Abstract
Myocardial dysfunction is a major cause of death in sepsis. MicroRNA-146b (miR-146b) has been reported to be related to myocardial disease. However, the role of miR-146b in sepsis as well as myocardial injury is still unclear. Septic cardiac dysfunction in mice was induced by cecal ligation and puncture (CLP) and miR-146b was found increased significantly in the myocardium tissue of CLP mice. It was found that up-regulation of miR-146b by agomiR injection suppressed expression of IL-1β in mice as well as myocardium apoptosis in CLP mice. However, suppression of miR-146b by antagomiR injection had inverse effects. Notch1 was identified as a target gene of miR-146b by bioinformatics analysis. And it was verified that in cardiomyocytes, the decrease of miR146b led to increase of both the mRNA and protein level of Notch1 and vice versa. In septic mice serum stimulated cardiomyocytes, up-regulation of miR-146b decreased the level of Notch1 and Hes1. The knockout of Notch1 in transgenic mice showed that the deficiency of Notch1 improved myocardial injury induced by CLP operation. The apoptosis of cardiomyocytes was relieved and the expression of IL-1β was decreased. In conclusion, miR-146b targets to Notch1 and protected cardiomyocytes against inflammation and apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is characterized as a systemic inflammatory response to severe infection and a progressive organ damage (Vincent et al. 2013). It affects more than 19 million people every year and is the leading cause of death in critical ill patients all over the world(Fleischmann et al. 2016; Hotchkiss et al. 2013). Cardiac dysfunction induced by sepsis is a closely associated with increased mortality (Chung et al. 2017; Landesberg et al. 2012). Sepsis patients experiencing cardiac dysfunction have a 70–90% mortality rate, while patients without cardiac dysfunction experience only a 20% mortality rate (Merx and Weber 2007; Parrillo et al. 1990). It is important to know the specific mechanism of the cardiac dysfunction during sepsis. There are two important mechanisms that responsible for septic cardiac dysfunction, uncontrolled immune and inflammatory responses.
MicroRNAs (miRs) are small noncoding RNAs on average 22 nucleotides long, which post-transcriptionally regulate gene expressions with important roles in numerous cellular processes. About half of the human transcriptome were regulated by miRs (Huntzinger and Izaurralde 2011). MiRs negatively regulate gene expression by targeting the 3′untranslated region (UTR) of specific mRNA for transcriptional degradation or translational repression (Krol et al. 2010; Lim et al. 2005). MiR-146a has been reported to have an effect in sepsis (Song et al. 2017; Wang et al. 2010a, 2013). And it has also been reported that the inhibition of miR-146b may increase hypoxia-induced cardiomyocytes apoptosis (Li et al. 2015). The specific mechanism is still unknown.
In this experiment, we focused on the level changes of miR-146b in sepsis as well as the relationship between miR-146b and myocardial injury induced by sepsis. We demonstrated that inhibition of miR-146b increased the apoptosis of cardiomyocytes and Notch1 acted as the downstream gene of it participating in sepsis induced cardiac dysfunction.
Material and method
Animals
Healthy 40 adult male C57BL/6 mice weighing 20–25 g and 8 myocardium specific Notch1-/- mice were included in this study. All animals were provided by Experimental Animal Center of The Fourth Military Medical University. All animals were housed separately and kept under standard conditions at room temperature (22–24 °C) under a 12:12 h light/dark cycle. All of the protocols were carried out in accordance with the approved guidelines in the ethical permit approved by the Ethics Committee of Xijing Hospital, affiliated with the Fourth Military Medical University (XJYYLL-2016209).
Reagents
Antibodies against Bcl-2, Bax, Notch1 and β-actin were purchased from Cell Signaling Technology (Beverly, MA, USA). The rabbit anti-goat, goat anti-mouse, and goat anti-mice secondary antibodies were purchased from Beyotime Institute of Biotechnology (Jiangsu, China).
Septic mouse model
The cecal ligation and puncture (CLP) was used to establish a septic mouse model as described in other literatures (Rittirsch et al. 2009). Briefly, mice were anesthetized with 0.1% pentobarbital sodium. The exposed cecum was ligated and then punctured two times with a 22 g needle in the middle and some feces were pressed out to ensure patency of the puncture. Then returned the bowel loops and closed the abdominal wall with 4–0 silk suture. Postoperatively, 1 ml of 0.9% saline was injected subcutaneously. In the sham group, cecum of mice was exposed and then returned without puncture and ligation and 1 ml 0.9% saline was also injected subcutaneously. The mortality rate of C57BL/6 mice in 72 h after CLP was approximately 60–80% in the experiment. Then tail vein intravenous injections of miR-146b agomiRs or their controls (NC agomiRs) at a dose of 30 mg/kg body weight to increase miR-146b before CLP operation. Inhibition of miR-146b was carried out by tail vein intravenous injections of miR-146b antagomiRs or their controls (NC antagomiRs) at a dose of 80 mg/kg body weight for three consecutive days before septic serum stimulation. The septic serum was acquired from CLP mice 48 h after surgery and then added into the dishes to form a concentration of 10% serum. The effects of miR-146b agomiR or antagomiR treatment were confirmed by measuring miR-146b level in myocardium through RT-PCR.
Septic cardiomyocytes model
Mouse cardiomyocytes were purchased from Sciencell, US. Cells were cultured in DMEM medium supplemented with 10% FBS (Gibco, US), 100U/mL penicillin, and 100U/mL streptomycin in a humidified 5% CO2, 95% air atmosphere. Cardiomyocytes (60–70%) were incubated for another 12 h in serum-depleted medium before stimulation. Twenty 60 mm dishes of cardiomyocytes were randomly divided into four groups (n = 4).
Blood and tissue acquirement and cytokines test
Blood was acquired 24 h after CLP or sham operation from left ventricle of mice under anesthesia for the measurements of IL-1β through a microplate reader (Infinite 200 PRO, Tecan, Switzerland). Myocardium tissue was obtained at the same time for pathological examination through H&E staining.
Quantitative real-time PCR (q-RT-PCR)
Total RNAs of cultured cells were extracted using RNA-isolation kit (Takara, Japan). 500 ng of total RNA was reverse-transcribed using Prime Script™ RT reagent Kit (Takara, Japan). The obtained cDNA was then amplified by the CFX Connect real-time system (BIO-RAD, US), using SYBR™ Premix Ex Taq™ Kit (Takara, Japan) with specific primers. The PCR conditions were 95 °C for 30 s, followed by 40 cycles of 95 °C for 30 s, 60℃for 10 s, and elongation at 72 °C for 15 s. The relative expression calculated using the 2 − ΔΔCT method was performed to analyze the results, with GAPDH as an internal control. The method to quantify miRNAs was performed by stem-loop RT-PCR. MiRNAs and reverse primers were put at 65 °C for 5 min to form highly target-specific stem-loop structure. Then reverse transcriptase, RNase inhibitor, dNTPs and buffer were added for reverse transcription. Primer oligonucleotides were synthesized by Invitrogen. MicroRNA Reverse Transcription Kit and TaqMan MicroRNA assay kits (Takara, Japan) were used for qRT-PCR determination of miR-146b, performed on CFX96 Real Time PCR Detection System (Bio-Rad, Hercules, CA, USA). U6 snRNA was used as the internal control to normalize sample input. The primer sequences for each gene were listed as follows: GAPDH-F: 5′-GTGTTCCTACCCCCAATGTG-3′, GAPDH-R: 5′-CATCGAAGGTGGAAGAGTGG-3′, Notch1-F: 5′-TGACAACTCCTACCTCTGCTTATG-3′, Notch1-R: 5′-GGTTCACAGGCACATTCGTA-3′, Hes1-F: 5′-GGTCTACACCAGCAACAGTG-3′, Hes1-R:5′-GGGCTAGGGACTTTACGGGT-3′, miR-146b:5′-TGAGAACTGAATTCCATAGGC-3′, u6: 5′-GTGCTCGCTTCGGCAGCACATAT-3′. The agomiR and antagomiR of miR-146b and the control fragment were purchased from GeneCopoeia (China).
Western blotting
For short, 50 µg of total protein were subjected to SDS-PAGE and transferred onto PVDF membranes. Membranes were blocked with 5% non-fat milk at room temperature for 3 h, incubated with primary antibodies specific to Bcl-2 (1:1000, Abcam, Cambridge, UK), Bax (1:1000, CST, USA), Notch1 (1:1000, CST, USA) and β-actin (1:1000, CST, USA) at 4 °C overnight. On the next day, membranes were incubated with HRP-conjugated secondary antibodies diluted at 1:3000 (Boster, Wuhan, China) at 37 °C for 1 h. Protein bands on the membrane were visualized with ECL Kit (Millipore, USA) using FluorChem FC system (Alpha Innotech). Results were presented as densitometric ratio between the protein of interest and the loading control (β-actin).
Cell transfection
Chemically modified sense RNAs (miR-146b agomiR) and antisense RNA (miR-146b antagomiR) and the cognate control RNA were synthesized by Qiagen. Mouse cardiomyocytes were transfected with miR-146b scrambled negative control, antagomiR or agomiRs using HiPerFect Transfection Reagent (Qiagen). Briefly, 20 µM of miR-146b antagomiR was mixed with 12 µL HiPerFect in 100 µL culture medium for 10 min at room temperature to form transfection complexes. The cells were incubated with the transfection complexes for 48 h.
Luciferase reporter assay
The3′untranslated region (UTR) fragments SIRT1 containing the miRNA binding sites were amplified by PCR using the cDNA template obtained from RNA sample of macrophages. The wide type 3′UTRs of SIRT1 as well as mutant 3′UTRs with nucleotide substitutions in the putative binding sites corresponding to the seed sequence of miR-146b was cloned downstream of the firefly luciferase gene in the pGL3 vector (Promega, Madison, WI). Cells were co-transfected with miR-146b or a control miRNA. 48 h after transfection, cells were rinsed in PBS and their luciferase activity was measured by a luminometer (Promega), using dual luciferase reporter assay system.
Statistical analysis
Results are presented as the means ± standard error of three independent experiments (SEM). Statistical differences between groups were analyzed by Student’s t test or the Mann–Whitney U test as appropriate using a SPSS 13.0 program. p < 0.05 was considered statistically significant.
Result
Myocardial injury was severe in CLP mice as indicated by H&E staining and miR-146b elevated in the myocardium of CLP mice
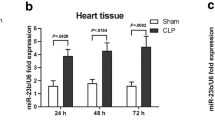
As shown in Fig. 1a, the myocardial sections were stained with hematoxylin and eosin to evaluate damage to the myocardium. In the sham group, the cardiomyocytes were intact and tidily arranged. In the CLP group, the inflammatory cells infiltration was evident and some of the nuclei were broken. The arrangements of the cardiomyocytes were in disorder. In Fig. 1b, the level of miR-146b was significantly increased in the myocardium of CLP mice compared with sham group.
Level of miR-146b elevated in septic mice. a Mice with or without CLP were divided into sepsis group or control group. The H&E staining of myocardium were examined by microscope. The intact of myocardium was greatly destroyed in CLP mice. b The level of miR-146b in myocardium was examined by RT-PCR. It showed that miR-146b in CLP mice were increased significantly. n = 4, **p < 0.01. Scale bar = 50 µm
The up-regulation of miR-146b could alleviate myocardium injury as well as reduce the expression of IL-1β
As shown in Fig. 2a, H&E staining showed that in CLP + agomiRs group, the level of miR-146b elevated compared with CLP group. Meanwhile the arrangements of cardiomyocytes were improved and the necrosis of nuclei reduced. The level of IL-1β was elevated significantly in mice in CLP group compared with CLP + agomiRs group. After the injection of agomiRs, the level of IL-1β reduced obviously (Fig. 2b) (p < 0.01). The level of Bax increased in the myocardium of CLP mice while decreased after agomiRs injection (p < 0.01). By contrast, the level of Bcl2 was decreased in CLP mice and upregulated with agomiRs injection (Fig. 2c) (p < 0.01).
Increased level of miR-146b alleviated organ injury induced by sepsis. a Mice suffering from CLP operation were injected with NC agomiR or miR-146b agomiR were divided into two groups. Mice suffering from sham operation were used as control group. The H&E staining of myocardium were examined by microscope. b The level of IL-1β in myocardium was examined by RT-PCR. It showed that IL-1β in CLP + NC agomiR mice was increased significantly while the injection of miR-146b agomiR decreased the level of it. c The protein levels of Bax and Bcl-2 in mice myocardium was tested by western blotting. It was found that sepsis increased level of Bax and decreased level of Bcl-2 while the injection of miR-146b agomiR revised the trend. n = 4, **p < 0.01 compared with CLP + NC agomiR. Scale bar = 50 µm
The down-regulation of miR-146b aggravated myocardium injury as well as increased the expression of IL-1β
As shown in Fig. 3a, H&E staining showed that in CLP + antagomiRs group, the level of miR-146b reduced compared with CLP group. The arrangements of cardiomyocytes were in great disorder and the necrosis of nuclei was more obvious compared with CLP group. The level of IL-1β was elevated significantly in CLP group. With the injection of antagomiRs, the level of IL-1β reached to an even higher level compared with CLP group (Fig. 3b) (p < 0.01). The level of Bax was lower in the myocardium of CLP mice than that in CLP + antagomiRs group (p < 0.01). The level of Bcl2 was higher in CLP group than in CLP + antagomiRs group (Fig. 3c) (p < 0.01).
Decreased level of miR-146b aggravated myocardium injury induced by sepsis. a Mice suffering from CLP operation were injected with NC antagomiR or miR-146b antagomiR were divided into two groups. Mice suffering from sham operation were used as control group. The H&E staining of myocardium were examined by microscope. b The level of IL-1β in myocardium was examined by RT-PCR. It showed that IL-1β in CLP + NC antagomiR mice was increased significantly while the injection of miR-146b antagomiR increased the level of it further. c The protein levels of Bax and Bcl-2 in mice myocardium were tested by western blotting. It was found that sepsis increased level of Bax and decreased level of Bcl-2 while the injection of miR-146b antagomiR intensified the trend. n = 4, **p < 0.01 compared with CLP + NC antagomiR. Scale bar = 50 µm
Bioinformatics analysis combined with reporter assay indicated that Notch1 was a potential target of miR-146b
To predict the potential targets of miR-146b, the databases of Target Scan, PicTar, and miRanda were used. As shown in Fig. 4a, Notch1 was a potential target of miR-146b as the seed region of 2–8nt in 3′UTR could be completely matched. Then luciferase reporter was used to confirm the binding of miR-146b and Notch1. As shown in Fig. 4b, the luciferase reporter assay showed a 50% decrease in the relative luciferase activity of the wild-type Notch1 3′-UTR vector after miR-146b agomiRs transfection in mouse cardiomyocytes (p < 0.05). In contrast, the mutant construction completely abolished this effect, suggesting that miR-146b could directly bind to Notch1 3′-UTR and inhibit Notch1 expression, which was consistent with Notch1 expression data in Fig. 4c, d. The level of Notch1 increased after the administration of miR-146bantagomiRs while decreased by miR-9 agomiRs. These findings indicated that miR-146b was able to negatively regulateNotch1 expression.
Bioinformatics analysis combined with luciferase reporter gene showed that Notch1 was the target gene of miR-146b. a The seed region, 2-8nt of Notch1 in 3′UTR could be completely matched with miR-146b. b Luciferase reporter assay showed a 54% decrease in the relative luciferase activity of Notch1 3′-UTR vector after miR-146b mimics transfection while the mutant construct completely abolished this effect. c and d In cardiomyocytes, the mRNA level of Notch1 was detected by RT-PCR and the protein level of Notch1 was detected by western blotting. It showed increased level of Notch1 increased with the administration of miR-146b antagomiR while decreased with miR-146b agomiR. n = 4, **p < 0.01 compared with NC group
The level changes of miR-146b affected the level of Notch1 and Hes1
As shown in Fig. 5, the mouse cardiomyocytes were transfected with agomiRs or the control RNA (NC) and then stimulated with septic mice serum or normal mice serum. The volume of serum added reached to 10% of the medium. The mRNA level of Notch1 and Hes1 were significantly increased in NC + septic mice serum group. With the transfection of miR-146b agomiR, the levels of Notch1 and Hes1 were down-regulated obviously (p < 0.01).
Notch signaling was activated with the stimulation of septic serum. Cardiomyocytes were stimulated with serum from CLP mice or normal mice. Then NC agomiR or miR-146b agomiR was transfected into the cells. The mRNA level of Notch1 and Hes1 were detected by RT-PCR. It was found that after the stimulation of septic serum, the level of Notch1 and Hes1 increased while with the transfection of miR-146b agomiR, the levels of these two decreased. n = 4, **p < 0.01 compared with sepsis + NC group
The inactivation of Notch signal improved myocardium injury and decreased the level of IL-1β
As shown in Fig. 6, the Notch1-/- mice and wild-type litter mates were used to go through CLP operation. It was found that the IL-1β level in blood was higher in WT mice than Notch1-/- mice. The level of Bax was lower and level of Bcl2 was higher in Notch1-/- mice compared with those in WT mice (p < 0.01).
Notch1 knockout alleviated the inflammation in mice after CLP operation. a The H&E staining of myocardium from Notch1-/- mice or WT mice suffering from CLP operation were examined by microscope. b The mRNA level of IL-1β was detected by RT-PCR. c The protein levels of Bax and Bcl-2 were detected by western blotting. n = 4, **p < 0.01 compared with WT group
Discussion
Myocardial dysfunction is regarded as a critical manifestation of sepsis and patients with myocardial injuries are at high risk of further detriment (Sergi et al. 2017). Cell death and inflammation are two important mechanisms that affect myocardial function (Lu et al. 2017; Rudiger and Singer 2007). However, the mechanism of sepsis-induced myocardial dysfunction is complex and still unclear. It has been reported that microRNAs plays an important role in sepsis (Ma et al. 2013). A recent study suggested that miR-146b was related to hypoxia-induced cardiomyocyte apoptosis. In this study, we were curious about whether miR-146b participated in sepsis induced myocardial dysfunction.
In the result, we found that miR-146b increased in CLP induced mice septic model. However, when we further upregulated the level of miR-146b, the sepsis was alleviated as shown in Fig. 2 that IL-1β, a typical inflammation cytokine, decreased and the myocardial injury improved. Apoptosis regulator Bax decreased and Bcl-2 increased. When we down-regulated the level of miR-146b, the sepsis aggravated and apoptosis increased. That was to say, during sepsis, miR-146b aroused reactively to keep a homeostasis. Another research reported that duing in vitro experiment, the inhibition of miR-146b could alleviate the expression of IL-6, which is an indicator of inflammation. However, since the in vivo condition is much more complex than in vitro, any breakthrough from cells should be carefully tested in vivo (Pfeiffer et al. 2017). And in other conditions, such as in hypoxic, the level of miR-146b was increased and the inhibition of miR-146b led to a much severe cell apoptosis (Li et al. 2015). That was to say, the effects of miR-146b varied in different conditions. So it is important to figure out the downstream mechanism of it.
Further, we predicted the target of miR-146b through bioinformatics analysis. Notch1 was predicted as a possible target gene of miR-146b and we used luciferase reporter assays to confirm that.
The Notch pathway plays a major role in the heart during development. Notch1 and Jagged1 are the pre-dominant forms of Notch receptors and ligands expressed in adult cardiomyocytes (Croquelois et al. 2008). In plenty of researches on Notch pathway and heart development, Notch is very important in maintain the normal development of heart (D’Amato et al. 2016; Grego-Bessa et al. 2007; Luxan et al. 2013). While there are few researches focused on the relationship between Notch and sepsis. It had been reported that Notch inhibition during the acute inflammatory response phase preventing the process of sympathetic hyper innervation (Yin et al. 2016). It has been reported that LPS could activate Notch signaling in RAW264.7 macrophage cells (Sangphech et al. 2014). Knockdown of Notch1 by Notch1 siRNA attenuated the inflammatory response in RAW264.7 macrophage cells after LPS stimulation in terms of reduced production of TNF-α and IL-6(Molderings and Schumann 1989; Tsao et al. 2011). So further, we examined whether the level changes of Notch1 affected the myocardial injury in mice. As shown in Fig. 4, Notch1 was negatively regulated by miR-146b in mouse cardiomyocytes. It is known that miRs could target to genes and then lead to the suppression of target gene expression. And studies conducted using genome-wide expression analyses and chromatin immunoprecipitation arrays have revealed that numerous genes can be directly regulated by Notch (Palomero et al. 2006; Wang et al. 2010b), and members of the Hairy enhancer of split (Hes) or Hairy related (Hey or Hrt) gene family have been identified as Notch target genes in several tissues. We found that the up-regulation of miR-146b led to the down-regulation of Notch1 and Hes1, which is the downstream gene in Notch pathway. The expression of IL-1β also decreased with the down-regulation of Notch1. We further stimulated cardiomyocytes with serum from septic mice or from normal mice to see whether the septic serum could affect the level of Notch1 and Hes1. It was found that after the stimulation of septic serum, the level of Notch1 and Hes1 increased dramatically. While with the transfection of miR-146b agomiR, the level of these two decreased. It was clearly to say that during sepsis, the cardiomyocytes suffered from over activation of Notch signaling. And increase the level of miR-146b could definitely improve that situation. Then we verified our hypothesis in vivo. In septic mice model, the knockout of Notch1 improved the myocardium injury as well as decreased the apoptosis of cardiomyocytes.
In conclusion, it was found that miR-146b increased in CLP mice, further increasing of miR-146b protected mice from myocardial injury. Notch1, which is critical in heart development, is a target gene of miR-146b. Inhibition of Notch1 in sepsis could alleviate the myocardial injury and improve sepsis.
References
Chung HY et al. (2017) Adjustment of dysregulated ceramide metabolism in a murine model of sepsis-induced cardiac dysfunction. Int J Mol Sci. https://doi.org/10.3390/ijms18040839
Croquelois A, Domenighetti AA, Nemir M, Lepore M, Rosenblatt-Velin N, Radtke F, Pedrazzini T (2008) Control of the adaptive response of the heart to stress via the Notch1 receptor pathway. J Exp Med 205:3173–3185. https://doi.org/10.1084/jem.20081427
D’Amato G et al (2016) Sequential Notch activation regulates ventricular chamber development. Nat Cell Biol 18:7–20. https://doi.org/10.1038/ncb3280
Fleischmann C et al (2016) Assessment of global incidence and mortality of hospital-treated sepsis: current estimates limitations. Am J Respir Crit Care Med 193:259–272. https://doi.org/10.1164/rccm.201504-0781OC
Grego-Bessa J et al (2007) Notch signaling is essential for ventricular chamber development. Dev Cell 12:415–429. https://doi.org/10.1016/j.devcel.2006.12.011
Hotchkiss RS, Monneret G, Payen D (2013) Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13:862–874. https://doi.org/10.1038/nri3552
Huntzinger E, Izaurralde E (2011) Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 12:99–110. https://doi.org/10.1038/nrg2936
Krol J, Loedige I, Filipowicz W (2010) The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11:597–610. https://doi.org/10.1038/nrg2843
Landesberg G et al (2012) Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur Heart J 33:895–903. https://doi.org/10.1093/eurheartj/ehr351
Li JW et al (2015) MicroRNA-146b inhibition augments hypoxia-induced cardiomyocyte apoptosis. Mol Med Rep 12:6903–6910. https://doi.org/10.3892/mmr.2015.4333
Lim LP et al (2005) Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433:769–773. https://doi.org/10.1038/nature03315
Lu Y, Yang Y, He X, Dong S, Wang W, Wang D, Zhang P (2017) Esmolol reduces apoptosis and inflammation in early sepsis rats with abdominal infection. Am J Emerg Med 35:1480–1484. https://doi.org/10.1016/j.ajem.2017.04.056
Luxan G et al (2013) Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat Med 19:193–201. https://doi.org/10.1038/nm.3046
Ma Y et al (2013) Genome-wide sequencing of cellular microRNAs identifies a combinatorial expression signature diagnostic of sepsis. PLoS ONE 8:e75918. https://doi.org/10.1371/journal.pone.0075918
Merx MW, Weber C (2007) Sepsis the heart. Circulation 116:793–802. https://doi.org/10.1161/CIRCULATIONAHA.106.678359
Molderings GJ, Schumann HJ (1989) Amplifying effects of several vasoconstrictor agents of alpha 1-adrenoceptor-mediated contraction of isolated thoracic aortae of the guinea pig. Pharmacology 39:373–382
Palomero T et al (2006) NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc Natl Acad Sci USA 103:18261–18266. https://doi.org/10.1073/pnas.0606108103
Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP (1990) Septic shock in humans: advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med 113:227–242
Pfeiffer D, Rossmanith E, Lang I, Falkenhagen D (2017) miR-146a, miR-146b, and miR-155 increase expression of IL-6 and IL-8 and support HSP10 in an In vitro sepsis model. PLoS ONE 12:e0179850 https://doi.org/10.1371/journal.pone.0179850
Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA (2009) Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 4:31–36. https://doi.org/10.1038/nprot.2008.214
Rudiger A, Singer M (2007) Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med 35:1599–1608. https://doi.org/10.1097/01.CCM.0000266683.64081.02
Sangphech N, Osborne BA, Palaga T (2014) Notch signaling regulates the phosphorylation of Akt and survival of lipopolysaccharide-activated macrophages via regulator of G protein signaling 19 (RGS19. Immunobiology 219:653–660. https://doi.org/10.1016/j.imbio.2014.03.020
Sergi C et al (2017) Cardiovascular dysfunction in sepsis at the dawn of emerging mediators. Biomed Pharmacother 95:153–160. https://doi.org/10.1016/j.biopha.2017.08.066
Song Y et al (2017) Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1beta-primed mesenchymal stem cells against sepsis. Stem cells 35:1208–1221. https://doi.org/10.1002/stem.2564
Tsao PN, Wei SC, Huang MT, Lee MC, Chou HC, Chen CY, Hsieh WS (2011) Lipopolysaccharide-induced Notch signaling activation through JNK-dependent pathway regulates inflammatory response. J Biomed Sci 18:56. https://doi.org/10.1186/1423-0127-18-56
Vincent JL, Opal SM, Marshall JC, Tracey KJ (2013) Sepsis definitions: time for change. Lancet 381:774–775. https://doi.org/10.1016/S0140-6736(12)61815-7
Wang JF, Yu ML, Yu G, Bian JJ, Deng XM, Wan XJ, Zhu KM (2010a) Serum miR-146a and miR-223 as potential new biomarkers for sepsis. Biochem Biophys Res Commun 394:184–188. https://doi.org/10.1016/j.bbrc.2010.02.145
Wang YC et al (2010b) Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res 70:4840–4849. https://doi.org/10.1158/0008-5472.CAN-10-0269
Wang L, Wang HC, Chen C, Zeng J, Wang Q, Zheng L, Yu HD (2013) Differential expression of plasma miR-146a in sepsis patients compared with non-sepsis-SIRS patients. Exp Ther Med 5:1101–1104. https://doi.org/10.3892/etm.2013.937
Yin J et al (2016) Inhibition of Notch signaling pathway attenuates sympathetic hyperinnervation together with the augmentation of M2 macrophages in rats post-myocardial infarction. Am J Physiol Cell Physiol 310:C41–C53. https://doi.org/10.1152/ajpcell.00163.2015
Acknowledgements
The authors thank Experimental Animal Center of The Fourth Military Medical University for providing the myocardium specific Notch1-/- mice and wild-type littermates.
Author information
Authors and Affiliations
Contributions
XW and YY designed the project. XW performed the experiments, and analyzed data. YY wrote the manuscript. XW and YY approved the final submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10735_2018_9781_MOESM1_ESM.tif
Supplementary Figure. Chemically modified sense RNAs (miR-146b agomiR) and antisense RNA (miR-146b antagomiR) were synthesized by Qiagen. Mouse cardiomyocytes were transfected with antagomiR or agomiRs using HiPerFect Transfection Reagent (Qiagen). The cells were incubated with the transfection complexes for 48h. Then the miRNA levels were tested by qRT-PCR. (TIF 160 KB)
Rights and permissions
About this article
Cite this article
Wang, X., Yu, Y. MiR-146b protect against sepsis induced mice myocardial injury through inhibition of Notch1. J Mol Hist 49, 411–417 (2018). https://doi.org/10.1007/s10735-018-9781-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-018-9781-4