Abstract
Integrin-linked kinase (ILK) plays a role in integrin signaling-mediated cell-extracellular matrix interactions and is involved in signal transduction pathways to control cell survival, differentiation, and proliferation in mammalian cells. ILK has been implicated in the progression of several human malignancies. However, its function in malignant tumors is not fully enunciated. Previous in vitro studies also implicated ILK in the regulation of E-cadherin expression and vascular endothelial growth factor expression. In the current study, we investigated the protein expression of ILK and its correlation with clinicopathological profiles, E-cadherin expression, microvessel density (MVD) and clinical outcome in 57 lung squamous cell carcinoma and 44 adenocarcinoma, using immunohistochemistry. No ILK was detected in normal bronchial epithelium, while it was positively expressed in 39 (68.42%) squamous cell carcinoma cases and 27 (61.36%) adenocarcinoma cases. Positive ILK expression was significantly associated with advanced TNM stage (P = 0.022) in adenocarcinoma, and associated with high MVD in lung squamous cell carcinoma (P < 0.001) and adenocarcinoma (P = 0.049). The Spearman's correlation test revealed that increased ILK expression was correlated with reduced E-cadherin expression in lung squamous cell carcinoma (correlation coefficient = 0.364, P = 0.005). Moreover, the Kaplan–Meier survival analysis showed that ILK, E-cadherin, and MVD were all statistically significant prognostic factors in patients with lung squamous cell carcinoma and adenocarcinoma. Measuring ILK and E-cadherin expression, and MVD may contribute to a better understanding of the prognosis of patients with lung squamous cell carcinoma and adenocarcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cell-extracellular matrix (ECM) interactions play an important role in cell survival, growth, differentiation, and migration [1]. As a key component of cell-ECM structures, integrin-linked kinase (ILK) associates with cytoplasmic domains of β1 and β3 integrins and couples them to the actin cytoskeleton [2]. In addition, ILK has been shown to act as a serine/threonine kinase originally, but there is an ongoing controversy if ILK is a kinase or not.
There is mounting experimental evidence indicating that ILK plays a pivotal role in many processes associated with oncogenesis. Overexpression and constitutive activation of ILK in epithelial cells suppresses anoikis and elicits anchorage-independent cell cycle progression, cell migration, and invasion [2–4]. Moreover, overexpression of ILK in epithelial cells also induces tumor formation in vivo [5]. Increased expression of ILK has also been reported in several malignancies, such as gastric carcinoma, prostate tumor, colon tumor, ovary carcinoma, melanoma, and non-small cell lung cancer [6–11]. However, inconsistent results with regard to ILK expression exist for lymphoma, retinoblastoma, and renal carcinoma [12]. Up to the current time, these discrepancies remain unsolved, and the function of ILK in tumor biology is still under debate.
E-cadherin is an important epithelial cell adhesion molecule mediating cell–cell interactions. Research revealed that overexpression of ILK leads to the loss of cell–cell adhesion because of downregulation of E-cadherin [13, 14]. Further evidence has indicated that ILK might play a key role in vascular endothelial growth factor (VEGF)-mediated endothelial activation and angiogenesis [15, 16]. However, currently, there is little evidence of the relationship between ILK expression and E-cadherin expression and microvessel density (MVD). Thus, we investigated the expression of ILK in lung squamous cell carcinoma and adenocarcinoma, and analyzed the relationship between ILK expression and some clinical pathological factors, E-cadherin expression, MVD, and clinical outcome.
Materials and methods
Tissue samples
One hundred one samples were collected for histological and immunohistochemical analysis. They included 57 lung squamous cell carcinomas and 44 lung adenocarcinomas with neighboring noncancerous tissues, which were obtained from patients who had surgery in the Liaoning Province Tumor Hospital and the First Affiliated Hospital of China Medical University between 1998 and 2002. All patients had no radiotherapy or chemotherapy before the operation, and median follow-up of the patients was 22.87 months (range 1–80 months), and their outcomes were known.
The histologic diagnosis was evaluated by examination of hematoxylin and eosin-stained sections according to the classification system of the World Health Organization (2004). All tumors were staged according to the 7th edition of TNM staging system for lung cancer of International Union Against Cancer (2010). There were 21 patients with stage I, 21 with stage II, 54 with stage III, and 5 with stage IV. Also, there were lymph node metastases in 58 cases.
Immunohistochemistry
Tissue sections (4-μm thick) of formalin-fixed, paraffin-embedded specimens were deparaffinized in xylene, rehydrated in graded alcohol, and transferred to phosphate buffered saline (PBS). The slides were rinsed three times with PBS, and antigen retrieval was performed with a pressure cooker for 90 s. Endogenous peroxidase was blocked by the use of 3% hydrogen peroxide in PBS for 20 min. The samples were washed three times with PBS and incubated for 30 min at room temperature with a protein blocking solution. Excess blocking solution was drained, and the samples were incubated overnight at 4°C with anti-ILK antibody (1:400) (Millipore, Billerica, MA, USA), anti-E-cadherin antibody (1:200) (Santa Cruz Biotechnology, CA, USA), and anti-CD34 antibody (ready-to-use) (MaiXin, Fuzhou, China). Bound primary antibodies were detected with biotin–streptavidin–peroxidase method. Immunodetection was performed with diaminobenzidine as the chromogen, and counterstained with hematoxylin and dehydrated in alcohol before mounting.
The results of immunohistochemical staining were evaluated independently by three observers with no prior knowledge of patients' clinical data. The smooth muscle cells, fibroblasts, and endothelial cells of blood vessels, all known to be abundant in ILK, were used as positive controls. For a negative control, PBS was used for the primary antibody. Cytoplasmic and nuclear expression of ILK was scored based on two parameters: the proportion of immunopositive cells and their intensity of immunoreactivity. The proportion of immunopositive cells was categorized as follows: 0: <10%; 1: ≥10% to <25%; 2: ≥25% to <50%; 3: ≥50% to <75%; and 4: ≥75%. The staining intensity was categorized by relative intensity as follows: 0, no positivity; 1, weak; 2, moderate; and 3, strong. A final immunoreactivity score of each section was obtained by multiplying the two individual scores. Cases with scores <2 were considered as negative expression, while cases with scores ≥2 were considered as positive expression. Membranous expression of E-cadherin showed no significant variations in intensity of staining, and the scoring system was applied based on the percentage of positive cells: 0: <10%; 1: ≥10% to <25%; 2: ≥25% to <50%; 3: ≥50% to <75%, and 4: ≥75%. Since the adjacent non-neoplastic bronchial epithelium had a score of 4 for membranous localization of E-cadherin, cases with scores <4 was defined as decreased E-cadherin expression, while cases with a score of 4 were defined as preserved E-cadherin expression.
Quantification of MVD
MVD was assessed by immunohistochemical analysis with antibodies to the endothelial marker CD34 and determined according to the method of Weidner [17]. Briefly, the immunostained sections were initially screened at low magnifications (×40 and ×100) to identify hot spots, which are the areas of highest neovascularization. Any yellow brown-stained endothelial cell or endothelial cell cluster that was clearly separate from adjacent microvessels, tumor cells, and other stromal cells was considered a single, countable microvessel. Within the hot spot area, the stained microvessels were counted in a single high-power (×200) field, and the average vessel count in three hot spots was considered the value of MVD.
Statistical analysis
All the data were analyzed with SPSS for Windows, version 13.0. The Pearson's chi-square test was used to analyze the relationship between ILK or E-cadherin expression and the clinicopathologic characteristics. The Spearman's correlation test was used to examine the correlation between the expression levels of ILK and E-cadherin. Correlations of MVD with clinicopathological factors and ILK expression were analyzed by T- test. Postoperative survival periods were calculated by the Kaplan–Meier method and compared by the log-rank test. The significance level was defined as P < 0.05.
Results
Expression of ILK and E-cadherin in normal lung tissues and lung squamous cell carcinoma, and adenocarcinoma
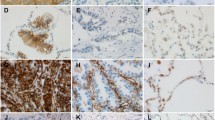
Immunohistochemistry of normal bronchial epithelium showed expression of E-cadherin primarily in the cell membrane, but the expression of ILK was not detected (Fig. 1a, b). Of the 57 squamous cell carcinoma samples stained, 34 (59.65%) cases showing decreased expression of E-cadherin (Fig. 1d), and 39 (68.42%) cases showing positive expression of ILK (Fig. 1c), including 11 (19.30%) cases showing both cytoplasmic and nuclear expressions of ILK (Fig. 2a). In 44 adenocarcinoma cases, there were 25 (56.82%) cases showing decreased expression of E-cadherin (Fig. 1f), and 27 (61.36%) cases showing positive ILK expression (Fig. 1e), including 6 (13.64%) cases showing both cytoplasmic and nuclear expressions of ILK (Fig. 2b). There was no case showing sole nuclear staining for ILK in squamous cell carcinoma and adenocarcinoma.
ILK and E-cadherin expression in bronchial mucosa epithelial cells and lung cancer tissues. Negative immunostaining for ILK in bronchial mucosa epithelial cells (a), cytoplasmic staining for ILK in squamous cell carcinoma (c), and adenocarcinoma (e). Membranous staining of E-cadherin in bronchial mucosa epithelial cells (b), decreased expression of E-cadherin in squamous cell carcinoma (d), and adenocarcinoma (f). (×400)
Relationship between ILK or E-cadherin expression and clinicopathological factors
As shown in Table 1, there was no statistically significant correlation between ILK expression and any clinicopathological parameters in squamous cell carcinoma (P > 0.05). The positive ILK expression was directly related to advanced TNM stage in adenocarcinoma (P = 0.022). There was no statistically significant correlation between nuclear ILK expression and any clinicopathological parameters in squamous cell carcinoma and adenocarcinoma (P > 0.05).
Decreased expression of E-cadherin was correlated with lymph node metastasis in squamous cell carcinoma (P = 0.033). In adenocarcinoma, decreased E-cadherin expression was correlated with advanced TNM stage (P = 0.020) and lymph node metastasis (P = 0.009; Table 2).
In addition, the Spearman's correlation test revealed that increased ILK expression was correlated with reduced E-cadherin expression in squamous cell carcinoma (correlation coefficient = 0.364, P = 0.005). In adenocarcinoma, ILK expression was not correlated with E-cadherin expression (P = 0.101).
Correlation of MVD with clinicopathological factors and ILK expression
Distribution of microvessels in sections of squamous cell carcinoma and adenocarcinoma is illustrated in Fig. 3. The average MVD in squamous cell carcinoma and adenocarcinoma cases were 43.19 ± 9.71 and 44.09 ± 8.19, respectively. The correlation of MVD with clinicopathological factors and ILK expression is shown in Table 3. In squamous cell carcinoma and adenocarcinoma, the MVD in positive ILK-staining cases was much higher than that in negative ILK-staining cases (squamous cell carcinoma 47.72 ± 7.10 vs.33.39 ± 7.03, T = 7.106, P < 0.001; adenocarcinoma 46.04 ± 7.83 vs 41.06 ± 8.09, T = 2.028, P = 0.049).
Cumulative Kaplan–Meier survival analysis
In squamous cell carcinoma, the 5-year survival rate for the patients with positive and negative ILK expression was 3% and 38%, respectively; the difference was statistically significant (P < 0.001; Fig. 4a). Furthermore, the 5-year survival rate for the patients with decreased E-cadherin expression was 9%, and that with preserved E-cadherin expression was 21% (P = 0.009; Fig. 4b). The Kaplan–Meier survival analysis also showed that TNM stage (P < 0.001), lymph node metastasis (P < 0.001), and MVD (P < 0.001) were all statistically significant prognostic factors.
Kaplan–Meier survival curve according to ILK and E-cadherin expression in lung squamous cell carcinoma and adenocarcinoma. Patients with negative ILK expression have a significantly better survival than those with positive ILK expression in squamous cell carcinoma (a) and adenocarcinoma (c), respectively. Patients with decreased E-cadherin expression have a significantly poorer survival than those with preserved E-cadherin expression in squamous cell carcinoma (b) and adenocarcinoma (d), respectively
In adenocarcinoma, the 5-year survival rate for the patients with positive and negative ILK expression was 4% and 18%, respectively; the difference was statistically significant (P < 0.001; Fig. 4c). The 5-year survival rate for the patients with decreased E-cadherin expression was 4%, and that with preserved E-cadherin expression was 16% (P = 0.046; Fig. 4d). The Kaplan–Meier survival analysis also showed that TNM stage (P < 0.001), lymph node metastasis (P = 0.002), and MVD (P < 0.001) were all statistically significant prognostic factors.
However, gender and age were not statistically significant prognostic factors in patients with squamous cell carcinoma and adenocarcinoma (P > 0.05).
Discussion
Cell-ECM interactions play an important role in cell survival, growth, differentiation, and migration [1]. As a key component of cell-ECM structures, ILK associates with cytoplasmic domains of β1 and β3 integrins, and apart from its unconventional kinase activity, it also functions as a scaffolding protein which mediates linkage of integrins to the actin cytoskeleton [2, 18]. As a scaffolding protein, the N-terminal domain of ILK associates with adapter proteins PINCH1 and PINCH2, while its C-terminal domain interacts with α/β-Parvin. This ternary PINCH/ILK/Parvin complex not only serves as a platform for structurally linking integrins with the actin cytoskeleton, but also interacts with specific additional partners and positively regulates PKB/Akt, MMP activity and negatively regulates GSK-3β activity, thus, implicating ILK in the pathologic processes of tumor invasion, progression, and metastasis [18–21].
In the current study, we detected ILK expression using immunohistochemistry in 57 lung squamous cell carcinomas and 44 lung adenocarcinomas. ILK was positively expressed in 39 (68.42%) squamous cell carcinoma cases and 27 (61.36%) adenocarcinoma cases, whereas there was no ILK expression in normal bronchial epithelium cells. Furthermore, our results showed that positive expression of ILK was correlated with advanced TNM stage in adenocarcinoma. In squamous cell carcinoma and adenocarcinoma, we also found that the cases with lymph node metastasis had higher ILK expression, though the differences were not significant (P = 0.075 and P = 0.055, respectively). These results are similar to the studies in gastric carcinoma, prostate tumor, colon cancer, ovary carcinoma, melanoma, and lung cancer [6–11]. The data indicate that increased ILK expression is associated with progression of lung squamous cell carcinoma and adenocarcinoma. In contrast, renal cell carcinoma and several non-epithelial tumors, such as lymphoma, retinoblastoma, mesenchymal chondrosarcomas, and sarcomas of bone and soft tissue have been reported to have decreased or no ILK expression [22, 23], which suggest that ILK may have different functions in tumors of different origins.
Interestingly, of the 66 cases with positive ILK expression, 17 cases showed both cytoplasm and nuclear staining of ILK, including 11 squamous cell carcinomas and 6 adenocarcinomas. However, there was no statistically significant correlation between nuclear ILK expression and any clinicopathological factors. Consistent with our findings, nuclear localization of ILK has been reported in non-small cell lung cancer and laryngeal carcinoma [11, 24]. Takanami did not investigate the correlation between nuclear ILK expression and clinicopathological parameters in non-small cell lung cancer [11]. There were no significant differences between nuclear ILK expression and clinicopathological parameters in laryngeal carcinoma [24]. It has been demonstrated that nuclear localization of ILK in breast cancer cell lines was attributed to a mechanism involving activation of PAK-1 [25]. Additionally, ILK has been shown to regulate the organization of the mitotic spindle [26]. Taken together, the nuclear function of ILK in lung squamous cell carcinoma and adenocarcinoma requires further study.
E-cadherin is an important epithelial cell adhesion molecule mediating cell–cell interactions. In epithelial derived tumors, loss of cell–cell adhesion is correlated with downregulation of E-cadherin, as well as increases proliferation, invasiveness, and metastasis. Consistent with these, we found that the expression of E-cadherin was downregulated in lung squamous cell carcinoma and adenocarcinoma, and reduced expression of E-cadherin was correlated with lymph node metastasis. In adenocarcinoma, decreased E-cadherin expression was also correlated with advanced TNM stage. Importantly, our data showed that increased ILK expression was correlated with reduced E-cadherin expression in squamous cell carcinoma. However, ILK expression was not correlated with E-cadherin expression in adenocarcinoma. Research revealed that overexpression of ILK leads to the loss of cell–cell adhesion because of the downregulation of E-cadherin, possibly by ILK-mediated activation of the E-cadherin repressor Snail [13, 14], or nuclear β-catenin stabilization by ILK-mediated inhibition on GSK-3 activity [21]. Our findings indicate that increased expression of ILK may be involved in the loss of E-cadherin expression in squamous cell carcinoma.
Tumor angiogenesis is critical to the growth and invasion of tumor cells, and MVD is accepted as a standard indicator of angiogenesis. In agreement with the published data, our study showed that high MVD was correlated with advanced TNM stage and lymph node metastasis in squamous cell carcinoma and adenocarcinoma. Previous studies indicate that ILK could positively regulate Akt activity, and, activation of Akt could also upregulate VEGF expression, thus, stimulating tumor angiogenesis [19, 27]. Inhibition of ILK expression or activity results in the inhibition of VEGF-mediated endothelial cell migration, capillary formation in vitro, and angiogenesis in vivo [15, 16]. For the first time, we demonstrated that MVD in positive ILK-staining cases was much higher than that in negative ILK-staining cases in squamous cell carcinoma and adenocarcinoma. These data probably indicate that ILK may be involved in angiogenesis, and thus, enhance tumor progression of lung squamous cell carcinoma and adenocarcinoma.
The overall prognosis of patients with positive ILK expression was poorer than those with negative ILK expression in squamous cell carcinoma and adenocarcinoma. The univariate survival analysis revealed that ILK was a significant prognostic factor in patients with lung squamous cell carcinoma and adenocarcinoma, as well as E-cadherin, TNM stage, lymph node metastasis, and MVD, which are consistent with previous studies [10, 11]. These data further support the notion that enhanced ILK expression may be involved in the malignant progression of lung squamous cell carcinoma and adenocarcinoma.
In conclusion, we found that overexpression of ILK is a common abnormality and correlates with high MVD in lung squamous cell carcinoma and adenocarcinoma. The findings also indicate a causative relation between loss of E-cadherin and overexpression of ILK in lung squamous cell carcinoma. The ILK, E-cadherin proteins and tumor angiogenesis appear to play important roles in the progression of lung squamous cell carcinoma and adenocarcinoma. Measuring ILK and E-cadherin expression, and MVD may contribute to a better understanding of the prognosis of patients with lung squamous cell carcinoma and adenocarcinoma.
References
Dedhar S (2000) Cell-substrate interactions and signaling through ILK. Curr Opin Cell Biol 12:250–256
Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L et al (1996) Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 379:91–96
Attwell S, Roskelley C, Dedhar S (2000) The integrin-linked kinase (ILK) suppresses anoikis. Oncogene 19:3811–3815
Radeva G, Petrocelli T, Behrend E et al (1997) Overexpression of the integrin-linked kinase promotes anchorage-independent cell cycle progression. J Biol Chem 272:13937–13944
Wu C, Keightley SY, Leung-Hagesteijn C et al (1998) Integrin-linked protein kinase regulates fibronectin matrix assembly, E-cadherin expression, and tumorigenicity. J Biol Chem 273:528–536
Ito R, Oue N, Zhu X et al (2003) Expression of integrin-linked kinase is closely correlated with invasion and metastasis of gastric carcinoma. Virchows Arch 442:118–123
Graff JR, Deddens JA, Konicek BW et al (2001) Integrin-linked kinase expression increases with prostate tumor grade. Clin Cancer Res 7:1987–1991
Bravou V, Klironomos G, Papadaki E et al (2003) Integrin-linked kinase (ILK) expression in human colon cancer. Br J Cancer 89:2340–2341
Ahmed N, Riley C, Oliva K et al (2003) Integrin-linked kinase expression increases with ovarian tumour grade and is sustained by peritoneal tumour fluid. J Pathol 201:229–237
Dai DL, Makretsov N, Campos EI et al (2003) Increased expression of integrin-linked kinase is correlated with melanoma progression and poor patient survival. Clin Cancer Res 9:4409–4414
Takanami I (2005) Increased expression of integrin-linked kinase is associated with shorter survival in non-small cell lung cancer. BMC Cancer 5:1
Eke I, Hehlgans S, Cordes N (2009) There's something about ILK. Int J Radiat Biol 85:929–936
Tan C, Costello P, Sanghera J et al (2001) Inhibition of integrin linked kinase (ILK) suppresses beta-catenin-Lef/Tcf-dependent transcription and expression of the E-cadherin repressor, snail, in APC-/- human colon carcinoma cells. Oncogene 20:133–140
McPhee TR, McDonald PC, Oloumi A et al (2008) Integrin-linked kinase regulates E-cadherin expression through PARP-1. Dev Dyn 237:2737–2747
Tan C, Cruet-Hennequart S, Troussard A et al (2004) Regulation of tumor angiogenesis by integrin-linked kinase (ILK). Cancer Cell 5:79–90
Edwards LA, Woo J, Huxham LA et al (2008) Suppression of VEGF secretion and changes in glioblastoma multiforme microenvironment by inhibition of integrin-linked kinase (ILK). Mol Cancer Ther 7:59–70
Weidner N (1995) Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat 36:169–180
McDonald PC, Fielding AB, Dedhar S (2008) Integrin-linked kinase–essential roles in physiology and cancer biology. J Cell Sci 121:3121–3132
McDonald PC, Oloumi A, Mills J et al (2008) Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res 68:1618–1624
Troussard AA, Costello P, Yoganathan TN et al (2000) The integrin linked kinase (ILK) induces an invasive phenotype via AP-1 transcription factor-dependent upregulation of matrix metalloproteinase 9 (MMP-9). Oncogene 19:5444–5452
Delcommenne M, Tan C, Gray V et al (1998) Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA 95:11211–11216
Haase M, Gmach CC, Eke I et al (2008) Expression of integrin-linked kinase is increased in differentiated cells. J Histochem Cytochem 56:819–829
Chung DH, Lee JI, Kook MC et al (1998) ILK (beta1-integrin-linked protein kinase): a novel immunohistochemical marker for Ewing's sarcoma and primitive neuroectodermal tumour. Virchows Arch 433:113–117
Goulioumis AK, Bravou V, Varakis J et al (2008) Integrin-linked kinase cytoplasmic and nuclear expression in laryngeal carcinomas. Virchows Arch 453:511–519
Acconcia F, Barnes CJ, Singh RR et al (2007) Phosphorylation-dependent regulation of nuclear localization and functions of integrin-linked kinase. Proc Natl Acad Sci USA 104:6782–6787
Fielding AB, Dobreva I, McDonald PC et al (2008) Integrin-linked kinase localizes to the centrosome and regulates mitotic spindle organization. J Cell Biol 180:681–689
Banerjee S, Saxena N, Sengupta K et al (2003) 17alpha-estradiol-induced VEGF-A expression in rat pituitary tumor cells is mediated through ER independent but PI3K-Akt dependent signaling pathway. Biochem Biophys Res Commun 300:209–215
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, J., Shi, R., Zhang, D. et al. Expression of integrin-linked kinase in lung squamous cell carcinoma and adenocarcinoma: correlation with E-cadherin expression, tumor microvessel density and clinical outcome. Virchows Arch 458, 99–107 (2011). https://doi.org/10.1007/s00428-010-1016-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-010-1016-3