Abstract
Integrin-linked kinase (ILK), an intracellular serine-threonine kinase, has been reported to be overexpressed in multiple types of human malignancies, including colorectal cancer (CRC). The prognostic value of ILK in CRC, however, remains unknown. In the present study, expression of ILK in 25 paired primary CRC samples and adjacent noncancerous tissues were quantified using real-time PCR and Western blotting. ILK protein expression was analyzed in 102 archived, paraffin-embedded CRC samples using immunohistochemistry. The correlation between ILK expression and clinicopathological factors was evaluated by the χ2 test. Patients’ overall survival was analyzed by Kaplan–Meier method. We found that both ILK mRNA and protein expression levels were significantly up-regulated in primary CRC samples compared with their corresponding normal tissues. Immunohistochemical analysis revealed relative high expression of ILK in 43 of 102 (42.2 %) primary CRC samples. Statistical analysis showed a significant correlation of ILK expression with tumor differentiation, lymph node metastasis, tumor invasion, and tumor-node-metastasis stage. Patients with tumors displaying high-level ILK expression showed significantly shorter overall survival (P = 0.028, log-rank test). More importantly, multivariate analysis indicated that high ILK protein expression was an independent prognostic factor for CRC patients (P = 0.026). Taken together, our data suggest that ILK overexpression is associated with tumor progression and a poor prognosis in CRC patients and may represent a novel potential prognostic marker for patients with CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the second most common malignant neoplasm and the fourth leading cause of cancer-related death worldwide. Estimated new cases of CRC in the world numbered 1.2 million in 2008, with deaths estimated at 0.6 million (Jemal et al. 2011). Currently, radical surgery represents the only potential curative treatment for CRC, but the 5-year survival rate for patients with the metastatic disease is approximately 19 % (Jemal et al. 2009). The tumor stage based on tumor-node-metastasis (TNM) classification is the most widely accepted predictor of prognosis and treatment in patients with CRC; however, considerable differences in recurrence and survival among patients with the same pathological stage of disease are frequently observed (O’Connell et al. 2004). Thus, there is an urgent demand of more robust markers that can precisely predict survival in patients with CRC.
Integrin-linked kinase (ILK) is a downstream substrate of the phosphoinositide 3-kinase (PI-3K) pathway located upstream of protein kinase B. It interacts with cytoplasmic domains of β-integrin subunits and regulates integrin dependent functions (Hannigan et al. 1996; Dedhar et al. 1999). ILK acts as an intracellular adaptor protein of growth factor signaling and cell–matrix interactions that regulates a diversity of fundamental cellular processes, such as proliferation, survival, apoptosis, migration, invasion and angiogenesis (Hannigan et al. 2005; McDonald et al. 2008). In recent years, accumulating evidence suggests that ILK is frequently activated and/or overexpressed in human malignancies (Hannigan et al. 2011), and overexpression of ILK is associated with tumor progression parameters and poor patient survival in multiple types of human tumors, including melanoma (Dai et al. 2003), non-small cell lung cancer (NSCLC) (Takanami 2005; Okamura et al. 2007; Yu et al. 2011), pancreatic cancer (Sawai et al. 2006), astrocytoma (Li et al. 2010), and oral squamous cell carcinoma (Zhao et al. 2012). In particular, Marotta and colleagues have reported dysregulation of ILK signaling in both familial adenomatous polyposis (Marotta et al. 2001) and sporadic human colon cancer (Marotta et al. 2003). Additionally, recent clinical evidence also indicates that ILK may play a critical role in human CRC progression, possibly through in vivo regulation of β-catenin, E-cadherin and Akt pathways (Bravou et al. 2006). However, there are no published reports that address a possible relationship between ILK expression and clinical outcomes in CRC patients.
In this study, we detected and compared the expression of ILK in primary cancer tissues and adjacent normal tissues by using real-time PCR and Western blotting. Moreover, we also evaluated ILK expression immunohistochemically in surgically resected CRC tissues to analyze its correlations with clinicopathological variables and prognosis of CRC patients.
Materials and methods
Patients, tissue specimens, and follow-up
A total of 102 primary tumor samples and 25 matched adjacent normal tissues were collected from patients with CRC who underwent complete surgical resection at our hospital from August 2005 to December 2006. None of these patients received any preoperative anticancer treatments (chemotherapy or radiotherapy), and all the patients were given routine adjuvant chemotherapy after the surgery. The study population consisted of 55 men and 47 women (mean age, 66 years; age range, 39–87 years). The histological type and grade of cell differentiation were classified according to the criteria established by the World Health Organization (WHO), and tumor staging was defined in accordance with the latest TNM classification system. The patients’ characteristics were retrieved from the medical records and are shown in Table 1. Follow-up data were obtained by phone, letter, and the outpatient clinical database. Follow-up time was defined as the interval between the date of initial surgery and the date of death, or the last visit date (September 30, 2011). Sixty-six (64.7 %) patients were alive at the last follow-up examination, while 36 patients (35.3 %) had died due to CRC-related disease. The mean follow-up time was 48 months (range, 4–65 months). After surgical resection, parts of the 25 paired primary tumor and adjacent normal tissues were preserved in liquid nitrogen immediately and then transferred to a −80 °C freezer for long-term storage. Informed consent was obtained from all the patients prior to the surgical operations, and this study was reviewed and approved by the Ethics Committee of China Medical University.
Immunohistochemistry and scoring
Formalin-fixed paraffin-embedded tissues were cut into 5 μm sections and mounted on glass slides. After dewaxing with xylene and rehydrating through descending ethanol, the tissue sections were heated at 100 °C for antigen retrieval in a steamer containing 10 mmol/l citrate buffer (pH 6.0) for 10 min. Thereafter, endogenous peroxidase activity was quenched using 3 % (v/v) hydrogen peroxide at room temperature for 15 min, and nonspecific binding of antibodies was blocked with 10 % normal goat serum. The sections were incubated at 4 °C overnight with a rabbit anti-ILK polyclonal antibody (dilution 1: 100, Cell Signaling, Danvers, MA, USA) and then with the secondary antibody at room temperature for 60 min, followed by incubation with streptavidin-horseradish peroxidase conjugate for 30 min. Finally, the antibody binding was visualized by using 3,3′-diaminobenzidine (DAB), and the sections were counterstained with hematoxylin, dehydrated, and mounted. The negative controls were prepared by replacing the primary antibody with an isotype matched IgG.
ILK staining was reviewed and scored independently by two pathologists who were blinded to clinical and outcome data. Score differences were discussed to obtain a consensus. The intensity of staining was scored according to 4 categories, where 0 indicates negative; 1, weak; 2, moderate; and 3, strong staining. The percentage of ILK-positive cells was also graded on a semiquantitative scale from 0 to 3, where 0 was given for 0 %, 1 for 1–10 %, 2 for 11–50 %, and 3 for 51–100 %. The sum of the percentage and intensity scores was used as the final ILK staining score, and defined as follows: 0–2, low expression, and 3–6, high expression.
Total RNA isolation and real-time PCR
Total RNA was extracted from frozen tissues by using TRIzol (Invitrogen, Carlsbad, CA, USA) following the supplier’s protocol. The amount of total RNA was determined by measuring absorbance at 260 nm. One microgram of total RNA was reverse transcribed using the PrimeScript II 1st Strand cDNA Synthesis Kit (TAKARA, Dalian, China) according to the manufacturer’s protocol. Real-time PCR was carried out in 25 μl reaction mixture containing 12.5 μl of SYBR® Premix Ex Taq™ (TAKARA), 0.2 μM of forward and reverse primers, and 2 μl template cDNA on a Real-Time Quantitative Thermal Block (Biometra, Göttingen, Germany). PCR primers were designed using Primer Express 2.0 software (Applied Biosystems, Foster City, CA, USA), and chemically synthesized by Sangon Biotechnology Co., Ltd. (Shanghai, China). The sequences of the primers are listed in Table 2. PCR amplification was initiated by preincubation for 30 s at 95 °C for activation, followed by 40 cycles of the protocol: denaturation at 95 °C for 5 s, annealing at 60 °C for 20 s, and elongation at 72 °C for 15 s with detection of fluorescence products. Melt-curve analysis was generated to identify specific reaction products. Data were analyzed through the comparative threshold cycle (CT) method (Livak and Schmittgen 2001).
Protein isolation and Western blotting
Total proteins were isolated from frozen tissues using lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 10 mM EDTA, 1 % NP-40, 0.1 % SDS, 1 mM PMSF, and 0.5 % sodium deoxycholate), and quantified by the Bradford method. Next, an equivalent amount of proteins was resolved on sodium dodecyl sulfate polyacrylamide gels (SDS-PAGE), and then electrotransferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA) under 70 V constant voltage conditions for 90 min. After blocking with 5 % fat-free milk at room temperature for 2 h, the membranes were immunoblotted overnight at 4 °C with anti-ILK polyclonal antibody (dilution 1: 1,000, Cell Signaling) or anti-β-actin antibody (dilution 1: 5,000, Sigma, MO, USA). Binding of the primary antibodies was detected with horseradish peroxidase (HRP)-conjugated secondary antibodies and visualized by an ECL plus chemiluminescence kit (Millipore).
Statistical analysis
Continuous data were compared using the Student’s t test. The relationship between ILK expression and clinicopathologic characteristics was analyzed by the Chi-square (χ2) test. Survival differences were estimated using the Kaplan–Meier method and compared by the log-rank test. Survival data were evaluated using univariate and multivariate Cox regression analysis. All statistical tests were two-sided, and P values less than 0.05 were considered to be statistically significant. Statistical analysis was conducted using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) for Microsoft Windows.
Results
ILK is frequently overexpressed in CRC tissues compared with the corresponding adjacent normal tissues
To determine whether ILK is overexpressed in CRC tissues, we measured ILK mRNA and protein expression levels in 25 fresh frozen CRC samples and their paired adjacent normal tissues by using real-time PCR and Western blotting, respectively. Our results showed that ILK mRNA expression level was significantly higher in CRC samples than adjacent noncancerous tissues (Fig. 1a, P = 0.016). Compared with the paired adjacent normal tissues, increased ILK mRNA expression (at least twofold change) was detected in 14 of 25 CRC samples (56.0 %). Additionally, changes observed by Western blotting were consistent with the results in real-time PCR study (Fig. 1b). These data not only indicate that ILK is overexpressed in CRC, but also suggest that ILK overexpression is due to the up-regulation of ILK mRNA.
ILK mRNA and protein expression levels in primary CRC samples and matched adjacent normal tissues measured by real-time PCR (a) and Western blotting analysis (b), respectively. a Data were normalized to expression of β-actin. Bars represent the means of ILK relative expression. b Representative blots are shown, and the protein size is expressed in kDa. T tumor, N normal
Overexpression of ILK is associated with clinicopathological characteristics of CRC patients
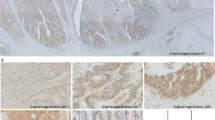
To further investigate the relationship between ILK expression and the clinicopathological features of CRC patients, ILK expression was examined in 102 paraffin-embedded, archived CRC tissues using immunohistochemical staining with an antibody against human ILK. Representative images of ILK staining are shown in Fig. 2. The immunostaining of ILK was mainly present the cytoplasm of tumor cells in CRC tissues. Of these 102 CRC tissues, high ILK protein expression was detected in 43 samples (42.2 %) and weak or negative staining was found in 59 tumor samples (57.8 %). The relationship between ILK expression and clinicopathological parameters of CRC patients was further analyzed. Significant correlations were found between ILK expression and four parameters, including tumor differentiation (P = 0.003), lymph node metastasis (P = 0.016), tumor invasion (P < 0.001), and TNM stage (P = 0.012). However, there were no statistical associations between ILK expression and the rest parameters, such as gender, age, tumor location, and tumor size (P > 0.05, Table 1).
Overexpression of ILK is associated with shorter overall survival in CRC patients
After operation, all patients were followed-up for overall survival, and the mean follow-up time was 48 months (range, 4–65 months). The relationship between ILK protein expression and survival of CRC patients was evaluated by Kaplan–Meier analysis and log-rank test. We found that the overall survival of patients with high ILK protein expression was significantly shorter than that of patients with low ILK protein expression (P = 0.028; Fig. 3). In addition, the 65-month overall survival rate was 47.9 months [95 % confidence interval (CI), 41.9–53.8 months] for patients with high ILK protein expression, compared with 54.8 months (95 % CI, 50.0–59.5 months) for patients with low ILK protein expression. To evaluate the possibility of ILK as an independent risk factor for poor prognosis, conventional clinicopathological factors and ILK protein levels were assessed by Cox’s univariate and multivariate hazard regression model (Table 3). In univariate analysis, tumor differentiation, lymph node metastasis, tumor invasion, TNM stage, ILK overexpression were significantly correlated with the overall survival of CRC patients (P = 0.013, 0.008, 0.023, 0.001, and 0.017, respectively). Moreover, multivariate analysis revealed that TNM stage and ILK expression were independent prognostic factors for overall survival (P = 0.011, and 0.026, respectively), suggesting that ILK may be a prognostic factor for survival in patients with CRC.
Discussion
In the current study, we demonstrated that ILK was expressed at both higher mRNA and protein levels in CRC tissues than corresponding noncancerous tissues. In agreement with these molecular biological findings, immunohistochemistry with an anti-ILK antibody showed that ILK was overexpressed in 42.2 % (43/102) primary CRC tissues, and ILK overexpression was significantly associated with tumor differentiation, lymph node metastasis, tumor invasion, and TNM stage, but not with gender, age, tumor location, and tumor size. More importantly, statistical analysis also indicated that patients with higher ILK expression had a shorter overall survival, whereas patients with lower ILK expression had a better survival. Altogether, our data indicate that overexpression of ILK is associated with an unfavorable prognosis and is an independent prognostic factor for survival in patients with CRC.
A considerable number of studies have demonstrated that ILK is frequently overexpressed in several solid tumors, including CRC (Marotta et al. 2003; Bravou et al. 2006).The prognostic significance of ILK in CRC, however, remains unclear. To address this issue, the present study analyzed the ILK expression level in CRC tissues using real-time PCR, Western blotting and immunohistochemistry. Meanwhile, we identified the relationship between ILK expression and clinicopathological features and evaluated its prognostic value in post-resection survival of CRC patients. The results showed that both ILK mRNA and protein levels were significantly higher in tumor tissues than in adjacent noncancerous tissues, which are reminiscent of previous findings in other malignancies, such as gastric (Ito et al. 2003) and hepatocellular carcinoma (Chan et al. 2011). In addition, ILK was associated with tumor differentiation, lymph node metastasis, tumor invasion, and TNM stage in these CRC patients. Our observations are consistent with a previous report from Bravou et al. (2006) who showing that ILK expression positively correlates these clinicopathological features in colon cancer patients. The present data, together with previous findings by others, suggest that ILK positively participates in tumorigenesis and progression of human CRC.
Despite extensive investigation, there is no general consensus as to whether ILK is an oncogene or a tumor suppressor (Durbin et al. 2009). On one hand, initial studies indicated that overexpression of ILK in colonic epithelial cells stimulates cellular transformation in vitro and in vivo (Hannigan et al. 1996; Wu et al. 1998). Subsequent studies demonstrated that ILK transgenic mice develop breast invasive ductal carcinoma and mice with conditional knockout ILK show a reduction in colonic tumorigenesis (White et al. 2001; Assi et al. 2008). Additionally, ILK is frequently overexpressed in a wide variety of human cancers and has been correlated with worse patient survival (Dai et al. 2003; Takanami 2005; Okamura et al. 2007; Yu et al. 2011; Sawai et al. 2006; Li et al. 2010; Zhao et al. 2012). These results support ILK as an oncogene. On the other hand, increased expression of ILK is found in breast epithelial cells and normal breast tissues in comparison to breast cancer cells and breast tumors (Chen et al. 2004; Troussard et al. 2006). Furthermore, stable overexpression of ILK in breast cancer cells suppresses the growth and invasion in vitro and tumor formation and lung metastases in vivo (Chen et al. 2004). These findings implicate ILK as a tumor suppressor. However, the most current available evidence suggests a pro-oncogenic function of ILK in tumorigenesis. Our data also support this notion, because a significant proportion of primary CRC tissues showed ILK overexpression, which was significantly associated with tumor differentiation, lymph node metastasis, tumor invasion, and TNM stage. Moreover, our findings agree with the fact that many previous studies have indicated a possible link between ILK expression and malignant behaviors of cancer cells. For instance, overexpression of ILK has been shown to promote invasion, migration, angiogenesis, and epithelial-mesenchymal transition (EMT) in various types of human cancer cells both in vitro and in vivo (Wani et al. 2011; Chan et al. 2011; Zhao et al. 2011; Chen et al. 2012). The most important finding of our study is that overexpression of ILK is associated with a poor prognosis and is an independent prognostic factor for the outcome in CRC patients. Nonetheless, further investigations are needed to confirm our observations in a larger series of CRC patients.
Several limitations of our study need to be pointed out. First, the relatively small number of analyzed patients may reduce the power to detect statistical associations and significantly affects survival analyses. Second, beyond cause of mortality, data on cancer recurrences were not available in these cohorts. Third, all patients were not given the same chemotherapy regimen both in terms of schedule and associated drug. Therefore, our observations should be further validated in a large number of patients with a uniform treatment protocol.
In conclusion, the data from the current study demonstrated that ILK is overexpressed in CRC tissues and associated with tumor progression and poor prognosis in CRC patients. Our study suggests that ILK overexpression is associated with tumor progression and a poor prognosis in CRC patients and may represent a novel potential prognostic marker for patients with CRC.
References
Assi K, Mills J, Owen D, Ong C, St Arnaud R, Dedhar S, Salh B (2008) Integrin-linked kinase regulates cell proliferation and tumour growth in murine colitis-associated carcinogenesis. Gut 57(7):931–940. doi:10.1136/gut.2007.142778
Bravou V, Klironomos G, Papadaki E, Taraviras S, Varakis J (2006) ILK over-expression in human colon cancer progression correlates with activation of beta-catenin, down-regulation of E-cadherin and activation of the Akt-FKHR pathway. J Pathol 208(1):91–99. doi:10.1002/path.1860
Chan J, Ko FC, Yeung YS, Ng IO, Yam JW (2011) Integrin-linked kinase overexpression and its oncogenic role in promoting tumorigenicity of hepatocellular carcinoma. PLoS ONE 6(2):e16984. doi:10.1371/journal.pone.0016984
Chen P, Shen WZ, Karnik P (2004) Suppression of malignant growth of human breast cancer cells by ectopic expression of integrin-linked kinase. Int J Cancer 111(6):881–891. doi:10.1002/ijc.20340
Chen D, Zhang Y, Zhang X, Li J, Han B, Liu S, Wang L, Ling Y, Mao S, Wang X (2012) Overexpression of integrin-linked kinase correlates with malignant phenotype in non-small cell lung cancer and promotes lung cancer cell invasion and migration via regulating epithelial-mesenchymal transition (EMT)-related genes. Acta Histochem. doi:10.1016/j.acthis.2012.05.004
Dai DL, Makretsov N, Campos EI, Huang C, Zhou Y, Huntsman D, Martinka M, Li G (2003) Increased expression of integrin-linked kinase is correlated with melanoma progression and poor patient survival. Clin Cancer Res 9(12):4409–4414
Dedhar S, Williams B, Hannigan G (1999) Integrin-linked kinase (ILK): a regulator of integrin and growth-factor signalling. Trends Cell Biol 9(8):319–323
Durbin AD, Hannigan GE, Malkin D (2009) Oncogenic ILK, tumor suppression and all that JNK. Cell Cycle 8(24):4060–4066
Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S (1996) Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 379(6560):91–96. doi:10.1038/379091a0
Hannigan G, Troussard AA, Dedhar S (2005) Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer 5(1):51–63. doi:10.1038/nrc1524
Hannigan GE, McDonald PC, Walsh MP, Dedhar S (2011) Integrin-linked kinase: not so ‘pseudo’ after all. Oncogene 30(43):4375–4385. doi:10.1038/onc.2011.177
Ito R, Oue N, Zhu X, Yoshida K, Nakayama H, Yokozaki H, Yasui W (2003) Expression of integrin-linked kinase is closely correlated with invasion and metastasis of gastric carcinoma. Virchows Arch 442(2):118–123. doi:10.1007/s00428-002-0718-6
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59(4):225–249. doi:10.3322/caac.20006
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90. doi:10.3322/caac.20107
Li J, Zhang H, Wu J, Guan H, Yuan J, Huang Z, Li M (2010) Prognostic significance of integrin-linked kinase1 overexpression in astrocytoma. Int J Cancer 126(6):1436–1444. doi:10.1002/ijc.24824
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408. doi:10.1006/meth.2001.1262
Marotta A, Tan C, Gray V, Malik S, Gallinger S, Sanghera J, Dupuis B, Owen D, Dedhar S, Salh B (2001) Dysregulation of integrin-linked kinase (ILK) signaling in colonic polyposis. Oncogene 20(43):6250–6257. doi:10.1038/sj.onc.1204791
Marotta A, Parhar K, Owen D, Dedhar S, Salh B (2003) Characterisation of integrin-linked kinase signalling in sporadic human colon cancer. Br J Cancer 88(11):1755–1762. doi:10.1038/sj.bjc.6600939
McDonald PC, Fielding AB, Dedhar S (2008) Integrin-linked kinase–essential roles in physiology and cancer biology. J Cell Sci 121(Pt 19):3121–3132. doi:10.1242/jcs.017996
O’Connell JB, Maggard MA, Ko CY (2004) Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 96(19):1420–1425. doi:10.1093/jnci/djh275
Okamura M, Yamaji S, Nagashima Y, Nishikawa M, Yoshimoto N, Kido Y, Iemoto Y, Aoki I, Ishigatsubo Y (2007) Prognostic value of integrin beta1-ILK-pAkt signaling pathway in non-small cell lung cancer. Hum Pathol 38(7):1081–1091. doi:10.1016/j.humpath.2007.01.003
Sawai H, Okada Y, Funahashi H, Matsuo Y, Takahashi H, Takeyama H, Manabe T (2006) Integrin-linked kinase activity is associated with interleukin-1 alpha-induced progressive behavior of pancreatic cancer and poor patient survival. Oncogene 25(23):3237–3246. doi:10.1038/sj.onc.1209356
Takanami I (2005) Increased expression of integrin-linked kinase is associated with shorter survival in non-small cell lung cancer. BMC Cancer 5:1. doi:10.1186/1471-2407-5-1
Troussard AA, McDonald PC, Wederell ED, Mawji NM, Filipenko NR, Gelmon KA, Kucab JE, Dunn SE, Emerman JT, Bally MB, Dedhar S (2006) Preferential dependence of breast cancer cells versus normal cells on integrin-linked kinase for protein kinase B/Akt activation and cell survival. Cancer Res 66(1):393–403. doi:10.1158/0008-5472.CAN-05-2304
Wani AA, Jafarnejad SM, Zhou J, Li G (2011) Integrin-linked kinase regulates melanoma angiogenesis by activating NF-kappaB/interleukin-6 signaling pathway. Oncogene 30(24):2778–2788. doi:10.1038/onc.2010.644
White DE, Cardiff RD, Dedhar S, Muller WJ (2001) Mammary epithelial-specific expression of the integrin-linked kinase (ILK) results in the induction of mammary gland hyperplasias and tumors in transgenic mice. Oncogene 20(48):7064–7072. doi:10.1038/sj.onc.1204910
Wu C, Keightley SY, Leung-Hagesteijn C, Radeva G, Coppolino M, Goicoechea S, McDonald JA, Dedhar S (1998) Integrin-linked protein kinase regulates fibronectin matrix assembly, E-cadherin expression, and tumorigenicity. J Biol Chem 273(1):528–536
Yu J, Shi R, Zhang D, Wang E, Qiu X (2011) Expression of integrin-linked kinase in lung squamous cell carcinoma and adenocarcinoma: correlation with E-cadherin expression, tumor microvessel density and clinical outcome. Virchows Arch 458(1):99–107. doi:10.1007/s00428-010-1016-3
Zhao G, Guo LL, Xu JY, Yang H, Huang MX, Xiao G (2011) Integrin-linked kinase in gastric cancer cell attachment, invasion and tumor growth. World J Gastroenterol 17(30):3487–3496. doi:10.3748/wjg.v17.i30.3487
Zhao D, Tang XF, Yang K, Liu JY, Ma XR (2012) Over-expression of integrin-linked kinase correlates with aberrant expression of Snail, E-cadherin and N-cadherin in oral squamous cell carcinoma: implications in tumor progression and metastasis. Clin Exp Metastasis. doi:10.1007/s10585-012-9485-1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, R., Liu, B., Yin, H. et al. Overexpression of integrin-linked kinase (ILK) is associated with tumor progression and an unfavorable prognosis in patients with colorectal cancer. J Mol Hist 44, 183–189 (2013). https://doi.org/10.1007/s10735-012-9463-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-012-9463-6