Abstract
Dental disease due to osteoclast (OC) overactivity reaches epidemic proportions in older domestic cats and has also been reported in wild cats. Feline odontoclastic resorptive lesions (FORL) involve extensive resorption of the tooth, leaving it liable to root fracture and subsequent loss. The etiopathogenesis of FORL remains unclear. Here, we explore the hypothesis that FORL is associated with hypoxia in the oral microenvironment, leading to increased OC activity. To investigate this, we developed a method of generating OCs from cat blood. Reducing O2 from 20% to 2% increased the mean area of OC eightfold from 0.01 to 0.08 mm2. In hypoxic cultures, very large OCs containing several hundred nuclei were evident (reaching a maximum size of ∼14 mm2). Cultures exposed to 2% O2 exhibited an increase of ∼13-fold in the area of bone slices covered by resorption lacunae. In line with this finding, there was a significant increase in cells differentiating under hypoxic conditions, reflected in increased expression of cathepsin K and proton pump enzymes. In conclusion, these results demonstrate that oxygen tension is a major regulator of OC formation in the cat. However, in this species, hypoxia induces the formation of “giant” OCs, which can be so large as to be visible with the naked eye and yet also actively resorb. This suggests that local hypoxia is likely to play a key role in the pathogenesis of FORL and other inflammatory conditions that are associated with bone resorption in cats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Acidosis has been implicated in the pathogenesis of various metabolic diseases [1], and acid ingestion is known to stimulate osteoclastic resorption, although the mechanism(s) remains unclear. Arnett and colleagues [2–4] have investigated the effects of small shifts in extracellular pH on the differentiation and resorptive activity of rodent osteoclasts in vitro. Similar responses are seen to hypoxia [5], a situation that would also occur at sites of “inflammatory” bone or tooth destruction. In normal tissues, interstitial oxygen partial pressure (pO2) is approximately in the range 30–80 mm Hg (∼4–10%). However, pO2 may be considerably lower in inflamed or infected tissues, e.g., in the oral environment at sites of periodontitis, caries, or abscesses [6]; in some diseased tissues, interstitial O2 may be <1% [7]. Hypoxia is known to act as a stimulator of the formation and activation of cells derived from marrow precursors, including cells of the monocyte-macrophage lineage that are closely related to osteoclasts [7–10]. Recent work has shown that hypoxia exerts an impressive stimulatory action on osteoclast formation in mouse marrow cultures over 7–14 days [5], and hypoxia increased both osteoclast number and size, resulting in large increases in resorption pit formation.
In the present study, we examined the effect of oxygen tension on the in vitro development of feline osteoclasts and mineralized tissue resorption because a dental disease involving osteoclast overactivity (feline odonto- clastic resorption lesions, FORL) reaches epidemic proportions in older domestic cats [11, 12]. FORL lead to pain, destruction of the periodontal attachment, and tooth loss [12–14]. A similar condition, multiple idiopathic root resorption (MIRR), occurs in humans; and it has been suggested that FORL provides a valuable model for the study of MIRR [15]. FORL involve multiple teeth; initiate on the external surface of the root, principally at the cementoenamel junction; and extend to involve dentine and enamel. FORL rarely involve the pulp, except in advanced stages of disease. Histological studies have demonstrated the presence of large numbers of osteoclasts on the external surface of the tooth root at early stages of the disease, whereas few osteoclasts are detected at advanced stages and during the initiation of repair response [16].
Despite it being a disease of significant veterinary importance, the etiopathogenesis of FORL, like MIRR in humans, remains unknown. Many factors have been implicated, such as food texture, mechanical stress, dietary deficiencies, excessive vitamin A intake, periodontal disease, structural features of the cementoenamel junction, and viral disease [16, 17]. However, none of these has been definitively proven to be the direct cause of tooth resorption; moreover, compared with other species, little is known about the biology of osteoclasts in the cat, contributing to the frustration in managing this disease. What is known is that FORL is associated with inflammation, and recently we showed increased mRNA expression of the inflammatory cytokines interleukin-1β and interleukin-6 in teeth affected with advanced FORL [18]. Because sites of inflammation in the mouth are likely to be hypoxic and hypoxia has striking effects on murine osteoclasts, we hypothesized that inflammatory change in the oral cavity of cats (e.g., as a consequence of viral or bacteriological challenge) could lead to the development of “acidic, hypoxic” conditions in the oral cavity and that these microenvironmental conditions would induce active tooth resorption and thus contribute to the pathogenesis of FORL.
For this study, we used a method we had previously established to generate feline osteoclasts from blood mononuclear cells stimulated by macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor κB ligand (RANKL) [19]. The osteoclasts arising in these cultures show all the hallmarks of genuine osteoclasts isolated directly from bone: pivotally, they are multinucleated, express tartrate-resistant acid phosphatase (TRAP) and F-actin rings, and resorb bone [such phenotypic characteristics are shown for feline osteoclasts in 19 and reviewed in 20]. Under hypoxic conditions, we found that gigantic osteoclasts developed in large numbers from feline peripheral blood monocytes and that these were impressively active in bone resorption. We conclude that these innate characteristics could account for the unique pathology of FORL in the absence of other causative factors and for the apparent rarity of this disease in other species such as humans.
Materials and Methods
Cat Peripheral Blood Mononuclear Cells
Peripheral blood was obtained from healthy adult cats, aged 2 years (Waltham Centre for Pet Nutrition, Waltham, UK). Ten milliliters of blood, obtained by jugular puncture, were heparinized (200 μL/10 mL of blood; PUMP-HEP, Leo Labs, Bucks, UK) and transported on wet ice. Peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation over Ficoll Hypaque (density 1.077; Amersham Pharmacia Biotech, Little Chalfont, UK) for 30 minutes at 1,460 × g at room temperature. Mononuclear cells were collected and washed twice in α-minimum essential medium (α-MEM; Sigma-Aldrich, Gillingham, UK) by centrifugation at 1,000 × g for 10 minutes.
Generation of Cat Osteoclasts from Peripheral Blood
Osteoclasts were generated from feline PBMCs using methods developed for human osteoclasts [21–23] and described previously [19]. Isolated PBMCs were resuspended in α-MEM supplemented with 10% heat-inactivated fetal bovine serum (Sera Laboratories, Crawley, UK), 2 mM L-glutamine, 100 IU benzyl penicillin/mL, and 100 mg streptomycin/mL (GIBCO BRL, Paisley, UK) and plated (2 × 105) on bovine bone slices (day 1) in 96-well plates in a final volume of 200 μL. Cultures were maintained for the first 4 days in culture medium and M-CSF (25 ng/mL; kindly provided by Genetics Institute, Boston, MA) at 37°C in 5% CO2/95% air and fed twice weekly. On day 4, 90% of the medium was removed and replaced with fresh medium containing additional soluble RANKL (30 ng/mL; kindly provided by Amgen, Thousand Oaks, CA); subsequently, cultures were fed twice weekly with both growth factors after demidepletion of medium and terminated after 14 days.
Growth Factor Manipulation
The growth factor concentrations used under “standard” culture conditions were found by prior experimentation [19]. Cultures were performed at ambient oxygen concentration in the absence of all growth factors or with the addition of M-CSF (concentration range 25–100 ng/mL) and RANKL (concentration range 30–120 ng/mL) to arrive at minimum factor doses for use in the study.
Induction of Hypoxia
The procedure reported by Arnett et al. [5] was followed. The oxygen tension of the cultures was varied from ambient (nominal 20% oxygen) to either 12% or 2% for the specified culture period and replenished at each medium change (medium pH was not altered by addition of extra acid or alkali) by transferring the dentine slices to 25 cm2 flasks with polyethylene plug-seal caps (Falcon, Becton Dickinson, Oxford, UK) containing 8 mL of the same medium (eight discs per flask). The flasks were then flushed for 2 minutes with gas mixtures containing 5% CO2 and 20%, 12%, or 2% O2 (balance N2) via a 21-gauge needle inserted through the loosened polyethylene cap. The cap was then tightened and the needle hub closed with a Luer plug. The sealed flasks were incubated at 37°C in a standard incubator containing 5% CO2/95% atmospheric air and regassed daily. Culture medium pH, pCO2, and pO2 were monitored at each medium change and at the end of experiments using blood gas analyzers (ABL 330 and ABL 705; Radiometer, Copenhagen, Denmark). pO2 was also monitored using a fluorescence-based oxygen probe (FOXY; Ocean Optics, Duiven, The Netherlands) as very low O2 levels are beyond the measurement range of clinical blood gas analyzers. The pH at the termination of the experiments did not differ from control values of ∼7.2.
Immunocytochemistry
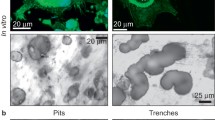
For confocal microscopy (Leica TCS NT, Heidelberg, Germany), osteoclasts on bone slices were fixed for 5 minutes in a 50:50 mixture of α-MEM with fixation buffer (3.5% paraformaldehyde and 2% sucrose in phosphate-buffered saline [PBS, Sigma-Aldrich]), washed in PBS, and placed in ice-cold permeabilization buffer (20 mM 4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid [HEPES], 300 mM sucrose, 50 mM NaCl, 3 mM MgCl2, 0.5% Triton X-100, and 0.5% sodium azide in PBS) for a further 5 minutes [24]. For staining of the osteoclast proton pump adenosine triphosphatase (ATPase), cells were fixed in ice-cold methanol for 5 minutes. Osteoclasts were then incubated in tetrarhodamine isothiocyanate (TRITC)-phalloidin conjugate (Molecular Probes, Eugene, OR; 5 U/mL) in PBS to identify resorbing osteoclasts by their characteristic F-actin “ring” structure [24]. Cell markers were identified using monoclonal antibodies: 23C6 for the human integrin αvβ3 vitronectin receptor (VNR) [24, 25], cathepsin K (gift of SmithKline Beecham, Philadelphia, PA), matrix metalloproteinase 9 (MMP-9) (Chemicon, Temecula, CA), TRAP (gift of S. A. Nesbitt, University College London, UK), and proton pump (gift of S. A. Nesbitt). The secondary antibody was fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG; Dako, Ely, UK). Osteoclasts were defined as cells expressing the VNR and F-actin rings [24]. Confocal micrographs shown are merged through-focus images for a stack of xy images (as in Fig. 1). Twenty osteoclasts were examined on each bone slice, and the intensity of staining was evaluated in arbitrary units (pixel intensity per unit area under standardized conditions as in Horton et al. [26], Table 1).
Effect of changing oxygen content to 2% in day 1–14 cultures on the expression of an osteoclast marker protein; changes in fluorescence assessed by immunocytochemistry and confocal microscopy. Immunostaining for cathepsin K in normoxic (A) and 2% oxygen-treated (B) osteoclasts; arrows in B mark nuclei and arrowheads, the location of multiple F-actin rings. Cells show typical changes in levels of cathepsin K as used for the analyses summarized in Table 1; cells of similar size were selected for illustrative purposes and solely for scaling of the figure. Scale bars = 20 μm.
TRAP Staining
Experiments were terminated by fixing the discs in 2% glutaraldehyde, followed by staining for 35 minutes to demonstrate TRAP (Sigma-Aldrich Kit 387-A).
Assessment of Osteoclast Numbers and Spread Area
The number of osteoclasts attached to the dentine substrate was determined after staining for TRAP by taking pictures of bone slices using a JVC (Tokyo, Japan) color video camera, with the images analyzed using Leica QWin software to give cell number; mean, maximum, and total cell area; and percent bone surface covered by osteoclasts.
Assessment of Osteoclastic Resorption
Devitalized cortical bovine bone slices (4 × 5 × 0.1 mm, 20 mm2) [19] were used as substrate for osteoclastic bone resorption. After counting and assessing the area of osteoclasts attached to the substrate, as above, cells were removed by rubbing on filter paper or by treatment with Trisol and rubbing on filter paper. Resorption lacunae were visualized by biotin-conjugated wheat germ agglutinin (WGA) lectin staining (Vector Laboratories, Burlingame, CA) after reaction with TRITC-streptavidin (Sigma-Aldrich). Fluorescent images were captured using a Leica fluorescence microscope and analyzed using Leica QWin software, as for the TRAP-stained cultures, producing values for resorption pit “area” assessed as “mean pixel intensity per unit area.”
Statistical Analysis
Data for osteoclast numbers and size and bone resorption are shown from one of three replicate experiments where values under each experimental condition tested represent data obtained from at least four bone slices. The results were analyzed using one-way analysis of variance, where significance was accepted at P < 0.05. Results are displayed as mean ± standard deviation. Control conditions, 20% oxygen and medium pH of ∼7.2, were used as baseline for statistical comparisons.
Results
General Characterization of Osteoclasts Generated from Feline PBMCs
PBMCs were cultured with M-CSF (25 ng/mL) and RANKL (30 ng/mL) and examined over a culture period of 7–14 days. Culture medium pH at the end of culturing remained at ∼7.2 (as in the murine system [5]). As in our earlier work [19], cells with osteoclastic morphology appeared from day 7. This coincided with the appearance of the first TRAP-positive polykaryons and small resorption lacunae, identified in bone slices by phase microscopy or WGA lectin staining for peroxidase-WGA (as in Fig. 4A).
By 14 days of culture [19], the cells were multinucleated, had F-actin rings (Fig. 1B), and expressed high levels of TRAP (Fig. 2C-E), the αvβ3 VNR, and the osteoclast enzyme cathepsin K (Fig. 1A,B and Table 1). The myeloid antigen CD18 and the megakaryocyte/platelet integrin CD41 were absent (data not shown, as [19]). Control cultures contained approximately 500 osteoclasts/20 mm2 bone slice, and these resorbed bone: they formed 4,620 ± 2,242 lacunae/20 mm2 bone slice and resorbed 6.1% ± 2.75% of the bone surface, with a mean pit area of 8.59 ± 3.46 μm2.
(A) Number of TRAP-positive osteoclasts per bone slice (20 mm2 area) in cultures exposed to 20%, 12%, and 2% oxygen from days 1 to 14. (B) Mean area of single osteoclasts in cultures exposed to 20%, 12%, and 2% oxygen from days 1 to 14, **P < 0.003, ***P < 0.0001. (C-E) Low-power charge-coupled device images showing examples of the effect of reducing culture oxygen content from 20% (C) to 2% (D, E) on the appearance of large TRAP-positive osteoclasts (D) or extensive syncytia (E) against a background of smaller TRAP-positive osteoclasts (as shown in C). Scale bars = 1 mm.
Effect of Hypoxia on Osteoclast Differentiation
An approximate 38% decrease in the number of osteoclasts was found if cultures were exposed to hypoxic conditions throughout a 14-day period (2% oxygen, P < 0.0001; Fig. 2A); a less dramatic effect was seen at 12% oxygen (P < 0.003). A concurrent increase in osteoclast area was observed, and this was especially marked in cultures performed at 2% oxygen (Fig. 2B): the mean osteoclast area was ∼0.01 mm2 at 20% oxygen, whereas an approximately eightfold increase was observed in cells cultured for 14 days in 2% O2 (∼0.08 mm2). Osteoclast fusion induced by hypoxia produced osteoclasts with a maximum cell area of 14 mm2 and diameter of ∼2 mm (significant differences vs. normoxia, data not shown). This effect is illustrated in Figure 2C-E. Osteoclasts under 2% oxygen conditions were generally larger, some extremely so (Fig. 2C,D), or developed into extensive syncytia visible to the naked eye (Fig. 2E).
Osteoclast numbers were not significantly altered if grown under normoxic conditions for the first 10 days and then switched to either 12% or 2% oxygen for the final 4 days, at which stage they were fully “mature” (Fig. 3A). A slight but significant increase in osteoclast area was seen after culture of 11–14 days at 2% oxygen (Fig. 3B), and although the maximum size of osteoclasts under these conditions increased threefold, these were still only one-third of the size of those produced in cultures exposed to hypoxia for a full 14 days.
Stimulation of Bone Resorption by Hypoxia
WGA lectin staining and quantification of fluorescence (Fig. 4A) were used to evaluate bone resorption. Hypoxia significantly stimulated bone resorption after exposure of culture of 1–14 days (Fig. 4B) and 11–14 days (Fig. 4C) to both 12% and 2% oxygen. Under normoxic conditions, osteoclasts resorbed ∼6.1% (1–14 days) and ∼5.5% (11–14 days) of the surface of bone slices; this increased ∼13- and ∼7.5-fold (P < 0.0001 and P < 0.0001) upon exposure to 2% oxygen during culture. Similar results were observed for resorption pit number (4,620 ± 2,242 to 10,190 ± 2,989 per 20 mm2 at 2% oxygen for 14 days, P < 0.0001, and 3,669 ± 1,766 to 10,800 ± 2,174/mm2 at 2% oxygen for 11–14 days, P < 0.0001; significant differences for both 12% and 2% oxygen for both culture periods; data not shown). Mean pit area increased 1.6-fold (8.59–13.72 μm2) in cultures exposed to 2% oxygen for 1–14 days.
Induction of Cathepsin K and Proton Pump in Osteoclasts by Hypoxia
Expression of cathepsin K, proton pump, TRAP, and VNR was analyzed in osteoclasts generated from PBMCs in 2% oxygen by immunostaining and confocal microscopy after 14 days of culture (Fig. 1, Table 1). VNR levels were not significantly altered by hypoxia, whereas two enzymes characteristic of osteoclast functional activation were greatly increased in line with the observed increase in bone resorption, cathepsin K by 87% (Fig. 1) and proton pump by 103% (both P = 0.035). TRAP enzyme levels were also increased (63%, P = 0.002).
Discussion
Relatively little research has been carried out on osteoclasts and the regulation of bone resorption in the cat even though diseases involving increased bone resorption are a significant cause of morbidity and mortality in this species [27]. These include metabolic bone disease [28, 29], neoplasia [30], arthritis [27], disuse osteopenia, non-union fractures [31], and FORL [11–14, 16, 17]. Early studies [32–36] investigated cat osteoclasts in situ or disaggregated directly from bone, and they were shown to express TRAP and αvβ3 integrin and to resorb bone. Recently [19], we demonstrated that the multinucleated cells produced by culture of feline PBMCs in the presence of M-CSF and RANKL, conditions inductive of osteoclast differentiation in other species, were genuine osteoclasts; they expressed TRAP, resorbed bone, and had high levels of αvβ3 and of the proteolytic enzymes cathepsin K and MMP-9. Bone resorption by feline osteoclasts was inhibited by two drugs in clinical use for bone disease, calcitonin and amino-bisphosphonate [19]. Cells with an identical phenotype were observed to form in vitro in the present study, confirming our earlier results. Under basal conditions, cultured feline osteoclasts were observed to be larger than human PMBC-derived osteoclasts, unless human cells were stimulated by transforming growth factor β (TGF-β) [37]. This is consistent with histological studies [38, 39] that have also shown feline osteoclasts to be significantly larger than those from other species. For example, murine osteoclasts isolated from neonatal mouse bone or grown in vitro frequently have fewer than five nuclei, while those from cats often have more than 100 nuclei. In humans, osteoclasts with a high number of nuclei are seen in Paget’s disease and in familial expansile osteolysis (a condition that is also associated with early tooth loss) [40], although whether this is related to local hypoxia has yet to be determined. The effects of hypoxia on feline osteoclast size are also dramatic; the largest osteoclasts induced under hypoxic culture conditions had a maximum cell area of approximately 14 mm2, a diameter of 2 mm, and often several hundred nuclei; i.e., they were visible to the naked eye (Fig. 2). These osteoclasts were also functionally highly active, containing multiple actin rings (Fig. 1B illustrates a “small” multinucleated osteoclast with several F-actin rings); and they frequently resorbed the entire surface of bone slices (mean area resorbed after 14 days in 2% O2 ∼80%, Fig. 4). The effects of hypoxia on bone resorption were most significant in differentiating osteoclasts exposed to hypoxia for all 14 days of culture compared to mature osteoclasts exposed to only 4 days of hypoxic conditions (days 11–14). The activity of culture-derived mature osteoclasts is also stimulated by extracellular acidification (unpublished observations and [41]). In vivo, the enhanced acid production in hypoxic tissues is likely to be compounded by the reduced vascular perfusion responsible for the hypoxia, resulting in complex local pH and pO2 gradients. Thus, the effects of hypoxia and acidosis are closely linked, causing mineralized tissue destruction by recruiting and then stimulating osteoclasts. Normal extracellular pH in bone or in the tooth environment has not been measured, but it is likely to be somewhat less than blood pH; e.g., in normal skin, interstitial pH has been measured at ∼7.1 [42], which approximates the half-maximal activation pH of dissociated rodent osteoclasts [2, 3, 5]. In our studies, hypoxia was not associated with acidosis, pH values of control, 12% and 2% oxygen cultures being identical (as in the mouse [5]).
Further studies will be needed to determine the mechanism by which the exaggerated effects of hypoxia upon feline osteoclast formation and function are mediated. Hypoxia may stimulate the expansion of the CD34/CD14-expressing precursor pool in bone marrow [43], but it could also increase osteoclast size by regulating fusion of osteoclast precursors since it is known to regulate trophoblast fusion [44, 45]. Intracellularly, the stimulatory action of hypoxia may be mediated by prolyl hydroxylases and hypoxia-induced factor (HIF). Cells sense oxygen using a family of oxygen-dependent enzymes that hydroxylate a proline residue on HIF, a constitutively produced transcription factor that mediates cellular responses to hypoxia, with proline hydroxylation in the presence of oxygen targeting HIF for intracellular destruction thence preventing its action [46, 47]. There are also a number of strong candidates for paracrine or autocrine mediators of the effect of hypoxia in the cat. Hypoxia-stimulated resorption in calvaria is dependent on prostaglandin synthesis [5], as is observed with acidosis [4], and prostaglandins stimulate osteoclast formation [21]. Hypoxia stimulates purine nucleotide release from endothelial cells [48], and Arnett et al. [49–51] have shown that ATP and adenosine diphosphate (ADP) are powerful osteolytic agents, acting through purinergic (P2) receptors on bone cells; this mechanism could account for some of the resorptive action of hypoxia in intact bone, but it remains to be investigated whether nucleotide release from other cell types in bone might also be enhanced by low pO2. Hypoxia has also been shown to activate nuclear factor κB in a variety of tissues, including CD14-positive cells which contribute to the osteoclast precursor population [52, 53]. Finally, one of the major effects of hypoxia on cells is to stimulate the production of potent angiogenic factors such as vascular endothelial growth factor (VEGF), tumor necrosis factor-α, and fibroblast growth factor [7]; these factors are also stimulators of the formation and/or function of osteoclasts in other species [54–56]. It is already well documented that hypoxia increases VEGF production by osteoblasts [57–59], and VEGF production by human peripheral blood-derived macrophages is also strongly upregulated by hypoxia [7]. Similarly, insulin-like growth factor I and TGF-β, produced by osteoblasts in response to low oxygen, are also stimulators of osteoclast formation [37, 60–63].
Some or all of these potential mechanisms could contribute locally active exogenous factors to the pathogenesis of FORL and to the general propensity of cat osteoclast precursors to develop into particularly large mature cells with especially high resorptive activity. Expression of these cytokines and growth factors in the feline oral microenvironment now needs to be investigated in normal and diseased animals suffering from FORL. Alternatively, there may be inherent, possibly genetic, differences in the domestic cat that result in a substantially different osteoclast response to environmental factors compared to that observed in other species. That is, the cat may react in a qualitatively or quantitatively different manner, whether this be in terms of an exaggerated osteoclast number and/or size, degree of activation (and hence resorption), growth factor sensitivity, or a response at a different range of pH or oxygen tension. We suggest that these innate characteristics could account for the unique pathology of FORL and for the apparent rarity of this disease in other species, including humans.
References
Arnett T (2003) Regulation of bone cell function by acid-base balance. Proc Nutr Soc 62:511–520
Arnett TR, Dempster DW (1986) Effect of pH on bone resorption by rat osteoclasts in vitro. Endocrinology 119:119–124
Arnett TR, Spowage M (1996) Modulation of the resorptive activity of rat osteoclasts by small changes in extracellular pH near the physiological range. Bone 18:277–279
Meghji S, Henderson B, Morrison MS, Arnett TR (2001) pH dependence of bone resorption: mouse calvarial osteoclasts are activated by acidosis. Am J Physiol 280:E112–E119
Arnett TR, Gibbons DC, Utting JC, Orriss IR, Hoebertz A, Rosendaal M, Meghji S (2003) Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol 196:2–8
Liu RS, Chu LS, Yen SH, Chang CP, Chou KL, Wu LC, Chang CW, Lui MT, Chen KY, Yeh SH (1996) Detection of anaerobic odontogenic infections by fluorine-18 fluoromisonidazole. Eur J Nucl Med 23:1384–1387
Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE (1999) Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukocyte Biol 66:889–900
Bradley TR, Hodgson GS, Rosendaal M (1978) The effect of oxygen tension on haemopoietic and fibroblast cell proliferation in vitro. J Cell Physiol 97:517–522
Broxmeyer HE, Cooper S, Lu L, Miller ME, Langefeld CD, Ralph P (1990) Enhanced stimulation of human bone marrow macrophage colony formation in vitro by recombinant human macrophage colony-stimulating factor in agarose medium and at low oxygen tension. Blood 76:323–329
Koller MR, Bender JG, Miller WM, Papoutsakis ET (1992) Reduced oxygen tension increases hematopoiesis in long-term culture of human stem and progenitor cells from cord blood and bone marrow. Exp Hematol 20:264–270
Lonmer MJ, Verstraete FJ (2000) Prevalence of odontoclastic resorption lesions and peripheral radiographic lucencies in cats: 265 cases (1995–1998) J Am Vet Med Assoc 217:1866–1869
Heaton M, Wilkinson J, Gorrel C, Butterwick R (2004) A rapid screening technique for feline odontoclastic resorptive lesions. J Small Anim Pract 45:598–601
Gengler W, Dubielzig R, Ramer J (1995) Physical examination and radiographic analysis to detect dental and mandibular bone resorption in cats: a study of 81 cases from necropsy. J Vet Dent 12:97–100
Lyon KF (1992) Subgingival odontoclastic resorptive lesions: classification, treatment, and results in 58 cats. Vet Clin North Am Small Anim Pract 22:1417–1432
Liang H, Burkes EJ, Frederiksen NL (2003) Multiple idiopathic cervical root resorption: systematic review and report of four cases. Dentomaxillofac Radiol 32:150–155
Okuda A, Harvey CE (1992) Etiopathogenesis of feline dental resorptive lesions. Vet Clin North Am Small Anim Pract 22:1385–1404
Reiter AM, Mendoza KA (2002) Feline odontoclastic resorptive lesions: an unsolved enigma in veterinary dentistry. Vet Clin North Am Small Anim Pract 32:791–837
DeLaurier A, Allen S, deFlandre C, Horton MA, Price JS (2002) Cytokine expression in feline osteoclastic resorptive lesions. J Comp Pathol 127:169–177
Muzylak M, Flanagan AM, Ingham K, Gunn N, Price J, Horton MA (2002) A feline assay using osteoclasts generated in vitro from peripheral blood for screening antiresorptive agents. Res Vet Sci 73:283–290
Horton MA, Nesbitt SA, Bennett JH, Stenbeck G (2002) Integrins and other cell surface attachment molecules of bone cells. In: Bilezikian JP, Raisz LG, Rodan GA (eds), Principles of Bone Biology, 2nd ed. Academic Press, San Diego, pp 265–286
Lader CS, Flanagan AM (1998) Prostaglandin E2, interleukin 1α, and tumor necrosis factor- increase human osteoclast formation and bone resorption in vitro. Endocrinology 139:3157–3164
Massey HM, Flanagan AM (1999) Human osteoclasts derive from CD14-positive monocytes. Br J Haematol 106:167–170
Lader CS, Scopes J, Horton MA, Flanagan AM (2001) Generation of human osteoclasts in stromal cell-free and stromal cell-rich cultures: differences in osteoclast CD11c/CD18 integrin expression. Br J Haematol 112:430–437
Nesbitt SA, Horton MA (1997) Trafficking of matrix collagens through bone-resorbing osteoclasts. Science 276:266–269
Horton MA, Lewis D, McNulty K, Pringle JA, Chambers TJ (1985) Monoclonal antibodies to osteoclastomas (giant cell bone tumours): definition of osteoclast-specific cellular antigens. Cancer Res 45:5663–5669
Horton MA, Massey HM, Rosenberg N, Nicholls B, Seligsohn U, Flanagan AM (2003) Upregulation of osteoclast α2β1 integrin compensates for lack of αvβ3 vitronectin receptor in Iraqi-Jewish-type Glanzmann thrombasthenia. Br J Haematol 122:950–957
Bennett D (1995) Diseases of the musculoskeletal system. In: Chandler EA, Gaskell CJ, Gaskell RM (eds), Feline Medicine and Therapeutics. Blackwell Science, Oxford, pp 150–191
Clark L (1970) The effect of excess vitamin A on long bone growth in kittens. J Comp Pathol 80:625–634
Barber PJ, Elliott J (1998) Feline chronic renal failure: calcium homeostasis in 80 cases between 1992 and 1995. J Small Anim Pract 39:108–116
Heldmann E, Anderson MA, Wagner-Mann C (2000) Feline osteosarcoma: 145 cases (1990–1995). J Am Anim Hosp Assoc 36:518–521
Hill FWG (1977) A survey of bone fractures in the cat. J Small Anim Pract 18:457–463
Addison WC (1978) Enzyme histochemical properties of kitten osteoclasts in bone imprint preparations. Histochem J 10:645–656
Allen TD, Testa NG, Suda T, Schor SL, Onions D, Jarrett O, Boyde A (1981) The production of putative osteoclasts in tissue culture – ultrastructure, formation and behaviour. Scanning Electron Microsc 3:347–354
Ibbotson KJ, Roodman GD, McManus LM, Mundy GR (1984) Identification and characterization of osteoclast-like cells and their progenitors in cultures of feline marrow mononuclear cells. J Cell Biol 99:471–480
Pharoah MJ, Heersche JN (1985) 1,25-Dihydroxyvitamin D3 causes an increase in the number of osteoclast like cells in cat bone marrow cultures. Calcif Tissue Int 37:276–281
Horton MA, Fowler P, Simpson A, Onions D (1988) Monoclonal antibodies to human antigens recognise feline myeloid cells. Vet Immunol Immunopathol 18:213–217
Massey HM, Scopes J, Horton MA, Flanagan AM (2001) Transforming growth factor-beta1 (TGF-β) stimulates the osteoclast-forming potential of peripheral blood hematopoietic precursors in a lymphocyte-rich microenvironment. Bone 28:577–582
Addison WC (1980) The effect of parathyroid hormone on the numbers of nuclei in feline odontoclasts in vivo. J Periodontal Res 15:536–543
De Vico G, Maiolino P (1997) “Osteoclast-like” giant cells in a feline mammary carcinoma. J Submicrosc Cytol Pathol 29:153–155
Reddy SV, Menaa C, Singer FR, Demulder A, Roodman GD (1999) Cell biology of Paget’s disease. J Bone Miner Res (suppl 2):3–8
Muzylak M, Arnett TR, Price J, Horton MA (2004) The effect of pH on osteoclast function in the cat. Bone 34:S25–S47
Martin GR, Jain RK (1994) Non-invasive measurement of interstitial pH profiles in normal and neoplastic tissue using fluorescence ratio imaging microscopy. Cancer Res 54:5670–5674
Ivanovic Z, Hermitte F, de la Grange PB, Dazey B, Belloc F, Lacombe F, Vezon G, Praloran V (2004) Simultaneous maintenance of human cord blood SCID-repopulating cells and expansion of committed progenitors at low O2 concentration (3%). Stem Cells 22:716–724
Chen EH, Olsen EN (2005) Unveiling the mechanisms of cell fusion. Science 308:369–373
Kudo Y, Boyd CA, Sargeant IL, Redman CW (2003) Hypoxia alters expression and function of syncytin and its receptor during trophoblast cell fusion of human placental BeWo cells: implications for impaired trophoblast syncytialisation in pre-eclampsia. Biochim Biophys Acta 1638:63–71
Bruick RK, McKnight SL (2001) A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294:1337–1340
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim AV, Hebestreit HF, Mukherji A, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ (2001) Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468–472
Bodin P, Burnstock G (1995) Synergistic effect of acute hypoxia on flow-induced release of ATP from cultured endothelial cells. Experientia 51:256–259
Morrison M, Turin L, King BF, Burnstock G, Arnett TR (1998) ATP is a potent stimulator of the activation and formation of rodent osteoclasts. J Physiol 511:495–500
Hoebertz A, Townsend-Nicholson A, Burnstock G, Arnett TR (2000) Expression of P2 receptors in bone and cultured bone cells. Bone 27:503–510
Hoebertz A, Meghji S, Burnstock G, Arnett TR (2001) Extracellular ADP is a powerful osteolytic agent: evidence for signaling through the P2Y1 receptor on bone cells. FASEB J 15:1139–1148
Hasegawa K, Ichiyama T, Isumi H (2003) NF-κB activation in peripheral blood mononuclear cells in neonatal asphyxia. Clin Exp Immunol 132:261–264
Lubec B, Labudova O, Hoeger H, Kirchner L, Lubec G (2002) Expression of transcription factors in the brain of rats with perinatal asphyxia. Biol Neonate 81:266–278
Simmons HA, Raisz LG (1991) Effects of acid and basic fibroblast growth factor and heparin on resorption of cultured fetal rat long bones. J Bone Miner Res 6:1301–1305
Nakagawa N, Yasuda H, Yano K, Mochizuki S, Kobayashi N, Fujimoto H, Shima N, Morinaga T, Chikazu D, Kawaguchi H, Higashio K (1999) Basic fibroblast growth factor induces osteoclast formation by reciprocally regulating the production of osteoclast differentiation factor and osteoclastogenesis inhibitory factor in mouse osteoblastic cells. Biochem Biophys Res Commun 265:158–163
Nakagawa M, Kaneda T, Arakawa T, Morita S, Sato T, Yomada T, Hanada K, Kumegawa M, Yakeda Y (2000) Vascular endothelial growth factor (VEGF) directly enhances osteoclastic bone resorption and survival of mature osteoclasts. FEBS Lett 473:161–164
Akeno N, Czyzyk-Krzeska MF, Gross TS, Clemens TL (2001) Hypoxia induces vascular endothelial growth factor gene transcription in human osteoblast-like cells through the hypoxia-inducible factor-2α. Endocrinology 142:959–962
Steinbrech DS, Mehrara BJ, Saadeh PB, Chin G, Dudziak ME, Gerrets RP, Gittes GK, Longaker MT (1999) Hypoxia regulates VEGF expression and cellular proliferation by osteoblasts in vitro. Plast Reconstr Surg 104:738–747
Steinbrech DS, Mehrara BJ, Saadeh PB, Greenwald JA, Spector JA, Gittes GK, Longaker MT (2000) VEGF expression in an osteoblast-like cell line is regulated by a hypoxia response mechanism. Am J Physiol 278:C853–C860
Steinbrech DS, Mehrara BJ, Saadeh PB, Greenwald JA, Spector JA, Gittes GK, Longaker MT (2000) Hypoxia increases insulin-like growth factor gene expression in rat osteoblasts. Ann Plast Surg 44:529–535
Warren SM, Steinbrech DS, Mehrara BJ, Saadeh PB, Greenwald JA, Spector JA, Buletreau PJ, Longaker MT (2001) Hypoxia regulates osteoblast gene expression. J Surg Res 99:147–155
Hill PA, Reynolds JJ, Meikle MC (1995) Osteoblasts mediate insulin-like growth factor-I and -II stimulation of osteoclast formation and function. Endocrinology 136:124–131
Fuller K, Lean JM, Bayley KE, Wani MR, Chambers TJ (2000) A role for TGFβ1 in osteoclast differentiation and survival. J Cell Sci 113:2445–2453
Acknowledgment
This work was carried out with the financial help of a grant from the Waltham Centre for Pet Nutrition (Waltham-on-the-Wolds, UK). M. A. H. is supported by a program grant from The Wellcome Trust. The authors are grateful for the expert advice and help of Timothy Arnett, Jenny Utting, and Andrea Brandao-Burch (University College London).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muzylak, M., Price, J.S. & Horton, M.A. Hypoxia Induces Giant Osteoclast Formation and Extensive Bone Resorption in the Cat. Calcif Tissue Int 79, 301–309 (2006). https://doi.org/10.1007/s00223-006-0082-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-006-0082-7