Abstract
Calcitonin gene-related peptide (CGRP) is a marked and important neuropeptide expressed in nerve fibers during bone repair. This study investigated the role of CGRP overexpression on osteogenic differentiation of rat bone mesenchymal stem cells (rBMSCs). rBMSCs were infected with viral stocks of pLenO-DCE-CGRP (CGRP group) or pLenO-DCE (Vector group), while normal rBMSCs were used as a control. Transfection efficiency of rBMSCs was analyzed by flow cytometry. Cell proliferation was examined using a Cell Counting Kit-8 and flow cytometry. Expressions of alkaline phosphatase(ALP), bone sialoprotein (BSP) and Runt-related transcription factor 2(Runx2) in rBMSCs were detected at 1 and 2 weeks after mineral induction by real-time PCR and western blotting. Alizarin Red staining was applied at 28 days. The ratio of osteoprotegerin (OPG) to receptor activator of nuclear factor kappa B ligand (RANKL) was also detected to determine the underlying mechanism. pLenO-DCE-CGRP-induced rBMSCs stably overexpressing CGRP were successfully established. Overexpression of the CGRP gene significantly promoted rBMSC proliferation (p < 0.05). In addition, expressions of osteogenesis-related indexes were upregulated in the CGRP group (p < 0.05) compared with vector and control groups, and more mineralization nodules were observed in the CGRP group (p < 0.05). CGRP gene increased OPG and reduced RANKL in rBMSCs. Hence, the OPG/ RANKL ratio was increased in the CGRP group compared with the other two groups. CGRP gene-modified rBMSCs show better osteogenic differentiation capacity compared with rBMSCs in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone transport and distraction osteogenesis have been widely used in clinical practice to stimulate or augment bone regeneration (Green et al. 1992). However, those approaches also result in healthy bone loss, significant donor site injury, scarring, deformity and surgical risks as well: infection, bleeding, inflammation and chronic pain (Pang et al. 2015). To overcome these limitations, mesenchymal stem cells transplantation has been investigated as a novel therapeutic for bone defect repair (Parekkadan and Milwid 2010), and as alternatives or adjuncts to the standard methods of bone regeneration. Bone mesenchymal stem cells (BMSCs) are one primary stem cell type applied in tissue engineering for translational research and clinical applications (Dong et al. 2014; Kon et al. 2012; Wang et al. 2013; Yang et al. 2014). Gene-modified BMSCs are better seed cells with great potential for repairing large bone defects (Deng et al. 2014; Sun et al. 2017; Xian et al. 2016; Zou et al. 2011).
Increasing evidence has demonstrated the involvement of nerve and related neuropeptides in bone metabolism (Uslu et al. 2016). The nervous system can influence osteoclast formation and osteogenic differentiation by producing several neuropeptides such as calcitonin gene-related peptide (CGRP), substance P and neurokinins, which regulate bone remodeling (Wang et al. 2017; Yu et al. 2015). CGRP is a highly expressed and important neuropeptide in nerve fibers during bone repair (Chen et al. 2017; Imai and Matsusue 2002). Both sensory nerve and its related neuropeptide CGRP have been implicated in the process of bone fracture healing (Lam et al. 2012; Li et al. 2007; Pang et al. 2015; Zhang et al. 2016). Targeted expression of CGRP to osteoblasts increases bone density in mice. Mice lacking alpha-CGRP show decreased bone formation and osteopenia (Schinke et al. 2004). Researches show that CGRP can promote osteoporotic rat-derived BMSC (rBMSC) proliferation and osteogenic differentiation (Liang et al. 2015). However, short half-life, high cost and multiple dosing restrict the application of CGRP protein (Chen et al. 2017; Xiang et al. 2017).
In this study, a recombinant lentiviral vector for overexpressing CGRP peptide, pLenO-DCE-CGRP, was constructed and transfected into rBMSCs to obtain stable CGRP expression. Effects of overexpressing CGRP on osteogenic differentiation of rBMSCs were examined in vitro.
Materials and methods
Cell culture
rBMSCs were harvested from Wistar rats (80–100 g) using the following method, for which use of animals was approved by Animal Care and Use Committee of School of Stomatology, Shandong University. Briefly, after anesthesia and euthanasia by intraperitoneal injection of 10% chloral hydrate (0.4 g/kg body weight), the body was soaked in 75% alcohol for 10 min. Under laminar flow room, tibiae and femora were excised and rinsed with 200 IU penicillin and 200 mg/mL streptomycin (Solarbio, Beijing, China) in phosphate-buffered saline (PBS). Both ends of the femora and tibiae were excised. α-MEM (Gibco, Grand Island, USA) supplemented with 20% fetal bovine serum (FBS; Gibco) was applied to wash marrow gently from the shafts into 10-cm-diameter culture ware with a 25-gauge needle. Cells were subsequently seeded into culture flasks (Takara Bio, Otsu, Japan) at a density of 5–10 × 105 cells/cm2. With the exception of primary cells, cells were cultured in α-MEM supplemented with 10% fetal bovine serum. When the density of BMSCs reached 80% confluence, they were passaged. After one subculture, adherent cells were termed rBMSCs. Cells (passage 3–4) were used for the following experiments.
The retroviral packaging cell line 293T (Clontech, Palo Alto, CA) was maintained in DMEM with 10% FBS. Subsequently, cells within 10 passages were used for the experiments.
Construction of pLenO-DCE-CGRP- transfected rBMSCs
pLenO-DCE-CGRP was provided by Shanghai Innovation Biotechnology (Shanghai, China) and identified by BioSUN Biotechnology (Beijing, China). pLenO-DCE-CGRP, pRsv-REV, pMDlg-pRRE and pMD2G, which contained essential components for viral packaging, were co-transfected into 293T cells using Lipofectamine (TM) 2000 (Invitrogen, Carlsbad, CA). In addition, pLenO-DCE was also transfected into 293T cells. After transfection for 4 h, media was replaced with α-MEM containing 20% FBS. After culture for 72 h, viral supernatants were collected and subjected to high-speed centrifugation to produce concentrated high-titer lentivirus solution. Transfection efficiency was subsequently determined using fluorescence microscopy.
Passage 3 rBMSCs were seeded in 6-well plates at a density of 1.5 × 105 cells/well and infected with viral stocks of pLenO-DCE-CGRP and pLenO-DCE in the presence of polybrene (4 µg/mL) for 6 h. After infection for 48 h and 72 h, fluorescence microscopy was employed to determine infection efficiency. The transfection rate was quantified by imaging five fields of view under 100–fold magnification. Numbers of green fluorescence positive cells and the total cell number were counted respectively under fluorescent and light microscope. rBMSCs with high infection efficiency were collected. Cells infected with pLenO-DCE-CGRP were included in the CGRP group, while cells infected with pLenO-DCE were in the Vector group. Uninfected cells served as the Control group.
Flow cytometry analysis
After infection with pLenO-DCE-CGRP or pLenO-DCE, the transfection efficiency of rBMSCs was analyzed by flow cytometry (FC500, Beckman Coulter). Briefly, 2 × 106 rBMSCs from each group were fixed in ice-cold 70% ethanol for 24 h. To stain for flow cytometry, 15 µL of propidiumiodide (PI) (20 µg/mL) was added and placed at 37 °C for 30 min in a dark environment.
Cell Counting Kit-8 assay
rBMSC proliferation was determined using a Cell Counting Kit-8 (CCK8; Biotech, Nanjing, China) according to the manufacturer’s instructions. Briefly, at 24, 48, and 72 h after transfection, 10 µL of CCK-8 (5 mg/mL) was added to each well. The absorbance was measured at 450 nm using a spectrophotometer (Bio-Rad, Hercules, CA, USA).
Osteogenic differentiation and Alizarin Red staining
rBMSCs from each group were inoculated in 6-well plates at a density of 1.8 × 105 cells/well. Once cells reached 80% confluence, the culture medium was replaced with 1.5 mL osteogenic medium containing α-MEM with 10% FBS, 10−8 mol/L dexamethasone (Sigma-Aldrich, St. Louis, MO), 10 mmol/L β-glycerol phosphate (Sigma-Aldrich) and 0.05 mmol/L ascorbic acid-2-phosphate (Sigma-Aldrich) for 28 days. Cells were subsequently fixed in 95% alcohol solution and then stained with 2% Alizarin red (Sigma-Aldrich) for 10 min. After staining, each well was eluted with 10% cetylpyridinium chloride (Sigma-Aldrich) for 1 h to determine the level of calcium salts within the cell matrix. Total absorbance of soluble calcium ions was measured with a spectrophotometer at a 562-nm wavelength to determine concentration.
Real-time PCR
Total RNA was isolated from cells with TRIZOL reagent (Invitrogen) according to the manufacturer’s protocol. Briefly, cells cultured in 6-well plates were mixed and treated with 1 mL TRIZOL reagent per well. Subsequently, 500 ng of total RNA was used for reverse transcription (Takara Bio, Dalian, Japan), and 80 ng of resulting cDNA samples was amplified with SYBR Premix Ex Taq (Takara Bio) in a Roche 480 Light Cycler (Roche, Mannheim, Germany). CGRP mRNA levels were tested after infection for 3 days. mRNA levels of ALP, BSP and Runx2 were tested after mineralization culture for 1 week and 2 weeks. Primers used were listed in Table 1.
Western blotting analysis
Western blotting analyses were performed as previously described. Briefly, protein concentrations were determined using a bicinchoninic acid assay protein assay kit (Beyotime, China). Equal amounts of total proteins were resolved by SDS—polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Invitrogen), which were washed three times with Tris-buffered saline/Tween-20 and blocked with 5% skim milk in Tris-buffered saline at room temperature for 1 h. The membranes were incubated with antibodies against CGRP (diluted 1:500; Abcam; Cambridge, UK), ALP (diluted 1:500; Abcam), BSP (1:1000; Cell Signaling Technology, Shanghai, China), Runx2 (1:1000; Cell Signaling Technology), OPG (diluted 1:500; Bioss, Beijing, China) and RANKL (diluted 1:500; Bioss). The secondary antibody was horseradish peroxidase-linked goat-anti rabbit IgG and anti-GAPDH or tubulin (1:10,000, Abcam).
Statistical analysis
All measurement results are presented as the mean ± SD. Statistically significant differences (p < 0.05) between the various groups (Control group, Vector group and CGRP group) were measured using one-way analysis of variance followed by Tukey’s test.
Results
Construction and identification of pLenO-DCE-CGRP transfected rBMSCs
293T cells were used for transfection. After transfection for 72 h, the transfection rate of pLenO-DCE was 90% (Fig. 1a), while that of pLenO-DCE-CGRP was 80% (Fig. 1b).
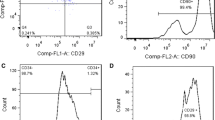
rBMSCs were used for infection. After infection for 48 h, the transfection rate of the CGRP group was 50%, while that of the Vector group was 60% (Fig. 2a). After 72 h, the transfection rate of the CGRP group was 65%, while that of the Vector group was 90% (Fig. 2b). Flow cytometry analysis showed the transfection rate of the Vector group (Fig. 3a) was 63.3%, while that of the CGRP group was 86% (Fig. 3b).
After infection for 3 days, CGRP mRNA levels were tested by real-time PCR. The results indicated higher CGRP mRNA levels in the CGRP group compared with Vector and Control groups (Fig. 4a) (p < 0.01). CGRP protein levels, as examined by western blotting, were also significantly increased in the CGRP group (Fig. 4b, c) (p < 0.01). Thus, successful transfection of pLenO-DCE-CGRP into rBMSCs was confirmed and shown to increase CGRP expression.
Cell proliferation
Statistical differences could be observed between the CGRP group and the other two groups (p < 0.05) at 48 and 72 h (Fig. 5), according to CCK-8 results.
Flow cytometry analysis also showed that CGRP promoted rBMSC proliferation. Indeed, the percentage of cells in S + G2 phase in the CGRP group was upregulated compared with Vector and Control groups (Fig. 6). These results indicated that CGRP overexpression promoted rBMSC proliferation.
Osteogenic ability detection
Western blotting results indicated upregulated protein levels of ALP, BSP and Runx2 in the CGRP group at different stages compared with Vector and Control groups (p < 0.05) (Fig. 7).
After mineral induction for 28 days, rBMSCs from each group were stained with Alizarin Red and observed under a microscope (Fig. 8). An increased number of calcified nodules was observed in the CGRP group compared with Vector and Control groups (p < 0.05). Collectively, all of the above results demonstrated that CGRP overexpression could promote osteogenic differentiation of rBMSCs. In addition, mRNA levels of ALP, BSP and Runx2 tested after mineral induction for 1 and 2 weeks (Fig. 9) by real-time PCR, showed upregulation after mineral induction.
OPG/RANKL ratio
Western blotting indicated significantly upregulated OPG protein levels after infection with pLenO-DCE-CGRP compared with Vector and Control groups (p < 0.05) (Fig. 10a). However, RANKL was decreased in the CGRP group (Fig. 10b), resulting in an increased OPG/RANKL ratio (Fig. 10c). Thus, CGRP overexpression in rBSMCs led to an increased OPG/RANKL ratio.
Discussion
Autologous bone marrow stem cell infusion for the treatment of multidrug-resistant tuberculosis (Zhu et al. 2016), multiple sclerosis (Nabavi et al. 2014), and Amyotrophic Lateral Sclerosis (ALS), has reliable clinical efficacy, good safety and tolerability. Intrathecal MSCs injection can slow disease progression and might be used as a disease modifying modality as an alternative treatment choice in patients with ALS (Kim et al. 2009). However, hematopoietic stem cells, bone marrow stem cells, peripheral blood stem cells and cord blood stem cells (CBSC), each have unique attributes related to collection methods and storage, which may potentially cause adverse reactions in stem cell infusion (Truong et al. 2016). As multipotent cells, BMSCs are an excellent candidate for cell therapy. BMSCs are easily accessible, isolated, and expanded to clinical scales (Colter et al. 2000; Sekiya et al. 2002). In addition to recent progress in stem cell separation and purification technologies, genetic engineering can also broaden the therapeutic capabilities of BMSCs (Deng et al. 2014; Xian et al. 2016).
In this study, BMSCs were selected as donor cells, and lentiviral vectors were adopted for transfection. Our flow cytometry results showed that transfection of BMSCs with lentiviral vectors is a desirable and efficient method. We observed no significant morphological changes in rBMSCs expressing CGRP or empty vector compared with normal rBMSCs. However, expression of CGRP in BMSCs was significantly upregulated after transfection according to real-time PCR and western blot results. pLenO-DCE-CGRP-transfected rBMSCs could obtain stable CGRP expression, which facilitated our examination of CGRP overexpression on osteogenic differentiation of rBMSCs and avoided the disadvantages of extrinsic recombinant CGRP protein such as short half-life, high cost, and multiple dosing.
Our previous study has identified CGRP receptors RAMP1 and CRLR in rBMSCs, which may combine with CGRP to play biological roles (Zhang et al. 2017). In our study, higher expressions of osteogenesis related indexes ALP, BSP and Runx2, and as well as mineralized nodules, were observed in the CGRP group after mineral-induction compared with the other two groups, demonstrating CGRP gene promoted rBMSCs osteogenetic differentiation and osteogenic ability in vitro. Genetic engineering broadens the therapeutic capabilities of MSCs. CGRP gene-modified rBMSCs show better osteogenic differentiation capacity compared with rBMSCs in vitro. Genetic engineering promotes osteogenic differentiation of rBMSCs. Our study provides a new foundation for further study in vivo. An in vivo study is necessary to verify whether CGRP gene-modified BMSCs are promising seed cells with great potential to repair bone defect (Salah et al. 2018).
Osteoclast differentiates from monocyte/macrophage precursors under the regulation of RANKL/RANK signaling. The OPG/RANKL ratio in bone is a major determinant of bone resorption and formation. Overexpressing CGRP in rBSMCs led to an increase of the OPG/RANKL ratio. The number and activity of osteoclasts could decrease if the OPG/RANKL ratio changes as the result of either an increase in the former or a decrease in the latter (or a change in both that leads to a change in the ratio in favor of OPG). However, whether CGRP-modified BMSCs can inhibit osteoclastogenesis by upregulating the OPG/RANKL ratio still needs further study. Co-culture of CGRP-modified BMSCs and osteoclast precursor cells might be helpful to verify this hypothesis in a future study.
References
Chen J, Liu W, Zhao J, Sun C, Chen J, Hu K et al (2017) Gelatin microspheres containing calcitonin gene-related peptide or substance P repair bone defects in osteoporotic rabbits. Biotechnol Lett 39(3):465–472
Colter DC, Class R, DiGirolamo CM, Prockop DJ (2000) Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA 97(7):3213–3218
Deng Y, Bi X, Zhou H, You Z, Wang Y, Gu P et al (2014) Repair of critical-sized bone defects with anti-miR-31-expressing bone marrow stromal stem cells and poly(glycerol sebacate) scaffolds. Eur Cells Mater 27(1):13
Dong Y, Long T, Wang C, Mirando AJ, Chen J, O’Keefe RJ et al (2014) NOTCH-mediated maintenance and expansion of human bone marrow stromal/stem cells: a technology designed for orthopedic regenerative medicine. Stem Cells Transl Med 3(12):1456–1466
Green SA, Jackson JM, Wall DM, Marinow H, Ishkanian J (1992) Management of segmental defects by the Ilizarov intercalary bone transport method. Clin Orthopaedics Related Res 280:136–142
Imai S, Matsusue Y (2002) Neuronal regulation of bone metabolism and anabolism: calcitonin gene-related peptide-, substance P-, and tyrosine hydroxylase-containing nerves and the bone. Microscopy Res Tech 58(2):61–69
Kim HY, Paek JY, Park HK, Mi RC, Yoon HS, Kim KS et al (2009) Efficacy and safety of autologous bone marrow-derived mesenchymal stem cell treatment in patients with amyotrophic lateral sclerosis. J Korean Neurol Assoc 27(2):163–169
Kon E, Filardo G, Roffi A, Di Martino A, Hamdan M, De Pasqual L et al (2012) Bone regeneration with mesenchymal stem cells. Clin Cases Miner Bone Metab 9(1):24–27
Lam WL, Guo X, Leung KS, Kwong KS (2012) The role of the sensory nerve response in ultrasound accelerated fracture repair. J Bone Joint Surg Br 94(10):1433–1438
Li J, Kreicbergs A, Bergstrom J, Stark A, Ahmed M (2007) Site-specific CGRP innervation coincides with bone formation during fracture healing and modeling: A study in rat angulated tibia. J Orthopaedic Res 25(9):1204–1212
Liang W, Zhuo X, Tang Z, Wei X, Li B (2015) Calcitonin gene-related peptide stimulates proliferation and osteogenic differentiation of osteoporotic rat-derived bone mesenchymal stem cells. Mol Cell Biochem 402(1–2):101–110
Nabavi SM, Aghdami N, Arab L, Hamzeloo A, IR) (2014). Safety and efficacy of intravenous injection of autologous bone marrow-derived mesenchymal stem cell in patients with multiple sclerosis: a double blind randomized semi-crossover clinical trial: preliminary report 1—safety issues. Mult Scler:935–935
Pang P, Shimo T, Takada H, Matsumoto K, Yoshioka N, Ibaragi S et al (2015) Expression pattern of sonic hedgehog signaling and calcitonin gene-related peptide in the socket healing process after tooth extraction. Biochem Biophys Res Commun 467(1):21–26
Parekkadan B, Milwid JM (2010) Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng 12:87–117
Salah RA, Mohamed IK, El-Badri N (2018) Development of decellularized amniotic membrane as a bioscaffold for bone marrow-derived mesenchymal stem cells: ultrastructural study. J Mol Histol. https://doi.org/10.1007/s10735-018-9768-1
Schinke T, Liese S, Priemel M, Haberland M, Schilling AF, Catala-Lehnen P et al (2004) Decreased bone formation and osteopenia in mice lacking alpha-calcitonin gene-related peptide. J Bone Miner Res 19(12):2049–2056
Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ (2002) Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem cells 20(6):530–541
Sun XK, Zhou J, Zhang L, Ma T, Wang YH, Yang YM, Tang YT, Li H, Wang LJ (2017) Down-regulation of Noggin and miR-138 coordinately promote osteogenesis of mesenchymal stem cells. J Mol Histol 48(5–6):427–436
Truong TH, Moorjani R, Dewey D, Guilcher GMT, Prokopishyn NL, Lewis VA (2016) Adverse reactions during stem cell infusion in children treated with autologous and allogeneic stem cell transplantation. Bone Marrow Transplant 51(5):680
Uslu S, Irban AG, Gereli A, Aydinlar EI, Elpen P, Ince U (2016) The effect of femoral nerve block on fracture healing via expressions of growth factors and beta-catenin. Folia Histochem Cytobiol 54(3):151–158
Wang L, Lin Z, Shao B, Zhuge Q, Jin K (2013) Therapeutic applications of bone marrow-derived stem cells in ischemic stroke. Neurol Res 35(5):470–478
Wang S, Xiao J, Li Z (2017) Role of substance P and calcitonin gene-related peptide in bone metabolism. Zhong nan da xue xue bao Yi xue ban = J Cent South Univ Med Sci 42(3):334–339
Xian L, Bao C, Xu HHK, Jian P, Jing H, Ping W et al (2016) Osteoprotegerin gene-modified BMSCs with hydroxyapatite scaffold for treating critical-sized mandibular defects in ovariectomized osteoporotic rats. Acta Biomater 42:378–388
Xiang L, Ma L, Wei N, Wang T, Yao Q, Yang B et al (2017) Effect of lentiviral vector overexpression alpha-calcitonin gene-related peptide on titanium implant osseointegration in alpha-CGRP-deficient mice. Bone 94:135–140
Yang Z, Zhu L, Li F, Wang J, Wan H, Pan Y (2014) Bone marrow stromal cells as a therapeutic treatment for ischemic stroke. Neurosci Bull 30(3):524–534
Yu X, Lv L, Zhang J, Zhang T, Xiao C, Li S (2015) Expression of neuropeptides and bone remodeling-related factors during periodontal tissue regeneration in denervated rats. J Mol Histol 46(2):195–203
Zhang Y, Xu J, Ruan YC, Yu MK, O’Laughlin M, Wise H et al. (2016). Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med 22(10):1160–1169
Zhu LY, Feng ZY, Zheng TY, Dan L, Zhou Q, Li FG et al (2016) Autologous bone marrow stem cells injection therapy resistant multi drug tuberculosis patients efficacy and safety. J Hunan Normal Univ (Med Sci) 13(1):40–42
Zou D, Zhang Z, He J, Zhu S, Wang S, Zhang W et al (2011) Repairing critical-sized calvarial defects with BMSCs modified by a constitutively active form of hypoxia-inducible factor-1α and a phosphate cement scaffold. Biomaterials 32(36):9707–9718
Acknowledgements
National Natural Science Foundation of China (81271138). Natural Science Foundation of Shandong Province (ZR2017QH007). Open Foundation of Shandong Provincial Key Laboratory of Oral Tissue Regeneration (SDKQ201704).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yu, X., Liu, S., Chen, H. et al. CGRP gene-modified rBMSCs show better osteogenic differentiation capacity in vitro. J Mol Hist 49, 357–367 (2018). https://doi.org/10.1007/s10735-018-9775-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-018-9775-2