Abstract
Protein tyrosine phosphatase 1B (PTP1B) is a non-transmembrane protein tyrosine phosphatase that has been implicated in cancer pathogenesis. However, the expression level and the role of PTP1B in the development and prognosis of colorectal cancer (CRC) remain unclear. In this study, the expression of PTP1B in CRC tissues and matched noncancerous tissues were detected by using immunohistochemistry, real-time PCR and Western blotting. The correlations between PTP1B expression level and clinicopathologic characteristics and patient survival were analyzed. We found that PTP1B expression was significantly higher in CRC tissues compared with matched non-tumour tissues. Statistical analysis showed that the PTP1B expression was correlated with tumor differentiation, tumor invasion, lymph node metastasis, and TNM stage. Patients with higher expressions of PTP1B had the lower survival (P = 0.012). Taken together, our results suggest that PTP1B expression might play a critical role in the progression of CRC and may serve as a valuable prognostic biomarker for CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common malignant tumors in the world (Bretthauer and Kalager 2012). It ranks second in Europe and third in the United States (de Wijkerslooth et al. 2011; Cummings and Cooper 2011). The incidence of CRC malignancy and mortality is increasing in China and other developing countries, and now it is the third most common cancer (Zhou et al. 2010; Mahmodlou et al. 2012; Shin et al. 2011; Moghimi-Dehkordi and Safaee 2012). Researches show that if the CRC is detected at an early stage, about 90 % of the patients can be cured by surgery, but unfortunately the disease is very often diagnosed only at an advanced stage, and so prognosis is poor (Walsh and Terdiman 2003). Therefore, it is critical for increasing our understanding of the molecular mechanisms leading to development, spread and metastasis of CRC and for identifying potentially prognostic and predictive biomarkers for the CRC.
Protein phosphorylation and dephosphorylation are fundamental cellular events mediated by kinases and phosphatases, respectively, which govern a host of cell functions such as growth, differentiation, adhesion and mobility (Soulsby and Bennett 2009). The protein tyrosine phosphatase 1B (PTP1B) is a classical non-transmembrane protein tyrosine phosphatase that is an important regulator of signaling pathways involved in human diseases such as obesity, diabetes, and cancer. Changes in expression and activity of PTP1B have been shown to be associated with various human cancers, and experimental evidences suggest that it can exert both tumor suppressing and tumor promoting effects (Lessard and Stuible 2010). On one hand, several reports suggest that PTP1B can promote apoptosis and might be a tumor suppressor gene (Zheng et al. 2012; Woodford-Thomas et al. 1992; Brown-Shimer et al. 1992; Sangwan et al. 2006; Gonzalez-Rodriguez et al. 2007). On the other hand, some studies show that PTP1B might act as a tumor promoter and has been correlated with cancer progression. For example, PTP1B is able to block anti-tumor signaling pathways in carcinoma and lymphoma cell lines (Lu et al. 2008). Some researches showed that PTP1B is frequently overexpressed in a wide variety of human cancers, such as ovarian carcinomas (Wiener et al. 1994), breast cancer Wiener et al. (1994), squamous cell carcinomas (Nanney et al. 1997), gastric cancer (Wang et al. 2012) and prostate cancer (Lessard et al. 2012; Taylor et al. 2010). In addition, some studies have indicated a possible link between PTP1B expression and malignant behaviors of cancer cells. For instance, overexpression of PTP1B has been shown to promote invasion, migration, and growth of tumor in human cancer cells both in vitro and in vivo (Wang et al. 2012; Lessard et al. 2012; Wang et al. 2010). About the role of PTP1B in CRC, a resesrch showed that upregulation of PTP1B was associated with increased tumor growth in colon cancer cell lines (Zhu et al. 2007). However, PTP1B expressions and the association of PTP1B expressions with the clinicopathological characteristics and prognosis in CRC tissue have not been investigated to date.
In order to investigate the role of PTP1B in the CRC, in the present study, real-time PCR, Western blotting and immunohistochemistry assays were performed to detect PTP1B expression in CRC tissues and paired non-tumour tissues. Meanwhile, we analyzed the correlation between PTP1B expression and clinicopathologic characteristics, including prognosis.
Materials and methods
Patients and tissue samples
96 primary tumor samples and matched adjacent non-tumour tissues were collected from patients with CRC who underwent complete surgical resection at the Department of General Surgery, the Fourth Hospital of Hebei Medical University from January 2005 to November 2006. All patients included in this study had not received preoperative radiotherapy, chemotherapy, or immunotherapy before surgery. CRC patients with diabetes and/or obesity have been excluded from the study. All tumors were reviewed and confirmed by two senior pathologists using the World Health Organization classification of tumors of the digestive system (Hamilton and Aaltonen 2000). The histological type and grade of cell differentiation were classified according to the criteria established by the World Health Organization (WHO). The depth of invasion and lymph node metastasis were graded according to the 2009 American Joint Committee on Cancer’s TNM system (Stephen et al. 2009). The patients’ characteristics were retrieved from the medical records and are shown in Table 1. Additionally, 30 surgical samples of CRC and matched non-tumour tissues were collected from December 2011 to September 2012, and they were frozen immediately in liquid nitrogen for real time PCR analysis and Western blot analysis, respectively. Informed consent was obtained from all the patients before surgery, and this study was authorised by the Ethics Committee of the hospital.
Immunohistochemical staining and scoring
Immunohistochemistry was performed by the Envision Systems (Gene Tech Company Limited, Shanghai, China), according to the kit manufacturer’s instructions. In brief, consecutive paraffin wax embedded, tissue sections (4 um) were deparaffinized in xylene for 1.5 h, dehydrated with graded ethanol washes (100–70 %). Antigen retrieval was performed by pretreament of the slides in citrate buffer (pH 6.0) in a microwave oven for 15 min. Thereafter, slides were cooled to room temperature and washed by PBS-T (PBS with 0.5 % tween 20) for 5 min × 3. Endogenous peroxidase activity was quenched by incubating the slides in 3 % hydrogen peroxide for 15 min, followed by washing in PBS-T for 5 min × 3, and incubated at 4 °C overnight in anti-PTP1B primary monoclonal antibody (1:200 dilution, Abcam). After that, the sections were rinsed with PBS-T for 5 min × 3, and incubated with horseradish peroxidase-linked goat anti-rabbit antibodies, followed by reaction with DAB (3,3′-diaminobenzidine) and counterstaining with Mayer’s haematoxylin.
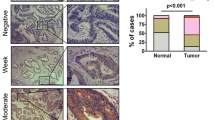
Each sample was scored by two independent investigators in a double-blind manner. Two independent investigators reviewed and scored the subcellular localization, staining intensity, and percentage of stained cells for each image. Score differences were discussed to obtain a consensus. PTP1B expression was evaluated as described previously (Li et al. 2012). The intensity of staining was scored according to 4 categories, where 0 indicates negative; 1, weak; 2, moderate; and 3, strong staining. The percentage of positive cells was also graded on a semiquantitative scale from 0 to 3, where 0 was given for 0 %, 1 for 1–10 %, 2 for 11–50 %, and 3 for 51–100 %. The sum of the percentage and intensity scores was used to identify two categories of expression: 0–2, low expression, and 3–6, high expression.
Quantitative real-time PCR
Total RNA was isolated from the fresh tumor and non-tumor tissues using the RNA simple total-RNA kit (Invitrogen, Carlsbad, CA, USA), and the purity of RNA was determined by detecting the absorbance at 260 and 280 nm with an ultramicrospectrophotometer (Thermo, NanoDrop 2000, America). One microgram of total RNA was reverse transcribed using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, America) according to the manufacturer’s protocol. Real-time PCR was carried out in 18ul reaction mixture containing 10ul of GoTaq® qPCR Master Mix(Promega, Beijing, China), 0.2uM of forward and reverse primers, 2ul template cDNA, and 7.2ul nuclease-free water. The thermal cycling program was set as follows: 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 1 min. PCR primers were designed using Primer-BLAST (NCBI, USA), and chemically synthesized by BGI (Beijing,China). The forward and reverse primers were 5′ -GACGAGGACCATGCACTGAG -3′ and 5′-GGAGGAGGGTCAGGCTATGT-3′ for the target gene PTP1B, and were 5′-CAACCGCGAGAAGATGACCCA-3′ and 5′-GTCACCGGAGTCCATCACGA-3′ for the reference gene β-actin. Melt-curve were generated to assure product specificity. Quantitative real-time PCR reactions were performed in triplicate on a Real-Time PCR systems (Agilent Technologies, Stratagene Mx3000P). Data were analyzed through the comparative threshold cycle (C T) method (Livak and Schmittgen 2001).
Western blotting
Total proteins were extracted from frozen tissues using lysis buffer (50 mM Hepes pH 7.5, 150 mM NaCl, 2.5 mM EDTA, 0.5 % NP-40, 0.1 % SDS, 1 mM PMSF, 1 mM DTT, 0.2 % aprotinin and 0.5 % leupetin), and quantified by BCA protein assay kit (Biomed Beijing China). Next, an equivalent amount of proteins was resolved on 10 % sodium dodecyl sulfate polyacrylamide gels (SDS-PAGE), and then transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, USA) under 100 V constant voltage conditions for 1 h. After blocking with 5 % fat-free milk at room temperature for 1.5 h, the membranes were immunoblotted overnight at 4 °C with rabbit anti-human monoclonal PTP1B antibody (dilution 1:5,000, Abcam) or anti-β-actin antibody (dilution 1:5,000, Sigma, MO, USA). Binding of the primary antibodies was detected with horseradish peroxidase (HRP)-conjugated secondary antibodies and visualized with enhanced chemiluminescence kit (ECL-kit, Santa Cruz) and exposed on X-ray films.
Statistical analysis
Statistical analysis was performed with SPSS16.0 software package (SPSS, Chicago, IL, USA). Chi squared test was used to test the correlation between PTP1B expression in tumors and clinicopathologic variables. Survival differences were estimated using the Kaplan–Meier method and compared by the log-rank test. Cox proportional hazards models were utilized for univariate and multivariate analyses. Continuous data were compared using the Student’st test. All statistical tests were two-sided, and P values less than 0.05 were considered to be statistically significant.
Results
Overexpression of PTP1B in CRC tissues
To explore the role of PTP1B in CRC, we measured the expression of PTP1B mRNA in 30 paired CRC tissues and their corresponding non-tumour tissues by real-time PCR. Our result showed that PTP1B mRNA expression level was significantly higher in CRC samples than paired non-tumour tissues (Table 1 P < 0.05). In 21 of 30 patients, the mRNA expression levels of PTP1B were relatively higher in the cancer tissues than that in the matched non-tumour tissues. In addition, the expressions of PTP1B protein in CRC tissues and paired non-tumour tissues were also detected by Western blotting. Compared with the paired non-tumour tissues, changes observed by Western blotting were consistent with the results in real-time PCR study (Fig. 1). Collectively, our data suggest that PTP1B is overexpressed in CRC, and that PTP1B overexpression is due to the up-regulation of PTP1B mRNA.
Associations of PTP1B expression with clinicopathological features of CRC patients
To further evaluate the correlation between PTP1B expression and the clinicopathological characteristics of CRC patients, we detected the expression of PTP1B in 96 cases of CRC tissues and paired non-tumour tissues using immunohistochemical staining. We found that 62 CRC tissues and 27 non-tumour tissues were shown to be high expression for PTP1B. However, the remaining 34 CRC tissues and 69 non-tumour tissues did not express or low expression of PTP1B. (Tables 2, 3). Representative immunostainings of PTP1B in paired non-tumour tissues and CRC tissues were shown in Fig. 2. Meanwhile, we analyzed the relationships between the expression of PTP1B and clinicopathologic characteristics, and significant correlations were found between PTP1B expression and four clinicopathologic parameters, including tumor differentiation (P = 0.043), tumor invasion (P = 0.032), lymph node metastasis (P = 0.024), and TNM stage (P = 0.017). However, there were no statistical associations between PTP1B expression and the rest parameters, such as gender, age, and tumor location (Table 3).
Relationship between expression of PTP1B and prognosis of CRC patients
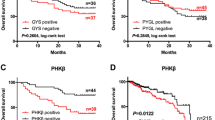
To investigate the prognostic relevance of PTP1B expression in CRC, all patients were followed-up for overall survival after operation. Follow-up time ranged from 6 to 65 months, and the mean follow-up time was 45.9 months. We evaluated the relationship between PTP1B protein expression and patient survival by using Kaplan–Meier analysis, and found that patients with high PTP1B expression had shorter survival times, however, those with low PTP1B expression had longer survival times (Fig. 3, log-rank, P = 0.012). Univariate analysis revealed that the patients with high PTP1B expression (P = 0.018), TNM stage (P < 0.001), lymph node metastasis (P < 0.001), Depth of invasion (P = 0.012), and tumor differentiation (P = 0.001) had significantly poorer survival (Table 4). The multivariate analysis indicated that PTP1B expression (P = 0.045), TNM stage (P = 0.015), lymph node metastasis (P = 0.014), were independent prognostic parameters for the CRC patients (Table 4).
Discussion
CRC is the most commonly leading causes of cancer-related death in the world, and the 5-year survival rate for CRC patients with the metastatic disease is approximately 19 % (Jemal et al. 2009). However, there are few tumor markers that have clinical utility in the management of CRC (Viana Lde et al. 2013). The founding of novel molecular markers indicating the progression and prognosis are thus becoming increasingly important for CRC patient. In the current study, we found that PTP1B expression was significantly associated with the progression and prognosis of human CRC.
A considerable number of researches have demonstrated that PTP1B is frequently overexpressed in several tumors (Lessard et al. 2012; Wang et al. 2012; Lessard et al. 2012). A study by Zhu S et al. (Zhu et al. 2007) showed that membrane PTP1B phosphatase activity levels were up-regulated in several naturally occurring human epithelial colon cancer cell lines, and inhibition of PTP1B can reduce the oncogenic properties of colon cancer cells. However, to the best of our knowledge, the PTP1B expression in human CRC tissues, the correlation between PTP1B expression and clinicopathologic parameters, and its prognostic significance in CRC have not been investigated. To address these issues, in the present study, we detected the expression of PTP1B in 30 CRC tissues and paired non-tumour tissues by quantitative real-time PCR and Western blot analysis. Our results showed that both PTP1B mRNA and protein levels were significantly higher in CRC tissues than in paired noncancerous tissues, suggesting that up-regulation of PTP1B may be an important event in the development and progression of CRC. Meanwhile, we detected the PTP1B protein expression in 96 primary CRC tissues and paired non-tumour tissues using immunohistochemistry, and analyzed the relationship between PTP1B expression and clinicopathological characteristics and evaluated its prognostic value in post-resection survival of CRC patients. We found that PTP1B was overexpressed in (62/96) 64.6 % primary CRC tissues, whereas (69/96)71.8 % non-tumour tissues didn’t display PTP1B expression or only exhibited faint expression. We also found that the overexpression of PTP1B was correlated with tumor differentiation, depth of invasion, lymph node metastasis, and TNM stage, but not with gender, age and tumor location. More importantly, our data showed that patients with higher PTP1B expression had a shorter overall survival, whereas patients with lower PTP1B expression had a better survival, and PTP1B is an independent prognostic factor for the outcome in CRC patients.
Nonetheless, Our study has other limitations. The number of investigated patients and the number of cases with positive staining for PTP1B are small, therefore, this may reduce the power to detect statistical associations and significantly affects survival analyses. So, further investigations are needed to confirm our observations in a larger series of CRC patients. In addition, the mechanism of action of PTP1B in the malignant cancer progression is still largely undefined. Wang et al. (2012) has reported that PTP1B might influence activities of Akt, Erk1/2, FAK, and Src to inhibit or enhance the proliferation, colony formation and in vivo tumorigenesis of gastric cancer cells. A study by Zhu et al. (2007) showed that elevated levels of PTP1B can increase tumorigenicity of colon cancer cells by activating Src. Thus, the molecular mechanism of PTP1B that is involved in the progression of CRC needs to be elucidated.
In summary, our study showed that PTP1B expression is up-regulated in CRC tissues and associated with tumor progression and poor prognosis in CRC patients. Our results provide novel evidence for a tumor-promoting role of PTP1B in CRC, and indicated that PTP1B may serve as a valuable prognostic biomarker and potential therapeutic target in CRC.
References
Bretthauer M, Kalager M (2012) Colonoscopy as a triage screening Test. N Engl J Med 366(8):759–760. doi:10.1056/NEJMe1114639
Brown-Shimer S, Johnson KA, Hill DE, Bruskin AM (1992) Effect of protein tyrosine phosphatase 1B expression on transformation by the human neu oncogene. Cancer Res 52(2):478–482
Cummings LC, Cooper GS (2011) Colorectal cancer screening: update for 2011. Semin Oncol 38(4):483–489. doi:10.1053/j.seminoncol.2011.05.002
de Wijkerslooth TR, Bossuyt PM, Dekker E (2011) Strategies in screening for colon carcinoma. Neth J Med 69(3):112–119
Gonzalez-Rodriguez A, Escribano O, Alba J, Rondinone CM, Benito M, Valverde AM (2007) Levels of protein tyrosine phosphatase 1B determine susceptibility to apoptosis in serum-deprived hepatocytes. J Cell Physiol 212(1):76–88. doi:10.1002/jcp.21004
Hamilton SR, Aaltonen LA (2000) Tumors of colon and rectum. World Health Organization classification of tumors: Pathology and genetics of tumors of digestive system. IARC, Lyon (in Press) 2000: 103–105
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics. CA Cancer J Clin 59(4):225–249. doi:10.3322/caac.20006
Lessard L, Stuible M (2010) Tremblay ML (2010) The two faces of PTP1B in cancer 1804. Biochim Biophys Acta 3:613–619. doi:10.1016/j.bbapap.2009.09.018
Lessard L, Labbé DP, Deblois G, Bégin LR, Hardy S, Mes-Masson AM, Saad F, Trotman LC, Giguère V, Tremblay ML (2012) PTP1B is an androgen receptor-regulated phosphatase that promotes the progression of prostate cancer. Cancer Res 72(6):1529–1537. doi:10.1158/0008-5472.CAN-11-2602
Li R, Liu B, Yin H, Sun W, Yin J, Su Q (2012) Overexpression of integrin-linked kinase (ILK) is associated with tumor progression and an unfavorable prognosis in patients. J Mol Hist 44(2):183–189. doi:10.1007/s10735-012-9463-6
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408. doi:10.1006/meth.2001.1262
Lu X, Malumbres R, Shields B, Jiang X, Sarosiek KA, Natkunam Y, Tiganis T, Lossos IS (2008) PTP1B is a negative regulator of interleukin 4-induced STAT6 signaling. Blood 112(10):4098–4108. doi:10.1182/blood-2008-03-148726
Mahmodlou R, Mohammadi P, Sepehrvand N (2012) Colorectal cancer in northwestern Iran. ISRN Gastroenterol 2012:968560. doi:10.5402/2012/968560
Moghimi-Dehkordi B, Safaee A (2012) An overview of colorectal cancer survival rates and prognosis in Asia. World J Gastrointest Oncol 4(4):71–75. doi:10.4251/wjgo.v4.i4.71
Nanney LB, Davidson MK, Gates RE, Kano M, King LE Jr (1997) Altered distribution and expression of protein tyrosine phosphatases in normal human skin as compared to squamous cell carcinomas. J Cutan Pathol 24(9):521–532
Sangwan V, Paliouras GN, Cheng A, Dube N, Tremblay ML, Park M (2006) Protein-tyrosine phosphatase 1B deficiency protects against Fas-induced hepatic failure. J Biol Chem 281(1):221–228. doi:10.1074/jbc.M507858200
Shin A, Joo J, Bak J, Yang HR, Kim J, Park S, Nam BH (2011) Site-specific risk factors for colorectal cancer in a Korean population. PLoS ONE 6(8):e23196. doi:10.1371/journal.pone.0023196
Soulsby M, Bennett AM (2009) Physiological signaling specificity by protein tyrosine phosphatases. Physiology (Bethesda) 24:281–289. doi:10.1152/physiol.00017.2009
Stephen BE, David RB, Carolyn CC et al (2009) AJCC Cancer Staging Manual, 7th edn. Springer, New York
Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL (2010) Integrative genomic profiling of human prostate cancer. Cancer Cell 18(1):11–22. doi:10.1016/j.ccr.2010.05.026
Viana Lde S, Affonso RJ Jr, Silva SR, Denadai MV, Matos D, de Salinas Souza C, Waisberg J (2013) Relationship between the expression of the extracellular matrix genes SPARC, SPP1, FN1, ITGA5 and ITGAV and clinicopathological parameters of tumor progression and colorectal cancer dissemination. Oncology 84(2):81–91. doi:10.1159/000343436
Walsh JM, Terdiman JP (2003) Colorectal cancer screening: scientific review. JAMA 289(10):1288–1296. doi:10.1001/jama.289.10.1288
Wang J, Chen X, Liu B, Zhu Z (2010) Suppression of PTP1B in gastric cancer cells in vitro induces a change in the genome-wide expression profile and inhibits gastric cancer cell growth. Cell Biol Int 34(7):747–753. doi:10.1042/CBI20090447
Wang J, Liu B, Chen X, Su L, Wu P, Wu J, Zhu Z (2012) PTP1B expression contributes to gastric cancer progression. Med Oncol 29(2):948–956. doi:10.1007/s12032-011-9911-2
Wiener JR, Hurteau JA, Kerns BJ, Whitaker RS, Conaway MR, Berchuck A, Bast RC Jr (1994a) Overexpression of the tyrosine phosphatase PTP1B is associated with human ovarian carcinomas. Am J Obstet Gynecol 170(4):1177–1183
Wiener JR, Kerns BJ, Harvey EL, Iglehart JD, Conaway MR, Berchuck A, Bast RC Jr (1994b) Overexpression of the protein tyrosine phosphatase PTP1B in human breast cancer: association with p185c-erbB-2 protein expression. J Natl Cancer Inst 86(5):372–378
Woodford-Thomas TA, Rhodes JD, Dixon JE (1992) Expression of a protein tyrosine phosphatase in normal and v-src-transformed mouse 3T3 fibroblasts. J Cell Biol 117(2):401–414
Zheng LY, Zhou DX, Lu J, Zhang WJ, Zou DJ (2012) Down-regulated expression of the protein-tyrosine phosphatase 1B (PTP1B) is associated with aggressive clinicopathologic features and poor prognosis in hepatocellular carcinoma. Biochem Biophys Res Commun 420(3):680–684. doi:10.1016/j.bbrc.2012.03.066
Zhou MG, Wang XF, Hu JP, Li GL, Chen WQ, Zhang SW, Wan X, Wang LJ, Xiang C, Hu YS, Yang GH (2010) Geographical distribution of cancer mortality in China, 2004-2005. Zhonghua Yu Fang Yi Xue Za Zhii 44(4):303–308
Zhu S, Bjorge JD, Fujita DJ (2007) PTP1B contributes to the oncogenic properties of colon cancer cells through Src activation. Cancer Res 67(21):10129–10137
Acknowledgments
The authors would like to thank all the hospital staff in the Department of General Surgery of the Fourth Affiliated Hospital of Hebei Medical University for their support of this research.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Q., Li, Y., Li, Z. et al. Overexpression of PTP1B in human colorectal cancer and its association with tumor progression and prognosis. J Mol Hist 45, 153–159 (2014). https://doi.org/10.1007/s10735-013-9536-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-013-9536-1