Abstract
Tail regeneration is a distinguishing feature of lizards; however, the mechanisms underlying tail regeneration remain elusive. Prostaglandin E2 (PGE2) is an arachidonic acid metabolite that has been extensively investigated in the inflammatory response under both physiological and pathological conditions. PGE2 also act as a regulator of hematopoietic stem cell homeostasis by interacting with Wnt signaling molecules. The present study aims to identify the effects of PGE2 on tail regeneration and the molecular mechanisms behind it. We initially found that PGE2 levels increased during the early stages of tail regeneration, accompanied by the up-regulated expression of cyclooxygenase 1 and cyclooxygenase 2. Next, we demonstrated that reduced PGE2 production leads to the retardation of tail regeneration. Subsequent experiments demonstrated that this effect is likely mediated by Wnt signaling, which proposing that the activation of the Wnt pathway is essential for the initiation of regeneration. The results showed that inhibition of PGE2 production could suppress Wnt activation and inhibit the proliferation of both epithelial and blastema cells. Furthermore, our findings indicated that forced activation of Wnt signaling could rescue the inhibitory effect of Cox antagonist on regeneration, suggesting a positive role of PGE2 on tail regeneration via a non-inflammatory mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Appendage regeneration is common in lower vertebrates, such as fish and amphibians (Alibardi 2018; Tanaka 2016). Among amniotes (reptiles and mammals), some lizards are capable of voluntarily shedding their tails to escape predation and subsequently regenerating a new tail, even though it is not a complete replica of the original one (Alibardi 2018; Simpson 1968; Zika 1969). Appendage regeneration involves a sequence of events involving wound healing, blastema formation, and the proliferation and differentiation of regenerated tissues, ultimately leading to the restoration of lost appendages (Jacyniak et al. 2017; Tanaka 2016). The cellular and molecular mechanisms underlying the initiation and progression of regeneration have been extensively investigated in zebrafish and axolotl (Haas and Whited 2017; Pfefferli and Jazwinska 2015). However, studies on regeneration in lizards are comparatively rare, which is partially due to the difficulty of genetic manipulation in reptiles. However, as an amniote model for regeneration, the mechanistic study of tail regeneration in lizards can not be ignored because lizard tail regeneration could be more closely related to regeneration in mammals. The morphologies of the initiation and progression stages of regeneration in reptiles have been reported in various species, including Anolis carolinensis (Zika 1969), Eublepharis macularius (Delorme et al. 2012; McLean and Vickaryous 2011), Podarcis muralis (Alibardi 2014), and Gekko japonicus (Zhou et al. 2013). Certain signaling molecules, such as TGF-beta, have also been implicated in wound healing in the leopard gecko (E. macularius) (Gilbert et al. 2016; Subramaniam et al. 2018). However, the cellular and molecular processes responsible for regeneration in reptiles remain elusive.
In the present study, we focused on investigating tail regeneration in G. japonicus. In our previous studies, we constructed the cDNA library (Liu et al. 2006) and sequenced the whole genome of G. japonicus (Liu et al. 2015). The comparative genomic analysis revealed specific genes that are undergoing positive selection for tail regeneration in G. japonicus and are thus likely to be important in tail regeneration. Prostaglandin endoperoxide synthase 1 (PTGS1), also known as cyclooxygenase 1 (COX1), has been proposed to be under positive selection. Prostaglandins are lipid-based bioactive molecules converted from arachidonic acid (AA) (Kawahara et al. 2015). COX is the key enzyme responsible for prostaglandin synthesis. Prostaglandin E2 (PGE2) is the most abundantly produced AA metabolite that has been extensively investigated in inflammation response. PGE2 is previously reported to be involved in acute inflammation as well as inflammatory immune diseases via eliciting vascular permeability, facilitating Helper T cells 1(Th1) differentiation and Helper T cells 17 (Th17) expansion (Kawahara et al. 2015). In recent years, PGE2 is also implicated in the regeneration of multiple tissues (Goessling et al. 2009; Tsujii et al. 1993; Zhang et al. 2015). The inhibition of prostaglandin-degrading enzyme 15-hydroxyprostaglandin dehydrogenase (15-PGDH) significantly potentiates tissue regeneration in multiple organs and strikingly points to the crucial role of PGE2 in regeneration. Sharma et al. reported the effect of PGE2 on the tail regeneration in Northern House gecko via COX inhibition (Sharma and Suresh 2008). However, the downstream targets of PGE2, and the mechanism underlying its observed effects have not been described.
In the present study, we first verified the effect of PGE2 on tail regeneration in G. japonicus. Furthermore, we investigated the potential signaling pathway involved in COX inhibition. Goessling et al. reported that PGE2 could alter Wnt-mediated regulation during hematopoietic stem cell (HSC) formation by inducing changes in β-catenin levels (Goessling et al. 2009). A study in Hemidactylus flaviviridis proposed that inhibition of cyclooxygenase-2 alters expression levels of some Wnt ligands in the regenerating tail (Buch et al. 2017). The Wnt pathway is the core signaling pathway involved in the initiation and progression of epimorphic regeneration processes, including fin regeneration in zebrafish, limb regeneration in axolotl (Kawakami et al. 2006) and tail regeneration in lizard (Vitulo et al. 2017). Therefore, we investigated whether PGE2 could modulate Wnt activation and affect tail regeneration in gecko.
Materials and methods
Animal treatment and tissue collection
Adult G. japonicus were housed in an air-conditioned room with controlled temperature (22–25 °C) and freely fed with mealworms and water in cages. All experimental protocols pertinent to the animals were given prior approval by the Laboratory Animal Care and Use Committee of the Nantong University. The tail autotomy of adult G. japonicus was performed according to previously published methods (Jiang et al. 2009). Briefly, caudatomy was conducted by inserting a nylon slipknot at the site of the sixth tail segment while pulling gently to mimic the conditions of autotomy in the natural environment of these lizards.
To evaluate the effects of activation or inhibition of Wnt pathway on tail regeneration, the N2-(2-(4-(2,4-dichlorophenyl)-5-(1H-imidazol-1-yl) pyrimidin-2-ylamino)ethyl)-5-nitropyridine-2,6-diamine (CHIR-98014) and inhibitor of Wnt production-4 (IWP-4) were applied respectively. Indomethacin was applied to evaluate the effect of COX inhibition on tail regeneration. CHIR-98014, IWP-4 and indomethacin were dissolved in 5% dimethylsulphoxide (DMSO) saline solution as stock solution. The concentration of stock solution of CHIR-98014, IWP-4 and indomethacin were 1 mM, 400 μM and 1 mM respectively. For the animal treatment, 50 μL stock solution of relevant reagents were injected intraperitoneally every 2 days. Control experiments were performed according to the same protocol with an equivalent amount of vehicle. To label the population of mitotically active cells in tail regenerates, 20 μL of 20 mM Brdu (5-bromo-2′-deoxyuridine, B5002, Sigma) diluted in phosphate-buffered saline (PBS) was injected intraperitoneally for 2 days before harvesting the regenerating tails. The harvested tails were fixed in 4% paraformaldehyde, and then dehydrated in a 10–30% sucrose gradient in PBS before being cut into 12-μm-thick slices. The Brdu labelled cells were counted and compared between the slices from the control and treated samples.
liquid chromatograph mass spectrometer (LC–MS) analysis
The PGE2 level in regenerated tails were determined at 0 day, 3 days, 7 days and 14 days post autotomy. For sample preparation, 0.2 cm tail tissue rostral to the plane of autotomy together with the regenerated tissue were collected for each gecko. To analyze the PGE2 level in each sample, 30 mg of tissue was added to a 400 μL mixture containing methanol and water (4/1, v/v, containing 0.01 mol/L butylated hydroxytoluene). Thereafter, the samples were ultrasonicated in ice water and centrifuged at 13,000 rpm at 4 °C for 15 min. Afterwards 200 μL of the supernatant was used for LC–MS analysis. The Waters Xevo® TQ-S mass spectrometry (MS) system with Waters ACQUITYTM UPLC chromatographic instrument control was used for the measurements. MassLynx4.1 (Waters Corporation, Milford, USA) software was used for data collection and analysis. The temperature of the Thermo Scientific Hypersil GOLD (2.1 mm × 100 mm, 1.9 µm) column was maintained at 40 °C, and separation was achieved using the following gradient: 70–70% B over 0 to 4 min, where A is 0.1% (v/v) formic acid aqueous solution and B is methanol (B). Multiple reaction monitoring (MRM) transition data were analyzed using MassLynx4.1 (Waters Corporation, Milford, USA) software using the default parameters. The standard curves were generated using a template based on the dilutions of PGE2 in methanol solution (1 mg/mL).
Western blotting
The COX1 and COX2 level in regenerated tails were determined at 0 day, 3 days, 7 days and 14 days post autotomy. The LEF1 and β-catenin level upon indomethacin treatment were evaluated at 14 days post autotomy. For sample preparation, 0.2 cm tail tissue rostral to the plane of autotomy together with the regenerated tissue were collected for each gecko. Total proteins from the regenerating tails were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were then transferred onto a polyvinylidene fluoride (PVDF) membrane and incubated separately with the following primary antibodies: COX1 (1:1000, ab13319, Abcam), COX2 (1:400, 12282, CST), LEF1 (1:250, HPA002087, Atlas Antibodies), and β-catenin (1:2500, ab32572, Abcam). β-actin (1:2000, 60008-1-Ig, Proteintech) was used as an internal control. Afterwards, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody, goat anti-rabbit IgG, or goat anti-mouse IgG at 25 °C for 2 h. The bands were visualized using chemiluminescence.
Hematoxylin–eosin (HE) staining
For hematoxylin and eosin (H&E) staining, the sliced tails were treated with HE staining kit (Solarbio, G1120). The slices were briefly rinsed with deionized water (ddH2O, 2 min), stained with Mayer’s hematoxylin (3 min), and subsequently rinsed in running water (5 min) and 0.5% concentrated HCl in 75% ethanol for 30 s. After washing in water for 30 min, the samples were stained in eosin (30 s) and subsequently dehydrated with 95% ethanol (1 min). Prior to overslipping, Samples were washed thrice with absolute ethanol (1 min each) and washed thrice with xylene (5 min each).
Immunofluorescence assay
Samples were permeabilized with 1% Triton X-100 and subsequently blocked with 10% bovine serum albumin in PBS. Afterwards, samples were incubated overnight at 4 °C with primary antibodies rabbit anti-catenin (1:200, ab32572, Abcam) or mouse anti-Brdu (1:200, 5292, Cell Signaling Technology). For detection of Brdu-labeled cells, samples were incubated in 1.2 M HCL in PBS for 20 min at 37 °C and rinsed in 0.02 M sodium tetra borate for 10 min at 25 °C before being permeabilized. The lizard tail slices were incubated with secondary antibodies goat anti-rabbit IgG (FITC) or goat anti-mouse (cy3) for 2 h at 25 °C. All samples were counterstained with Hoechst (Byotime biotechnology). Signals were visualized using fluorescent microscopy (Zeiss) or confocal microscopy (Leica).
Results
Early regeneration after tail autotomy in Gekko japonicus
Tail regeneration is a well-known phenomenon in lizards, and the morphological changes involved have been extensively characterized for various species, such as A. carolinensis, Podarcis sicula, and E. macularius. The gross view of the regenerated tail of G. japonicus is similar to that of A. carolinensis and E. macularius (McLean and Vickaryous 2011), and we defined the early stage of regeneration based on the variations in the morphological features (Fig. 1). Stage I (~ 0 to 6 days) is the earliest stage and comprises sequential occurred events, including bleeding, exposure of autotomized vertebra, retraction of adjacent tissues, formation of exudate clot, and proliferation of epithelium. To distinguish the regeneration process in a simple manner, we termed this stage based on the presence of a featured scab while ignoring other more complex events (Fig. 1a, b). Stage II (~ 7 to 11 days) was a period of rapid increase in cell population that begins with detachment of scab (Fig. 1c), subsequent exposure of the epithelium, and the formation of a dome-shaped regenerated tail (Fig. 1d). The length of regenerated tail is also an important index of regeneration stage, and length was normalized by the ratio of length to the diameter of amputation plane of tail in individual gecko. In stage II, normalized length is less than 0.5. Stage III (~ 12 to 16 days) is marked by a dome-shaped tail (normalized length is greater than 0.5 and less than 1) as showed in Fig. 1e, f. We categorized the early period of regeneration into three stages with the aim of documenting and analyzing the regeneration state in a simple manner through gross observation of the regenerated tails under normal conditions or following specific treatments. However, the changes in the wound epithelium and the blastema are more complicated than the events involved in the stage categorization. Actually, the wound epithelium continues to proliferate and subsequently thickens during these stages as denoted in Fig. 1g–j.
The early stage of regeneration after tail autotomy in G. japonicus. a–f Six individuals were used for the tail autotomy. The gross view and morphological observation were applied to define the stage of regeneration. The early periods of regeneration were categorized into three stages. a, b Stage I (~ 0 to 6 days) is characterized by the presence of a covered scab. c, d Stage II (~ 7 to 11 days) is defined based on the normalized length of regenerated tail. The normalized length was ratio of the length of regenerated tail to the diameter of amputation plane of tail in individual gecko. In stage II, normalized length is less than 0.5. e, f Stage III (~ 12 to 16 days), the normalized length is greater than 0.5 and less than 1. g–l During these stages, a blastema mass becomes visible in the epithelium. The epithelium at 3 days (g–i) and 7 days (j–l) are shown. H is the enlarged view of g and j is the enlarged view of I
PEG2 levels are up regulated during the early stage of tail regeneration
PGE2 has been described to augment the regeneration of multiple tissues by facilitating the expansion of several types of tissue stem cells. Tail regeneration is a process that accompanies rapid expansion of stem/progenitor cells (Ho et al. 2017; Truntipakorn et al. 2017). In this study, we asked whether PGE2 is involved in tail regeneration in gecko. We first evaluated PGE2 levels at 0, 3, 7, and 14 days after tail amputation. Results showed that PGE2 levels continuously increased and peaked at 14 days (Fig. 2a), which proposed PGE2 might be a vital molecule for the tail regeneration for gecko. We further detected the expression level of cyclooxygenase (COX) that is essential for PGE2 production. Cyclooxygenase has two isoforms, namely, COX1 and COX2, also known as prostaglandin-endoperoxide synthase (PTGS), which are responsible for the synthesis of prostanoids. Both the expression levels of COX1 and COX2 evaluated in intact and regenerated tail tissues. Usually, the COX1 is constitutive isoform in tissues and the COX2 is inducible isoform upon stimulus. However, the results of western blotting showed that COX1 and COX2 were both present in the intact and regenerated tail. After tail autotomy, both the expression levels of two isoforms were elevated during regeneration (Fig. 2b–d). COX1 level was continually increased, while the COX2 increased dramatically at 3 days and maintained a high level in the rest time. The expression of COX1 and COX2 after tail autotomy were also reported in another species H. flaviviridis (Buch et al. 2018). The results highlighted potential effect of PGE2 on wound healing and blastema formation.
PEG2 levels were up regulated during the early stage of tail regeneration. a PGE2 level were determined at 0 day, 3 days, 7 days and 14 days post autotomy by LC–MS analysis, and six individuals were used for each time point. PGE2 levels continuously increased and reached the peak levels at 14 days. b–d The expression level of COX1 and COX2, which are responsible for the synthesis of PGE2 precursor were determined by Western blotting at 0 day, 3 days, 7 days and 14 days post autotomy. Three individuals were used for each time point. The results showed that the expression of both COX1 and COX2 were up-regulated in regenerated tail tissues. Data were analyzed by ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001 versus control
COX inhibition decreased PGE2 levels and inhibited tail regeneration
To evaluate the effects of PGE2 levels on the regeneration process, we attenuated PGE2 production by limiting the enzyme required for the synthesis of prostaglandin and further observed whether regeneration was affected. The exogenous administration of indomethacin, an inhibitor of both COX-1 and COX-2, dramatically reduced PGE2 levels in the regenerated tissues (Fig. 3b), and delayed the progression of regeneration (Fig. 3c) as expected. In control group, the number of geckos progressed into stage I, II and III were 3, 4 and 22 out of 29 individuals at 14 days after tail autotomy, while the number in stage I, II and III after indomethacin treatment were 18, 8 and 2 out of 28 individuals (Fig. 3c). These data indicated a positive role of PGE2 on regeneration. PGE2 is a well-known molecule to promote inflammatory response, while our previous study has revealed that the inflammation of regenerated tissues after tail autotomy is limited in gecko (Dong et al. 2013). Therefore, the continually increased PGE2 might have alternative pathway to regulated regeneration. The robust proliferation of epithelial and blastema cells is a prominent feature during the early stages of regeneration. We evaluated whether PGE2 contributed to cell proliferation during tail regeneration. Results of the Brdu incorporation assay at 14 days post autotomy revealed that indomethacin administration (Fig. 3h–j) significantly reduced the number of Brdu-labeled cells in both epithelial and blastema cells compared with that in control (DMSO) group (Fig. 3e–g), indicating that PGE2 is essential for the rapid cell expansion during the early stage of regeneration.
COX inhibition retarded regeneration and decreased PGE2 levels. a Schematic diagram of experimental design showed the time points for the indomethacin treatment and Brdu incorporation. b PGE2 levels were evaluated at 14 days post autotomy after indomethacin treatment by LC–MS analysis. Six geckos were used for control (DMSO) and indomethacin (Indo) group respectively. The result showed that exogenous administration of indomethacin significantly reduced PGE2 levels in the regenerated tissue. Data were analyzed by Student’s t test. **p < 0.01 versus control. c To evaluate the effect of indomethacin on tail regeneration, 29 and 28 geckos were used for control and indomethacin group, and categorization of regeneration stage after 14 days after autotomy indicated that PGE2 inhibition led to the delayed tail regeneration. Data were analyzed by χ2 test. ***p < 0.001 versus control. d–j COX inhibition decreased proliferation of epithelium and blastema cells. To evaluate the effect of COX on cell proliferation, Brdu-labeled cells were determined at 14 days post tail autotomy in control (DMSO) and indomethacin (Indo) group. f and i were enlarged view of e and h; g and j were enlarged view of f and i. The wounding epithelium (WE) was the area between dashed line and full line. The results showed that indomethacin treatment significantly reduced Brdu incorporation into both wound epithelial and blastema cells. Data were analyzed by Student’s t test. *p < 0.05 versus control
Wnt activation is essential for tail regeneration in geckos
We further aimed to identify the molecular mechanism behind the positive effects of PEG2 production on the regeneration process. The Wnt signaling pathway has been reported to be able to interact with PGE2 signaling molecules to regulate the developmental specification and repopulation of hematopoietic stem cells (Goessling et al. 2009). Wnt is a core signaling molecule for the regeneration of multiple tissues, including appendage regeneration in zebrafish, limb regeneration of axolotl (Kawakami et al. 2006; Wehner and Weidinger 2015) and tail regeneration in lizard (Vitulo et al. 2017; Hutchins et al. 2014). Then, we investigated whether Wnt pathway is activated during regeneration and whether the activation is essential for tail regeneration in geckos. We first detected the β-catenin signal in the regenerated tissues. The β-catenin accumulation and nuclear translocation are required for the activation of classical Wnt pathway. The results showed that β-catenin signal could be observed at 10 days post autotomy (Fig. 4a–c), and the nuclear localized β-catenin was also observed (Fig. 4c), which indicated that β-catenin dependent classical Wnt pathway was activated during regeneration. Then, we further applied IWP-4, a potent Wnt antagonist (Chen et al. 2009) to the geckos and evaluated the effect of IWP-4 on tail regeneration at 10 days after tail autotomy. The results demonstrated that IWP-4 treatment notably hampered the regeneration process (Fig. 4d) supporting the point that Wnt activation is essential for tail regeneration in G. japonicus. We further detected whether the IWP-4 treatment decreased the β-catenin levels after tail autotomy. The data showed that IWP-4 treatment significantly reduced β-catenin level during regeneration.
Wnt activation is essential for tail regeneration. a, c β-catenin accumulation and nuclear translocation were observed at 10 days post autotomy. The wounding epithelium (WE) was defined as the area between dashed line and full line. The arrowhead indicated the nuclear location of β-catenin. B was the enlarged view of a, and c was the enlarged view of b. d IWP-4 (inhibitor of Wnt signaling pathway) was used to determine the effect of Wnt signal on tail regeneration. The regeneration stage were observed at 10 days after autotomy. In control (DMSO) group, nine were in stage I and 25 were progressed in stage II, while in IWP-4 group, 25 were in stage I and 8 were in stage II. The results showed that exogenous administration of indomethacin hampered tail regeneration. Data were analyzed by χ2 test. **p < 0.01 versus control. e–j Significant accumulation of β-catenin in the wound epithelium (WE) was observed at 10 days after tail autotomy, and the β-catenin level reduced after IWP-4 treatment. f and i were enlarged view of e and h, g and j were enlarged view of f and i
COX inhibition attenuated Wnt activation during regeneration
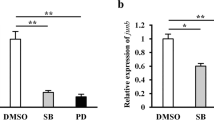
PGE2 signal is crucial for tail regeneration. However, the mechanisms through which PGE2 affects regeneration remain elusive. The Wnt pathway is a potential target for PGE2 regulation, which indicated in the study of regeneration of hematopoietic stem cells (Goessling et al. 2009). We had performed a COX inhibition assay and found that regeneration delayed upon COX treatment. We further investigated whether Wnt signaling activation responded to indomethacin treatment. Results of immunofluorescence assay showed that the β-catenin signals significantly reduced in epithelial cells relative to the control cells at 14 days after tail autotomy (Fig. 5a–f). Furthermore, results of the western blot assay at the same time point revealed that β-catenin levels were also decreased in these cells (Fig. 5g, i). In addition, the expression levels of lymphoid enhancer-binding factor 1 (LEF1), a downstream transcription factor responding to β-catenin dependent Wnt pathway, was also reduced upon indomethacin treatment (Fig. 5h, j), suggesting that Wnt pathway activation was significantly inhibited when COX activity was blocked. Our findings indicated that the positive effects of PGE2 on tail regeneration are likely mediated by β-catenin dependent Wnt pathway. To verify the above hypothesis, we tested whether the forced activation of Wnt could attenuate COX inhibition-induced delay in regeneration. CHIR-98104 is a specific GSK3β inhibitor that can increase β-catenin and activate the classical Wnt pathway (Moore et al. 2013). We, therefore, evaluated the regeneration state of the control, indomethacin, and indomethacin + CHIR-98104 groups at 14 days post autotomy. In control group, the number of geckos progressed into stage I, II and III were 3, 7 and 19 out of 29 individuals at 14 days after tail autotomy. However, the number in stage I, II and III after indomethacin treatment were 14, 12 and 5 out of 31 individuals. The treatment of Wnt agonist successfully rescued the COX inhibition-induced delay in regeneration, led to the number of geckos in stage I, II and III as 5, 6 and 21 respectively, indicating the important role of the PGE2-Wnt signaling axis in G. japonicus tail regeneration (Fig. 5k).
COX inhibition attenuated Wnt activation during regeneration. a–f β-catenin signals were detected in regenerated tail after treatment of indomethacin by immuofluorescence assay. β-catenin were presented in both the wound epithelium (WE) and blastema, and the signal significantly reduced in the epithelial cells of indomethacin (Indo) group relative to those in the control (DMSO) group at 14 days after tail autotomy. The WE was defined as the area between dashed line and full line. b and e were enlarged view of a and e, c and f were enlarged view of b and e. g–j To further investigate the effect of COX inhibition on Wnt pathway, the β-catenin and LEF1 levels were investigated after indomethacin treatmen by Western blot assay at 14 days after tail autotomy. The results revealed that both β-catenin and LEF1were down-regulated following COX inhibition. Data were analyzed by Student’s t test. **p < 0.01 versus control. k To test whether the forced activation of Wnt pathway could rescue the retard of regeneration mediated by COX inhibition, CHIR-98104 was applied together with the indomethacin treatment. The number of geckos used in control (DMSO), indomethacin (Indo) and rescue (CHIR+Indo) group were 29, 31 and 32 respectively, and the regeneration stage was analyzed at 14 days after tail autotomy. The results showed that CHIR-98104 successfully rescued COX inhibition-induced delay in regeneration. Data were analyzed by χ2 test. **p < 0.001 versus control
Discussion
PGE2 was previously considered as a lipid signaling molecule that could mediate inflammatory responses under physiological and pathological conditions (Kawahara et al. 2015). In recent years, PGE2 was also reported to act as a mediator for potentiating regeneration in multiple tissues of mice (Zhang et al. 2015). The stimulatory role of PGE2 in regeneration was also proposed in tail regeneration of H. flaviviridis (Sharma and Suresh 2008; Buch et al. 2017). The present study revealed that PGE2 was essential for the tail regeneration of G. japonicus, and the underlying mechanism might be associated with the interaction between PGE2 and the molecules of Wnt pathway during tail regeneration.
PGE2 is converted from PGH, which is synthesized by COX-1 or COX-2, COX1 is ubiquitously expressed, and COX2 is usually regarded as an injury-induced enzyme for prostaglandin synthesis. The current findings revealed that COX1 and COX2 levels were both up regulated following tail autotomy, indicating an increased PGE2 levels was maintained during the wound re-epithelia and blastema formation. The administration of exogenous PGE2 has been shown to facilitate the wound healing response by stimulating epithelial cell migration (Carolina et al. 2018). Our study demonstrated that PGE2 promoted the proliferation of wound epithelium and blastema cells, which is an important finding of the molecular mechanisms underlying appendage regeneration. Given that the PGE2 receptors includes EP1, EP2, EP3, and EP4, the expression profiles of these receptors in cell type-specific resolution should be verified to shed light on the cellular mechanisms underlying regeneration. Although PGE2 is the most abundant AA metabolite, there is the alternative possibility that other AA metabolites, such as PGI2, PGF2, and PGD2 (Smith et al. 1991), could additionally play specific roles in regeneration. The inhibition of COX activity could decrease not only PGE2 levels, but also the levels of the other AA metabolites. Therefore, a pertinent metabolomics investigation on the AA metabolite is an interesting topic for future work.
The Wnt pathway is a core signaling axis for development and regeneration, and the essential roles of the Wnt pathway are elicited in the regeneration of zebrafish fins, axolotl limbs and lizard tail. Hutchins et.al reported the differentially expressed genes of Wnt pathway in the 25 dpa regenerating tail of A. carolinensis (Hutchins et al. 2014). A comparative transcriptomes analysis of the regenerating tail vs. the scarring limb in P. muralis further revealed that Wnt signals including Wnt6, Wnt10 were exclusively up regulated in regenerating tail while not in scarring limb (Vitulo et al. 2017). In addition, a study for the tail regeneration of P. muralis indicated that Wnt1 is present in the apical region of the blastema and around the regenerating spinal cord (Alibardi 2017). Here, we report that Wnt activation is also a critical signal for tail regeneration in gecko, and that Wnt activation was inhibited following indomethacin treatment. More importantly, the indomethacin-mediated retardation of regeneration could be rescued by treatment of Wnt agonist, suggesting an interaction between PGE2 and the Wnt pathway in tail regeneration. The detailed molecular mechanism underlying this interaction was not revealed in present work. An earlier study in stem cells showed that PGE2 could modulate the Wnt pathway by altering the cellular levels of β-catenin (Castellone et al. 2005). In our study, β-catenin signals presented in both epithelial and blastema cells, and a significant accumulation of β-catenin signal was observed in keratinocytes. However, most BrdU positive cells were basal epidermal cells and suprabasal keratinocytes, which showed relative weak β-catenin signal. This observation is similar with that in fin regeneration of zebra fish. Their results showed that Wnt signaling in the nonproliferative distal blastema is required for cell proliferation in the proximal blastema (Wehner et al. 2014). The beta-catenin immune-staining in regenerating lizard epidermis and scales were previously described in A. carolinensis (Wu et al. 2014). Their finding for the localization of β-catenin and BrdU signals in regenerated tail at 15 days after autotomy were consistent with that of our data. These data indicated a sophisticated role of Wnt signals in regenerated tissues. A more meticulous expression profile with detailed spatial and temporal information on the Wnt activated cells could improve our understanding of the basic principles and pathways underlying caudal regeneration.
References
Alibardi L (2014) Immunolocalization of nestin in the lizard Podarcis muralis indicates up-regulation during the process of tail regeneration and epidermal differentiation. Ann Anat 196(2–3):135–143. https://doi.org/10.1016/j.aanat.2013.12.004
Alibardi L (2017) Wnt-1 immunodetection in the regenerating tail of lizard suggests it is involved in the proliferation and distal growth of the blastema. Acta Histochem 119(3):211–219. https://doi.org/10.1016/j.acthis.2017.01.001
Alibardi L (2018) Perspective: appendage regeneration in amphibians and some reptiles derived from specific evolutionary histories. J Exp Zool B Mol Dev Evol. https://doi.org/10.1002/jez.b.22835
Buch PR, Sarkate P, Uggini GK, Desai I, Balakrishnan S (2017) Inhibition of cyclooxygenase-2 alters Wnt/beta-catenin signaling in the regenerating tail of lizard Hemidactylus flaviviridis. Tissue Eng Regen Med 14(2):171–178. https://doi.org/10.1007/s13770-017-0037-2
Buch PR, Desai I, Balakrishnan S (2018) COX-2 activity and expression pattern during regenerative wound healing of tail in lizard Hemidactylus flaviviridis. Prostaglandins Other Lipid Mediat 135:11–15. https://doi.org/10.1016/j.prostaglandins.2018.01.002
Carolina E, Kato T, Khanh VC, Moriguchi K, Yamashita T, Takeuchi K et al (2018) Glucocorticoid impaired the wound healing ability of endothelial progenitor cells by reducing the expression of CXCR6 in the PGE2 pathway. Front Med (Lausanne) 5:276. https://doi.org/10.3389/fmed.2018.00276
Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS (2005) Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science 310(5753):1504–1510. https://doi.org/10.1126/science.1116221
Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW et al (2009) Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 5(2):100–107. https://doi.org/10.1038/nchembio.137
Delorme SL, Lungu IM, Vickaryous MK (2012) Scar-free wound healing and regeneration following tail loss in the leopard gecko Eublepharis macularius. Anat Rec (Hoboken) 295(10):1575–1595. https://doi.org/10.1002/ar.22490
Dong Y, Gu Y, Huan Y, Wang Y, Liu Y, Liu M et al (2013) HMGB1 protein does not mediate the inflammatory response in spontaneous spinal cord regeneration: a hint for CNS regeneration. J Biol Chem 288(25):18204–18218. https://doi.org/10.1074/jbc.M113.463810
Gilbert RWD, Vickaryous MK, Viloria-Petit AM (2016) Signalling by transforming growth factor beta isoforms in wound healing and tissue regeneration. J Dev Biol 4(2):E21. https://doi.org/10.3390/jdb4020021
Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL et al (2009) Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136(6):1136–1147. https://doi.org/10.1016/j.cell.2009.01.015
Haas BJ, Whited JL (2017) Advances in decoding axolotl limb regeneration. Trends Genet 33(8):553–565. https://doi.org/10.1016/j.tig.2017.05.006
Ho ATV, Palla AR, Blake MR, Yucel ND, Wang YX, Magnusson KEG et al (2017) Prostaglandin E2 is essential for efficacious skeletal muscle stem-cell function, augmenting regeneration and strength. Proc Natl Acad Sci USA 114(26):6675–6684. https://doi.org/10.1073/pnas.1705420114
Hutchins ED, Markov GJ, Eckalbar WL, George RM, King JM, Tokuyama MA et al (2014) Transcriptomic analysis of tail regeneration in the lizard Anolis carolinensis reveals activation of conserved vertebrate developmental and repair mechanisms. PLoS ONE 9(8):e105004. https://doi.org/10.1371/journal.pone.0105004
Jacyniak K, McDonald RP, Vickaryous MK (2017) Tail regeneration and other phenomena of wound healing and tissue restoration in lizards. J Exp Biol 220(Pt 16):2858–2869. https://doi.org/10.1242/jeb.126862
Jiang M, Gu X, Feng X, Fan Z, Ding F, Liu Y (2009) The molecular characterization of the brain protein 44-like (Brp44l) gene of Gekko japonicus and its expression changes in spinal cord after tail amputation. Mol Biol Rep 36(2):215–220. https://doi.org/10.1007/s11033-007-9169-0
Kawahara K, Hohjoh H, Inazumi T, Tsuchiya S, Sugimoto Y (2015) Prostaglandin E2-induced inflammation: relevance of prostaglandin E receptors. Biochim Biophys Acta 1851(4):414–421. https://doi.org/10.1016/j.bbalip.2014.07.008
Kawakami Y, Rodriguez Esteban C, Raya M, Kawakami H, Marti M, Dubova I et al (2006) Wnt/beta-catenin signaling regulates vertebrate limb regeneration. Genes Dev 20(23):3232–3237. https://doi.org/10.1101/gad.1475106
Liu Y, Ding F, Liu M, Jiang M, Yang H, Feng X et al (2006) EST-based identification of genes expressed in brain and spinal cord of Gekko japonicus, a species demonstrating intrinsic capacity of spinal cord regeneration. J Mol Neurosci 29(1):21–28
Liu Y, Zhou Q, Wang Y, Luo L, Yang J, Yang L et al (2015) Gekko japonicus genome reveals evolution of adhesive toe pads and tail regeneration. Nat Commun 6:10033. https://doi.org/10.1038/ncomms10033
McLean KE, Vickaryous MK (2011) A novel amniote model of epimorphic regeneration: the leopard gecko Eublepharis macularius. BMC Dev Biol 11:50. https://doi.org/10.1186/1471-213x-11-50
Moore SF, van den Bosch MT, Hunter RW, Sakamoto K, Poole AW, Hers I (2013) Dual regulation of glycogen synthase kinase 3 (GSK3)alpha/beta by protein kinase C (PKC)alpha and Akt promotes thrombin-mediated integrin alphaIIbbeta3 activation and granule secretion in platelets. J Biol Chem 288(6):3918–3928. https://doi.org/10.1074/jbc.M112.429936
Pfefferli C, Jazwinska A (2015) The art of fin regeneration in zebrafish. Regeneration (Oxf) 2(2):72–83. https://doi.org/10.1002/reg2.33
Sharma P, Suresh S (2008) Influence of COX-2-induced PGE2 on the initiation and progression of tail regeneration in Northern House Gecko Hemidactylus flaviviridis. Folia Biol (Praha) 54(6):193–201
Simpson SB Jr (1968) Morphology of the regenerated spinal cord in the lizard Anolis carolinensis. J Comp Neurol 134(2):193–210. https://doi.org/10.1002/cne.901340207
Smith WL, Marnett LJ, DeWitt DL (1991) Prostaglandin and thromboxane biosynthesis. Pharmacol Ther 49(3):153–179
Subramaniam N, Petrik JJ, Vickaryous MK (2018) VEGF, FGF-2 and TGFbeta expression in the normal and regenerating epidermis of geckos: implications for epidermal homeostasis and wound healing in reptiles. J Anat 232(5):768–782. https://doi.org/10.1111/joa.12784
Tanaka EM (2016) The molecular and cellular choreography of appendage regeneration. Cell 165(7):1598–1608. https://doi.org/10.1016/j.cell.2016.05.038
Truntipakorn A, Makeudom A, Sastraruji T, Pavasant P, Pattamapun K, Krisanaprakornkit S (2017) Effects of prostaglandin E2 on clonogenicity, proliferation and expression of pluripotent markers in human periodontal ligament cells. Arch Oral Biol 83:130–135. https://doi.org/10.1016/j.archoralbio.2017.07.017
Tsujii H, Okamoto Y, Kikuchi E, Matsumoto M, Nakano H (1993) Prostaglandin E2 and rat liver regeneration. Gastroenterology 105(2):495–499
Vitulo, N., Dalla Valle, L., Skobo, T., Valle, G., & Alibardi, L. (2017). Transcriptome analysis of the regenerating tail vs. the scarring limb in lizard reveals pathways leading to successful vs. unsuccessful organ regeneration in amniotes. Dev Dyn 246(2):116–134
Wehner D, Weidinger G (2015) Signaling networks organizing regenerative growth of the zebrafish fin. Trends Genet 31(6):336–343. https://doi.org/10.1016/j.tig.2015.03.012
Wehner D, Cizelsky W, Vasudevaro MD, Ozhan G, Haase C, Kagermeier-Schenk B et al (2014) Wnt/beta-catenin signaling defines organizing centers that orchestrate growth and differentiation of the regenerating zebrafish caudal fin. Cell Rep 6(3):467–481. https://doi.org/10.1016/j.celrep.2013.12.036
Wu P, Alibardi L, Chuong CM (2014) Regeneration of reptilian scales after wounding: neogenesis, regional difference, and molecular modules. Regeneration (Oxf) 1(1):15–26. https://doi.org/10.1002/reg2.9
Zhang Y, Desai A, Yang SY, Bae KB, Antczak MI, Fink SP et al (2015) TISSUE REGENERATION. inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science 348(6240):aaa2340. https://doi.org/10.1126/science.aaa2340
Zhou Y, Xu Q, Li D, Zhao L, Wang Y, Liu M et al (2013) Early neurogenesis during caudal spinal cord regeneration in adult Gekko japonicus. J Mol Histol 44(3):291–297. https://doi.org/10.1007/s10735-012-9466-3
Zika JM (1969) A histological study of the regenerative response in a lizard Anolis carolinensis. J Exp Zool 172(1):1–8. https://doi.org/10.1002/jez.1401720102
Funding
This study was supported by the National Natural Science Foundation of China (Grant Nos. 31472004 and 31071874), and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, M., Wang, T., Li, W. et al. PGE2 facilitates tail regeneration via activation of Wnt signaling in Gekko japonicus. J Mol Hist 50, 551–562 (2019). https://doi.org/10.1007/s10735-019-09847-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-019-09847-7