Abstract
This study aims to elucidate the mechanisms of Wnt/β-catenin signaling pathway in the development of preeclampsia (PE). The mRNA levels of Wnt1, β-catenin, c-myc and cyclinD1 were determined by real-time PCR in the placentas. Moreover, the expression levels of Wnt1, β-catenin, Dickkopf-1 (DKK1) and glycogen synthase kinase 3β (GSK-3β) proteins were detected by Western blot. Immunohistochemistry was used in placental tissue microarray to localize the expression of Wnt1, β-catenin, DKK1 proteins in the placentas of two groups. Compared with the control placentas, the mRNA levels of Wnt1, β-catenin, c-myc and cyclinD1 were decreased in the severe preeclamptic placentas. The Western blot results showed that the expression levels of Wnt1, β-catenin, and GSK-3β proteins were significantly elevated in the control group, while the expression level of DKK1 was significantly decreased. In addition, the staining intensity of Wnt1, β-catenin were weaker in the placentas of the severe PE group while the staining intensity of DKK1 was significantly stronger in the placentas of the severe PE group. Wnt/β-catenin signaling pathway may play a significant role in the pathogenesis of PE by regulating the invasion and proliferation of trophoblast.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preeclampsia (PE) is a pregnancy-specific disease which is characterized by hypertension, proteinuria and other systemic disorders (Mol et al. 2016). The PE, especially severe PE (sPE), contribute to the fetal and maternal morbidity and mortality, and the incidence of PE is 3–5% among pregnancy women (Tranquilli et al. 2012; Ananth et al. 2013). Although numerous studies have investigated the mechanism of PE, but its cause has yet to be defined (Redman et al. 1999; Kaufmann et al. 2003; Romero and Chaiworapongsa 2013). It is a widely accepted hypothesis that placenta play a significant role in the development of PE (Zhang et al. 2013a). Trophoblast cells are the major cell type in placental tissue. The proliferation, differentiation and apoptosis of trophoblasts may contribute to the development of PE. Multiple signaling pathways involved in regulating the proliferation and apoptosis of trophoblast during the placentation process, including Wnt signaling pathway.
It was reported that Wnt signaling could regulate cell proliferation, migration, and invasion from hydras to humans (Zhang et al. 2017). Canonical “Wnt/β-catenin” pathway, noncanonical pathway and the “adjust the spindle orientation and asymmetric cell division” pathway are three Wnt signaling pathways in human (Kestler and Kuhl 2008). Among the three Wnt signaling pathways, the Wnt/β-catenin signaling pathway is the most widely studied. Wnt1 is one of the most important Wnt ligands, and it is demonstrated that Wnt1 is overexpressed in various human cancers. Compared with term trophoblasts, the expression level of Wnt1 was detected to be higher in first trimester trophoblasts, suggesting that Wnt1 may regulate trophoblast invasion (Sonderegger et al. 2007). Dickkopf-1 (DKK1) is a secreted glycoprotein which can binds to Wnt ligands and thereby affects a variety of biological responses. Wnt/β-catenin signaling pathway mainly depends on the level of β-catenin in the cell. In the absence of Wnt, the level of β-catenin is low in cytoplasm. The β-catenin is phosphorylated by a multiprotein destruction complex, which includes glycogen synthase kinase 3β (GSK3β), thus contributing to the reduction of β-catenin (Valenta et al. 2012). In the end, the level of β-catenin remains low, and cannot activate nuclear transcription, such as c-myc, cyclinD1. In contrast, in the presence of Wnt ligands, Wnt signalling components bind to membrane receptor Frizzled, thereby inhibiting the multiprotein destruction complex, resulting in an increase of β-catenin. Then the increased β-catenin in the cytoplasm is transferred to the nucleus followed by the activation of downstream target genes.
It was demonstrated that the expression levels of DKK1 and sFRP4 were increased in the placentas of patients with severe PE compared with the normal placental tissues, while the levels of Wnt2 and β-catenin were reduced (Zhang et al. 2013a, b), suggesting that the Wnt/β-catenin signal pathway may serve an important role in the development of PE. To elucidate Wnt/β-catenin involved in the pathogenesis of PE, we detected the expression of Wnt/β-catenin signal pathway and its downstream target gene in the placentas of patients with severe PE in this study.
Materials and methods
Participators
First, 30 severe preeclamptic patients and 32 normal pregnancy women from The Third Affiliated Hospital of Zhengzhou University, from October 2015 to March 2016, were enrolled in this study. Then 32 women with normal pregnancy constituted the control group while 30 women with severe PE were classified as the severe PE group. This study was approved by the Ethics Committee of Zhengzhou University School of the Third Clinical Medicine. All participators signed informed consent. The criteria for diagnosis of severe PE were strictly according to the American College of Obstetricians and Gynecologists Practice Bulletin (Committee on Obstetric Practice 2002). Patients with heart, liver, kidney and endocrine diseases were excluded from this study.
Sample collection
The placental tissues were obtained within 15 min after delivery. Five small pieces of biopsies (2 cm × 2 cm × 1 cm) were collected from the placental center, as well as from each quadrant to avoid sampling bias. Samples with infarction, hemorrhage and calcification area were excluded in this study. The phosphate buffer was used to rinse tissue and then sterile filter paper was used to remove the blood in tissue. A part of placentas was fixed in 10% formalin for construction of tissue microarray, and the other samples were immediately frozen in liquid nitrogen and stored at − 80 °C.
RNA extraction and quantitative RT-PCR
Total RNA was extracted from 62 placental tissues using Trizol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturers’ protocols. Ethidium bromide staining of the nucleic acids before agarose gel electrophoresis was used to evaluate the RNA integrity. According the manufacturer’s protocols, Reverse Transcriptase M-MLV kit was used to prepare complementary DNA (CWBIO, Beijing, China). The expression levels of Wnt1, β-catenin, c-myc, cyclinD1 in the two groups were performed using the Ultra SYBR Mixture (with ROX) (CWBIO, Beijing, China) on an Applied Biosystems 7500 (ABI, Foster City, California). The utilized primers are shown in Table 1. Cycling conditions were as follows: denaturation at 95 °C for 10 min, followed by cycling (35 times) at 95 °C for 15 s and 60 °C for 1 min. In order to ensure the amplification of the product, the temperature was increased by increments of 0.2 °C from 60 to 95 °C during the melt cycle. All samples were run in triplicate. The comparative method 2−ΔΔCT was used for the relative quantification of Wnt1, β-catenin, c-myc, cyclinD1 transcription in the two groups.
Western blotting analysis
Western blotting was used to detect the expression of Wnt1, DKK1, GSK-3β and β-catenin proteins in the placentas of two groups as we described in the previous study (Zhang et al. 2018). In brief, total proteins were extracted from frozen tissue with radio immunoprecipitation assay lysate buffer (Solarbio, Beijing, China). The total protein concentration was measured by a BCA assay (Sangon, Shanghai, China) according to the manufacturers’ instructions. For Western blot analyses, the samples containing 30 mg of protein were separated by 10% SDS polyacrylamide gel electrophoresis, and then gels were electrophoretically transferred to the nitrocellulose membrane (Millipore, MA, USA). After the transmembrane is completed, the membranes were blocked for 1 h at room temperature with blocking buffer (5% nonfat milk, 0.1% Tween 20). After the blocking, the membranes were incubated overnight at 4 °C with anti-Wnt1 (1:2000 dilution, Abcam), anti-β-catenin (1:4000 dilution, Abcam), anti-GSK-3β (1:1000 dilution, Abcam), anti-DKK1 (1:1000 dilution, Abcam) respectively. Then the membranes were incubated for 2 h with the secondary fluorescent antibodies (1:4000 dilution, Odyssey CIX) at room temperature. The fluorescence intensity was detected by the Infrared Laser Scanning Imaging System (Odyssey CIX, LINCOLN, USA).
Immunohistochemistry (IHC) in placental tissue microarray
IHC was used to evaluate the localization and expression of Wnt1, DKK1, and β-catenin proteins in placental tissue microarray (TMA) as we described in the previous study (Zhu et al. 2015). In brief, placental TMA sections were deparaffinized two times with xylene for 10 min each, and then hydrated through graded alcohol. After deparaffinization and hydration to distilled water, antigen recapture was performed. The sections were infiltrated into the prepared antigen repair solution, heated in a microwave oven above 90 °C first, and then low-fire 20 min. After antigen recapture, the sections were incubated with 3% hydrogen peroxide for 15 min to block endogenous. Furthermore, the sections were incubated with anti-Wnt1 (Abcam, USA) at a dilution of 1:200, anti-β-catenin (Abcam, USA) at a dilution of 1:150 and anti-DKK1(Abcam, USA) at a dilution of 1:200. Then the slices were incubated with IHC detection reagent after being washed in PBS three times. The reaction was visualized by DAB. Finally, the slices were counterstained with hematoxylin for 5 min. Placental TMA stained with PBS served as the controls. The slides were examined by the inversion fluorescence microscope (OLYMPUS IX-71, Tokyo, Japan). Immunostaining of Wnt1, β-catenin and DKK1 in the placental TMA were graded on a semiquantitative scale: 0, absent staining/no color; 1, weak staining/pale brown color; 2, moderate staining/dark brown color; 3, strong staining/brownish-black color. There were two investigators to separately assess the intensity of the slices (XF and XX).
Statistical analysis
Statistical analyses were performed by SPSS 24.0. The data were showed as the means ± SD. Student t test, Manne–Whitney and Chi square test were used to compare the differences between the two groups. P < 0.05 was considered statistically significant.
Results
Clinical characteristics in normal controls and PE groups
No significant differences were shown in maternal age, BMI between two groups (Table 2). However, it is demonstrated that the systolic blood pressure and diastolic blood pressure, as well as the level of urine protein (g/24 h) in normal women is significantly lower than that of the women with sPE. On the contrary, gestational age of mother, birth weight and status of fetus in control group were significantly higher compared with the sPE group (P < 0.001).
Wnt1, β-catenin, c-myc and cyclinD1 mRNA expression in the placenta
Compared with the sPE group, the expression levels of Wnt1, β-catenin were significantly increased in the normal pregnancy group (P < 0.01, Fig. 1). It has been proven that c-myc and cyclinD1 are the target genes of Wnt signal pathway (Hubel et al. 2007). Quantitative RT-PCR was performed to elucidate the expression level of c-myc and cyclinD1 genes in the two groups. The results suggested that there were statistically significant increase of c-myc mRNA and cyclinD1 mRNA expression in the normal pregnancy placentas compared with the preeclamptic specimens (P < 0.01, Fig. 1).
Quantitative real-time PCR of Wnt1, β-catenin, c-myc and cyclinD1 mRNA expression in the placentas of the two groups. Compared with the control group, mRNA of Wnt1, β-catenin, c-myc and cyclinD1 are decreased in the severe PE group. Bars indicate the means ± SD. GAPDH was used as an internal control. *P < 0.05, **P < 0.01
Wnt1, DKK1, β-catenin and GSK-3β protein expression in the placentas of two groups
Western blot was then performed to verify the protein expression levels of Wnt1, DKK1, β-catenin and GSK-3β in the placentas of two groups. Compared with the normal pregnancy group, significantly decreased expression levels of Wnt1, β-catenin and GSK-3β were detected in the placentas of the sPE group (0.952 ± 0.129 vs. 0.531 ± 0.104, P < 0.001; 0.876 ± 0.101 vs. 0.534 ± 0.127, P < 0.001; 0.406 ± 0.061 vs. 0.326 ± 0.070, P = 0.002 respectively; Fig. 2). However, the expression level of DKK1 was significantly higher in the preeclamptic placentas than that of the normal pregnancy women (0.921 ± 0.086 vs. 0.497 ± 0.110, P < 0.001. Fig. 2). β-actin (43KD) and GAPDH (37KD) were used as internal control.
The protein levels of Wnt1, β-catenin, GSK-3β, and DKK1 in the placentas of two groups by Western blot. a Representative examples of four proteins in the placentas from normal pregnancies and the severe PE. 1–2, normal control; 3–4, severe PE. b Quantification of Wnt1, β-catenin, GSK-3β, and DKK1 Western blot data obtained from the two groups. Bars represent the mean values; error bars indicate SD. **P < 0.01, ***P < 0.001
Localizations of Wnt1, DKK1 and β-catenin protein expression in the placentas
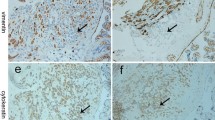
IHC was performed in placental TMA to localize the expression of Wnt1, DKK1 and β-catenin in the two groups. Hematoxylin and eosin (H&E) staining were used to examine the placental TMA before the IHC analysis (as shown in Fig. 3). Moreover, H&E staining demonstrated villous trophoblast cells (VT) and extravillous cytotrophoblast cells (EVT) were well separated by TMA. Wnt1, DKK1 and β-catenin were mainly expressed in the cytoplasm and the membrane of the EVT and VT (Figs. 4, 5, 6). It was showed that Wnt1, β-catenin revealed weaker staining intensity in the placentas of the sPE group (P < 0.01, Figs. 4c, d, 5c, d) than the normal pregnancy group (Figs. 4a, b, 5a, b) in both EVT and VT. On the contrary, the staining intensity of DKK1 was significantly stronger in the placentas of the sPE group (Fig. 6c, d) than that of the normal placentas (Fig. 6a, b) (P = 0.01). The immunostaining intensities were then analyzed in Table 3. Moreover, negative control was shown in Figs. 4e, f, 5e, f, 6e, f.
β-catenin staining of placental TMA in the two groups. a, b β-Catenin staining of the normal pregnancy placental TMA. c, d β-Catenin staining of the preeclamptic placental TMA. Compared with the severe PE group, β-catenin expression is significantly increased in the normal pregnancy group. e, f Negative control. The arrows point to the positive cells. VT villous trophoblast, EVT extravillous trophoblast
DKK1 staining of placental TMA in the two groups. a, b DKK1 staining of the normal pregnancy placental TMA. c, d DKK1 staining of the preeclamptic placental TMA. Compared with the normal controls, DKK1 expression is significantly increased in the sPE group. e, f Negative control. VT villous trophoblast, EVT extravillous trophoblast
Discussion
PE is the leading cause of maternal and perinatal morbidity and mortality in the developing countries. There are various theories in the pathogenesis of PE. It is recognized that sPE is a placenta-derived disease, and abnormal trophoblast invasion is the key link in the pathogenesis (Sabai et al. 2005). Abnormal differentiation of trophoblasts plays a significant role in placenta formation, fetal growth and pregnancy success, lead to variety of gestational diseases such as PE (Goldman-Wohl and Yagel 2002).
It is a widely accepted classification of PE that it is divided to early-(< 34 weeks of gestation) and late-onset (> 34 weeks of gestation) PE (Valensise et al. 2008). Studies found that the pathogenesis of early-and late-onset PE were different. Early-onset PE may be caused by placental disorder of insufficient perfusion of the spiral artery while late-onset PE seems to be a manifestation of metabolic disorders (Von Dadelszen et al. 2003; Herzog et al. 2017). Recent studies have shown that late-onset PE may be associated with trophoblastic dysfunction, resulting in decreased intervillus perfusion and increased hypoxia (Redman et al. 2014). In the present study, the gestational age of sPE group is 36.45 ± 1.35 weeks, indicating that most preeclamptic patients enrolled this study are late-onset PE. This study focuses on the abnormal differentiation of trophoblast to elucidate the mechanisms of the development of PE.
Studies have shown that there are multiple signaling pathways involved in the regulation of the proliferation, differentiation, apoptosis, and invasion of trophoblasts during placenta formation. It was demonstrated that Wnt3a could stimulate trophoblast invasion of the basement membrane matrix, suggesting that Wnt signaling pathway may be involved in the proliferation, differentiation, and invasion of trophoblast cells (Bryan et al. 2010). In the present study, our results demonstrated that the mRNA expression of Wnt1 in placentas of the sPE patients was significantly lower than that of the normal group by qRT-PCR, and the difference between the two groups was statistically significant. The Wnt1 protein expression level measured by Western blot is consistent with the mRNA expression. Furthermore, we identified the localization of the Wnt1 protein in the placental TMA using IHC. Our results indicated that Wnt1 was mainly expressed in the cytoplasm and cell membrane of villous VT and the EVT, whereas Wnt1 showed a stronger staining intensity in the normal pregnancy group than that of the sPE group. It was reported that the expression of Wnt1 was higher in first trimester trophoblasts than late trimester trophoblasts, indicating that Wnt1 may regulate trophoblast invasion (Sonderegger et al. 2007). Wnt1 is an important ligand of Wnt/β-catenin signaling pathway. The higher expression of Wnt1 in the VT and EVT probably indicated Wnt1 might contribute to the abnormal invasion and apoptosis of trophoblast.
DKK1 is a secreted glycoprotein that binds to Wnt ligands and plays an important role in diverse developmental processes. The mRNA expression of DKK1 was increased during decidualization of endometrial stromal cells, indicating Wnt pathway may play a role in endometrial differentiation (Tulac et al. 2003). It was reported that DKK1 reduced the proliferation of cytotrophoblasts in human villous explants (Pollheimer et al. 2006). Our results showed that the expression of DKK1 protein was significantly risen in the sPE group. Moreover, the staining intensity of DKK1 by IHC was stronger in the preeclamptic TMA, which is in accordance with our previous study (Zhang et al. 2013b).
β-catenin and GSK-3β are considered to be the switch of Wnt/β-catenin signaling pathway. It has been reported that reduced level of β-catenin could lead to decreased in the invasion ability of trophoblast in HTR8/Svneo cells, suggesting abnormal activation of Wnt/β-catenin signaling contribute to the development of PE (Rao et al. 2018). In this study, a significant decrease of β-catenin and GSK-3β protein expression were observed in the sPE group. Moreover, β-catenin mRNA expression level was determined by using qRT-PCR. The results showed that there was a significant decrease of β-catenin mRNA expression in the sPE group (Fig. 1). In addition, we also found that β-catenin localized at the VT and the EVT in placenta, however, the staining intensity of β-catenin in the normal pregnancy group was significantly stronger than that of the sPE group. The expression of DKK1 and β-catenin agreed with our previous study, the main difference was that we used placental TMA to localize the expression of both proteins in this study. However, TMA technology allows a uniform analysis of placental tissue in a large scale which is more efficient, scientific, and more convincing.
C-myc and cyclinD1, downstream target genes of Wnt/β-catenin signaling pathway, were investigated in this study. When there is a certain number of β-catenin in the cytoplasm, it will be transferred into the nucleus, followed by activating the downstream target gene, such as c-myc and cyclinD1. In the present study, compared with the normal pregnancy group, the expression levels of c-myc mRNA and cyclinD1 mRNA were decreased in the sPE group. C-myc and cyclinD1 are both oncogene that can stimulation of cell cycle progression, cell proliferation and apoptosis (Liao et al. 2007). It was demonstrated that c-myc can inhibit trophoblast differentiation (Kumar et al. 2013). The decreased expression of c-myc and cyclinD1 in the placentas with sPE indicate that Wnt/β-catenin signaling pathway may play an essential role in the development of PE by regulating the invasion and proliferation of trophoblast.
However, we only detected the expression of Wnt/β-catenin signaling pathway related proteins and genes in human placenta. Further research will focus on how the Wnt/β-catenin signaling pathway contributes to the development of PE by using cell lines.
Conclusion
In summary, compared with the normal pregnancy, the expression levels of Wnt1, β-catenin, GSK-3β, c-myc and cyclinD1 were decreased in the placentas of patients with severe PE, while the expression level of DKK1 was higher in the severe preeclamptic placentas. Abnormal activation of Wnt/β-catenin may contribute to the development of PE by regulating the invasion and proliferation of trophoblast.
Change history
29 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10735-021-10043-9
References
Ananth CV, Keyes KM, Wapner RJ (2013) Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ 347:1–9. https://doi.org/10.1136/bmj.f6564
Committee on Obstetric Practice A (2002) Practice bulletin #33: diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol 99(2):347–365
Goldman-Wohl D, Yagel S (2002) Regulation of trophoblast invasion: from normal implantation to pre-eclampsia. Mol Cell Endocrinol 187:233–238. https://doi.org/10.1016/S0303-7207(01)00687-6
Herzog EM, Eggink AJ, Reijnierse A et al (2017) Impact of early- and late-onset preeclampsia on features of placental and newborn vascular health. Placenta 49:72–79. https://doi.org/10.1016/j.placenta.2016.11.014
Hubel CA, Wallukat G, Wolf M et al (2007) Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension 49:612–617
Kaufmann P, Black S, Huppertz B (2003) Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod 69:1–7. https://doi.org/10.1095/biolreprod.102.014977
Kestler HA, Kuhl M (2008) From individual Wnt pathways towards a Wnt signalling network. Philos Trans R Soc B Biol Sci 363:1333–1347. https://doi.org/10.1098/rstb.2007.2251
Kumar P, Luo Y, Tudela C et al (2013) The c-Myc-regulated microRNA-17 92 (miR-17 92) and miR-106a 363 clusters target hCYP19A1 and hGCM1 to inhibit human trophoblast differentiation. Mol Cell Biol 33:1782–1796. https://doi.org/10.1128/MCB.01228-12
Liao DJ, Thakur A, Wu J et al (2007) Perspectives on c-Myc, cyclin D1, and their interaction in cancer formation, progression, and response to chemotherapy. Crit Rev Oncog 13:93–158. https://doi.org/10.1615/CritRevOncog.v13.i2.10
MacDonald BT, Tamai K, He X (2010) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Biol 17:9–26. https://doi.org/10.1016/j.devcel.2009.06.016.
Mol BWJ, Roberts CT, Thangaratinam S et al (2016) Pre-eclampsia. Lancet 387:999–1011. https://doi.org/10.1016/S0140-6736(15)00070-7
Pollheimer J, Loregger T, Sonderegger S et al (2006) Activation of the canonical wingless/T-cell factor signaling pathway promotes invasive differentiation of human trophoblast. Am J Pathol 168:1134–1147. https://doi.org/10.2353/ajpath.2006.050686
Rao H, Bai Y, Zhang F et al (2018) The role of SATB1 in HTR8/SVneo cells and pathological mechanism of preeclampsia. J Matern Fetal Neonatal Med 16:1–10. https://doi.org/10.1080/14767058.2018.1425387
Redman CWG, Sacks GP, Sargent IL (1999) Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol 180:499–506. https://doi.org/10.1016/S0002-9378(99)70239-5
Redman CW, Sargent IL, Staff AC (2014) IFPA senior award lecture: making sense of pre-eclampsia—two placental causes of preeclampsia? Placenta 35:S20–S25. https://doi.org/10.1016/j.placenta.2013.12.008
Romero R, Chaiworapongsa T (2013) Preeclampsia: a link between trophoblast dysregulation and an antiangiogenic state. J Clin Invest 123:2775–2777. https://doi.org/10.1172/JCI70431
Sabai B, Dekker G, Kupferminc M (2005) Pre-eclampsia. Lancet 365:785–799. https://doi.org/10.1016/S0140-6736(05)17987-2
Sonderegger S, Husslein H, Leisser C, Knöfler M (2007) Complex expression pattern of Wnt ligands and frizzled receptors in human placenta and its trophoblast subtypes. Placenta 28:1–12. https://doi.org/10.1016/j.placenta.2006.11.003
Tranquilli AL, Landi B, Giannubilo SR, Sibai BM (2012) Preeclampsia: no longer solely a pregnancy disease. Pregnancy Hypertens 2:350–357. https://doi.org/10.1016/j.preghy.2012.05.006
Tulac S, Nayak NR, Kao LC et al (2003) Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. J Clin Endocrinol Metab 88:3860–3866. https://doi.org/10.1210/jc.2003-030494
Valensise H, Vasapollo B, Gagliardi G, Novelli GP (2008) Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension 52:873–880. https://doi.org/10.1161/HYPERTENSIONAHA.108.117358
Valenta T, Hausmann G, Basler K (2012) The many faces and functions of Î 2-catenin. EMBO J 31:2714–2736. https://doi.org/10.1038/emboj.2012.150
Von Dadelszen P, Magee LA, Roberts JM (2003) Subclassification of preeclampsia. Hypertens Pregnancy 22:143–148. https://doi.org/10.1081/PRG-120021060
Zhang Z, Li H, Zhang L et al (2013a) Differential expression of beta-catenin and dickkopf-1 in the third trimester placentas from normal and preeclamptic pregnancies: a comparative study. Reprod Biol Endocrinol 4:17. https://doi.org/10.1186/1477-7827-11-17
Zhang Z, Zhang L, Zhang L et al (2013b) Association of Wnt2 and sFRP4 expression in the third trimester placenta in women with severe preeclampsia. Reprod Sci 20:981–989. https://doi.org/10.1177/1933719112472740
Zhang Z, Wang X, Zhang L et al (2017) Wnt/β-catenin signaling pathway in trophoblasts and abnormal activation in preeclampsia (Review). Mol Med Rep 16:1007–1013. https://doi.org/10.3892/mmr.2017.6718
Zhang Z, Wang X, Wang J, Zhang L (2018) The decreased expression of Stat3 and p-Stat3 in preeclampsia-like rat placenta. J Mol Histol 49:175–183. https://doi.org/10.1007/s10735-018-9757-4
Zhu L, Zhang Z, Zhang L et al (2015) HMGB1-RAGE signaling pathway in severe preeclampsia. Placenta 36:1148–1152. https://doi.org/10.1016/j.placenta.2015.08.006
Acknowledgements
The present study was supported by the National Natural Science Foundation (Grant Number 81501285).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, X., Zhang, Z., Zeng, X. et al. Wnt/β-catenin signaling pathway in severe preeclampsia. J Mol Hist 49, 317–327 (2018). https://doi.org/10.1007/s10735-018-9770-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-018-9770-7