containing 10% fetal bovine serum (FBS) (Gibco BRL, Sydney, Australia) and 30 ng/ml macrophage colony stimulating factor (M-CSF) at 37 °C in an incubator with hypoxic atmosphere of 2% O2, 5% CO2, 94% N2. The cells were also incubated at normoxic conditions of 20% O2, 5% CO2, 75% N2 as a control. We added 50 ng/ml RANKL for 5 days to induce BMMs to differentiate into osteoclasts and then stained for TRAP. Recombinant mouse M-CSF and RANKL were obtained from R&D Systems (Minneapolis, MN, USA).
TRAP staining
The cells were fixed with 4% paraformaldehyde and then stained for TRAP using a commercially available kit (Joy Tech Bio. Co., Hangzhou, China). Osteoclasts were identified as TRAP-positive multinucleated cells containing three or more nuclei.
Bone resorption assay
Corning Osteo Assay Surface plates (Corning, NY, USA) with bone biomimetic synthetic surface were applied. BMMs (2 × 104 cells/well) were cultured in complete α‐MEM (10% FBS, 30 ng/ml M‐CSF and 50 ng/mL RANKL) for 7 days. Equal number of BMMs were seeded to bone biomimetic synthetic surface and cultured in normal and hypoxic atmospheres. The total resorption areas were analyzed using the Image J software.
Immunohistochemistry and immunofluorescence
The method was the same as our previously (Xue et al. 2018; Yu et al. 2017). Polyclonal antibody HIF-1ɑ (dilution 1:100, Abcam, UK) were applied. The secondary antibody was fluorescein rhodamine (TRI-TC)-conjugated goat anti-rabbit IgG. Nuclei were stained with DAPI solution.
Real-time PCR analysis
The method was the same as our previously (Chen et al. 2019). PrimeScript™ RT Reagent Kit (TaKaRa Biotechnology) was then used to obtain cDNA. TB Green® Premix Ex TaqTM Kit (TaKaRa Biotechnology) was applied for qPCR. The primers used were the followings: Mouse HIF-1α forward primer 5′-GTCGGACAGCCTCACCAAACAG -3′ and reverse primer 5′- TAGGTAGTGAGCCACCAGTGTCC-3′. Mouse GAPDH forward primer 5′- AGGTCGGTGTGAACGGATTTG-C-3′ and reverse primer 5′-TGTAGACCATGTAGTTGAGGTCA-3′.
Western blotting analysis
RIPA lysis buffer (Solarbio) was used to extract the total proteins. A bicinchoninic acid (BCA) assay kit (Solarbio) was used to measure protein concentrations. Equal loading quantities of proteins were separated by 10% SDS-PAGE and electroblotted to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, USA). After blocked using 5% nonfat milk, primary antibodies against HIF-1α (diluted 1:500, Abcam), cathepsin K(CTSK) (diluted 1:500, Abcam), NFATc1 (nuclear factor-activated T cells c1) (diluted 1:500; CST) and c-Fos (diluted 1:500; CST) were incubated overnight at 4 °C. After washing with TBST, secondary horseradish peroxidase (HRP)-linked goat-anti rabbit IgG antibody (diluted 1:10,000, CWBiotech, Beijing, China) was incubated at room temperature for 1 h. The blots were visualized by using an ECL kit (Millipore).
Statistical analysis
All data are expressed as the mean ± SD. Unpaired Student’s t-tests were conducted with GraphPad Prism 5 software. The results for multiple group comparisons were analyzed using one-way analysis of variance (ANOVA) followed by a Newman–Keuls post hoc test. A value of P < 0.05 was considered statistically significant.
Results
Histological observation of pulps of a tooth with severe periodontitis
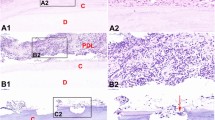
Normal dental pulp showed loose connective tissues, containing fibroblasts, nerve fiber tissues, blood vessels, and the surrounding pseudostratified odontoblastic layer. Few inflammatory cells were observed (Fig. 1B1). Inflammatory cells were observed in the dental pulps of teeth with severe periodontitis. Red blood cells were increased heavily (Fig. 1A1). A large number of inflammatory infiltration cells were observed in remnant periodontal ligament (Fig. 3A1). The fibrous tissues were obviously increased (Fig. 3A2).
Hypoxia occurred in the dental pulps in the dental pulps of teeth with severe periodontitis. Histological observation of pulps of a tooth with severe periodontitis by HE. Red blood cells (black arrows) were increased heavily (A1). Normal pulp tissue as control (B1). HIF-1α positive inflammatory cells (red arrows) were observed in the dental pulps (A2). Higher magnification (A3). Few positive HIF-1ɑ expressions were detected in healthy dental pulps. Higher magnification (the arrows show HIF-1α positive expressions) (B3). D dentin, PD predentine, P pulp. (Color figure online)
Expressions of HIF-1α in the dental pulps of teeth with severe periodontitis
Positive HIF-1ɑ expressions were observed in odontoblasts, pulp fibroblasts, vascular endothelial cell and infiltrating inflammatory cells in the dental pulps of teeth with severe periodontitis (Fig. 2A1–B3). Western blotting showed that the dental pulps of teeth with severe periodontitis expressed higher HIF-1α compared with that of normal gingiva (*P < 0.05) (Fig. 2C).
Expressions of HIF-1α in the dental pulps of teeth with severe periodontitis by immunofluorescence and western blotting. Immunofluorescence showed HIF-1α positive expressions in the dental pulps of teeth with severe periodontitis (A1–A3). Higher magnification (B1–B3). Higher HIF-1α was detected in the dental pulps of teeth with severe periodontitis by western blotting compared with that of normal pulps (C). (***P < 0.01)
Tooth internal resorption sites were found in some teeth with severe periodontitis. Odontoblastic layer (black arrows) and dentin (red arrows) were destroyed in the resorption sites. The fibrous tissues were significantly increased in the dental pulps (Fig. 3A1–A4). Multinucleate odontoclasts/osteoclast (red arrows) were observed around the dentin resorption sites in the dental pulps (Fig. 3B1 and B2). Many HIF-1α positive inflammatory cells (red arrows) were found around the dentin resorption sites (Fig. 3C1 and C2).
Tooth internal resorption was observed in some teeth with severe periodontitis. A large number of inflammatory infiltration cells were observed in remnant periodontal ligament of teeth with severe periodontitis (A1). The fibrous tissues were significantly increased (A2). Tooth internal resorption sites were found. Odontoblastic layer (black arrows) and dentin were destroyed in the resorption sites (red arrows) (A3 and A4). Multinucleate cells (red arrows) were observed around the resorption sites (B1 and B2) in the dental pulps. Many HIF-1α positive cells (red arrows) were observed around the dentin resorption sites (C1 and C2). C cementum, D dentin, P pulp, PD predentine, PL periodontal ligament. (Color figure online)
A large number of red blood cells were observed in the blood vessels, which showed negative expression of HIF-1α (Fig. 4A1 and A2). HIF-1α positive inflammatory cells could been observed around blood vessels (Fig. 4B1–C2). The result showed HIF-1α positive inflammatory cells might originate from the peripheral blood.
Effect of hypoxia on RANKL-induced osteoclast differentiation in vitro
To investigate whether hypoxic got involved in macrophages fusion to form odontoclasts/osteoclast, which participated in the tooth internal resorption, BMMs were cultured in an incubator with hypoxic atmosphere of 2% O2, 5% CO2, 37 °C. Western blotting (Fig. 5a), real-time PCR (Fig. 5b) and immunofluorescence results (Fig. 5c) confirmed HIF-1α expressions in BMMs under hypoxia.
Hypoxia promoted RANKL-induced osteoclast differentiation in vitro. BMMs were cultured in an incubator with hypoxic atmosphere of 2% O2, 5% CO2, 37 °C for 48 h. The protein expression of HIF-1α was detected by western blotting (a). Realtime PCR was carried out to determine HIF-1α mRNA (b). The expression of HIF-1α was observed in BMMs under hypoxia by immunofluorescence (c). Compared with the normal condition (D1), more TRAP positive osteoclasts were detected in the hypoxic conditions (D2). Increased HIF-1α under hypoxia upregulated the number and area of TRAP‐positive osteoclasts (E1 and E2). (*P < 0.05, ***P < 0.01)
More TRAP positive osteoclasts were observed in the hypoxic conditions (Fig. 5D2), compared with the normal condition (Fig. 5D1), Increased HIF-1α under hypoxia upregulated number and area of TRAP‐positive osteoclasts (Fig. 5E1and E2).
Effect of hypoxia on RANKL-induced osteoclast bone resorption in vitro
Increased resorption area was observed in the hypoxic conditions (Fig. 6A2), compared with the normal condition (Fig. 6A1). Hypoxia upregulated resorption area of biomimetic synthetic surface of Osteo Assay Stripwell Plates (Fig. 6A3).
Hypoxia promoted RANKL-induced osteoclast resorption in vitro. Osteo Assay Strip well 96 Plates were applied to determine bone resorption in vitro. Equal number of BMMs were seeded to bone biomimetic synthetic surface of well plates and cultured in normal and hypoxic atmospheres. Hypoxia upregulated resorption area of biomimetic synthetic surface. (*P < 0.05)
Effect of hypoxia on the activation of NFATc1, CTSK and c-fos signaling
The protein expressions of osteoclast-specific factors were also detected by western blotting. The NFATc1, CTSK and c-fos expressions were higher in the hypoxic conditions than that in the normal atmosphere. Hypoxia aggravated the expression of NFATc1 (Fig. 7a and d), CTSK (Fig. 7b and d) and c-fos (Fig. 7c and d).
Hypoxia promoted osteoclastogenesis by activation of NFATc1, CTSK and c-fos signaling. Total cellular proteins extracted from BMMs stimulated with 50 ng/ml RANKL for 5 days in normal and hypoxic atmospheres. Hypoxia aggravated the expression of NFATc1 (a and d), CTSK (b and d) and c-fos (c and d) (*P < 0.05, ***P < 0.01)
Discussion
In this study, we observed the effect of severe periodontitis on pulp tissues, which is helpful to elucidate the complex pathogenesis of periodontal-resource lesions in combined periodontal-endodontic lesions. The results presented here supported the assumption that severe periodontitis could disturb dental pulps. Pathological changes of the dental pulps further confirmed the positive association between periodontitis and changes of the pulps. Increased red blood cells and inflammatory infiltratory cells were observed in the dental pulps of teeth with severe periodontitis, which was consistent with the results of Wan (Wan et al. 2015).
The changes of the pulps may be caused by several factors such as caries, restoration, microcracks, occlusal trauma, and orthodontic movement (Pan et al. 2014). Teeth with these factors were excluded in this study. Teeth with endodontic lesions were also excluded by histological observation of the pulps. The dental pulps of teeth with severe periodontitis expressed higher HIF-1α compared with that of normal pulps. Increased expressions of HIF-1α identified that hypoxia occurred in the dental pulps in response to severe periodontitis. Positive HIF-1ɑ expressions were observed in odontoblasts, pulp fibroblasts, vascular endothelial cell and inflammatory infiltrating cells in the dental pulps. These results showed that the presence of hypoxic environment in the pulps associated with severe periodontitis.
Hypoxia may influence normal and different pathological processes. In the regulation of oxygen homeostasis, it has been fully testified that HIF-1α is an essential transcriptional regulator (Semenza 2014). Under normal oxygen atmospheres, HIF-1α is hardly undetectable. When cells are in hypoxic conditions, the level of the HIF-1α protein obviously increases (Blouin et al. 2004). We previously investigate the impact of hypoxia on human periodontal ligament cells (hPDLCs) under this hypoxic atmosphere in vitro (Yu et al. 2015). It has been reported that hypoxia present in the microenvironment of periodontitis (Gölz et al. 2015; Yu et al. 2015). Hypoxic environment was observed in the pulps of teeth with severe periodontitis. Combined periodontal-endodontic lesions coexist with periodontal lesions and pulpal diseases. Increased HIF-1α present in both periodontal and pulpal lesions, indicating hypoxia might get involved in the pathogenesis of periodontal-resource lesions in combined periodontal-endodontic lesions.
Tooth internal resorption is a pathologic condition leading to destruction of dentin. It may be due to insult to pulp. It can be secondary to trauma, luxation, orthodontic movement, or chronic pulpal and periodontal diseases (Pan et al. 2014; Patni et al. 2018). Interestingly, tooth internal resorption sites were found in some teeth with severe periodontitis. Multinucleate odontoclasts/osteoclast were observed around the dentin resorption sites. Osteoclasts play an indispensable role in hard tissues (bone, dentin and cementum) resorption (Muzylak et al. 2006). Macrophage is one of main inflammatory infiltratory cells and differentiates into osteoclasts. To elucidate the mechanisms of odontoclasts/osteoclast activation in hypoxic pulpal environment. Effect of hypoxia on osteoclast differentiation of BMMs was observed in vitro. Our results showed that HIF-1α expressions in BMMs increased heavily under hypoxic atmosphere of 2% O2, 5% CO2, 37 °C and HIF-1ɑ promotes osteoclastogenesis in vitro. The expressions of osteoclast-specific factors NFATc1, CTSK and c-fos expressions were higher in the hypoxic conditions than that in the normal atmosphere. Hypoxia and HIF-1α could regulate angiogenic-osteogenic coupling and osteoclast-mediated bone resorption (Knowles et al. 2010; Knowles 2017). Under hypoxia, upregulated HIF-1α protein accelerated the differentiation of RAW264.7 cells into osteoclasts (Zhu et al. 2019). Teeth with severe periodontitis show increased potential of osteoclastic differentiation. Hypoxic microenvironment is a common feature of these osteolytic diseases such as periodontitis (Gölz et al. 2015; Yu et al. 2015), rheumatoid arthritis and cancer metastasis to bone (Knowles and Athanasou 2008), in all of which hypoxia and/or level of HIF-1α correlates with over-activation of osteoclasts (Knowles 2015, 2017).
Finally, High levels of HIF-1ɑ in hypoxic dental pulps associated with teeth with severe periodontitis. Inhibition of HIF-1ɑ as alternative therapeutic agents targeting periodontal-resource lesions in combined periodontal-endodontic lesions.
References
Blouin CC, Page EL, Soucy GM, Richard DE (2004) Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia-inducible factor 1alpha. Blood 103(3):1124–1130
Chen LL, Yan J (2001) Porphyromonas gingivalis lipopolysaccharide activated bone resorption of osteoclasts by inducing IL-1, TNF, and PGE. Acta Pharmacol Sin 22(7):614–618
Chen X, Chen X, Zhou Z, Mao Y, Wang Y, Ma Z et al (2019) Nirogacestat suppresses RANKL-induced osteoclast formation in vitro and attenuates LPS-Induced bone resorption in vivo. Exp Cell Res 382(1):111470
Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N et al (2003) HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 112(5):645–657
Gölz L, Memmert S, Rath-Deschner B, Jäger A, Appel T, Baumgarten G et al (2015) Hypoxia and P. gingivalis synergistically induce HIF-1 and NF-κB activation in PDL cells and periodontal diseases. Med Inflamm. https://doi.org/10.1155/2015/438085
Knowles HJ, Athanasou NA (2008) Hypoxia-inducible factor is expressed in giant cell tumour of bone and mediates paracrine effects of hypoxia on monocyte-osteoclast differentiation via induction of VEGF. J Pathol 215(1):56–66
Knowles HJ, Cleton-Jansen AM, Korsching E, Athanasou NA (2010) Hypoxia-inducible factor regulates osteoclast-mediated bone resorption: role of angiopoietin-like 4. FASEB J 24(12):4648–4659
Knowles HJ (2015) Hypoxic regulation of osteoclast differentiation and bone resorption activity. Hypoxia (Auckland, NZ) 3:73–82
Knowles HJ (2017) Hypoxia-induced fibroblast growth factor 11 stimulates osteoclast-mediated resorption of bone. Calcif Tissue Int 100(4):382–391
Liu F, Wang Y, Xu J, Liu F, Hu R, Deng H (2016) Effects of Porphyromonas gingivalis lipopolysaccharide on the expression of key genes involved in cholesterol metabolism in macrophages. Arch Med Sci 12(5):959–967
Muzylak M, Price JS, Horton MA (2006) Hypoxia induces giant osteoclast formation and extensive bone resorption in the cat. Calcif Tissue Int 79(5):301–309
Pan H, Cheng L, Yang H, Zou W, Cheng R, Hu T (2014) Lysophosphatidic acid rescues human dental pulp cells from ischemia-induced apoptosis. J Endod 40(2):217–222
Patni PM, Jain P, Jain S, Hiremath H, Agarwal R, Patni MJ (2018) Internal tunneling resorption associated with invasive cervical resorption. J Conserv Dent 21(1):105–108
Semenza GL (2014) Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol 9:47–71
Sunitha VR, Emmadi P, Namasivayam A, Thyegarajan R, Rajaraman V (2008) The periodontal—endodontic continuum: a review. J Conserv Dent 11(2):54–62
Takedachi M, Iyama M, Sawada K, Mori K, Yamamoto S, Morimoto C et al (2017) Hypoxia-inducible factor-1alpha inhibits interleukin-6 and -8 production in gingival epithelial cells during hypoxia. J Periodontal Res 52(1):127–134
Wan L, Lu HB, Xuan DY, Yan YX, Zhang JC (2015) Histological changes within dental pulps in teeth with moderate-to-severe chronic periodontitis. Int Endod J 48(1):95–102
Xia M, Qi Q (2013) Bacterial analysis of combined periodontal-endodontic lesions by polymerase chain reaction-denaturing gradient gel electrophoresis. J Oral Sci 55(4):287–291
Xue L, Su L, Zhao L, Li J, Du Y, Yu X (2018) Cyclophilin a increases CD68(+) cell infiltration in rat experimental periodontitis. J Mol Histol 49(2):157–164
Yu X, Gong Z, Lin Q, Wang W, Liu S, Li S (2017) Denervation effectively aggravates rat experimental periodontitis. J Periodontal Res 52(6):1011–1020
Yu XJ, Xiao CJ, Du YM, Liu S, Du Y, Li S (2015) Effect of hypoxia on the expression of RANKL/OPG in human periodontal ligament cells in vitro. Int J Clin Exp Pathol 8(10):12929–12935
Zhu J, Tang Y, Wu Q, Ji YC, Kang FW (2019) Mechanism of participation of osteocytes in the formation of osteoclasts under hypoxia. Hua Xi Kou Qiang Yi Xue Za Zhi 37(5):463–468
Acknowledgements
China Postdoctoral Science Foundation (2019M651537). Clinical medicine science and technology innovation plan of Jinan (201907099).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, X., Jiang, H., Cheng, G. et al. High levels of HIF-1ɑ in hypoxic dental pulps associated with teeth with severe periodontitis. J Mol Hist 51, 265–275 (2020). https://doi.org/10.1007/s10735-020-09878-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-020-09878-5