Abstract
Among raised dermal scar types, keloid (KS) and hypertrophic scars (HS) are considered to present clinical similarities, but there are no known specific biomarkers that allow both scar types to be easily distinguished. Development and progression of raised dermal scars comprises the activation of several molecular pathways and cell defence mechanisms leading to elevated extracellular matrix component synthesis, delayed apoptosis, altered migration and differentiation. Therefore, the aim here was to identify biomarkers that may differentiate between KS and HS compared to normal skin (NS). To achieve this aim, NS (n = 14), KS (n = 14) and HS (n = 14) biopsies were evaluated using histology by H&E staining. Tissue biopsies and primary fibroblasts (passages 0–4) were employed to assess the gene expression levels of 21 biomarkers selected from our previous microarray studies using qRT-PCR. Finally, protein expression was evaluated using In-Cell Western Blotting in primary fibroblasts (p 0–4). Our results demonstrated that out of the 21 biomarkers screened at mRNA and protein levels, α2β1-integrin, Hsp27, PAI-2, MMP-19 and CGRP showed significantly higher expression (p < 0.05) in KS compared to NS and HS. Additionally, these five key biomarkers were found to be significantly higher (p < 0.05) at mRNA level in KS taken from the sternum, a region known to be subjected to high mechanical forces in the body during the performance of daily movements. In conclusion, our findings offer potential molecular targets in raised dermal scars differentiation. Future targeted research may allow provision of diagnostic and prognostic markers in keloid versus hypertrophic scars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cutaneous wound healing is a complicated, multistep process that involves the combination of several molecular, cellular, physiological, biochemical and mechanical factors [9, 13]. Abnormal wound healing occurs as a result of perturbation of the intricate balance during the process of repair. Environmental factors such as the nature of injury, severity, depth of injury, anatomical location, tensional stress, infection, and genetic factors including heritable predisposition to scarring, sex and hormone levels have been proposed to contribute to abnormal skin scar formation [8, 59]. This may result in the formation of raised dermal scars in the form of hypertrophic and keloid scars [28, 58, 59]. Hypertrophic scars (HS) are raised dermal scars that remain within the boundary of the original injury, and can regress over time [8, 25]; whereas keloid scars (KS) are raised dermal lesions that spread beyond the boundaries of the original wound and invade the healthy surrounding tissue [7, 58].
To date, no mechanisms have been identified that clearly and comprehensively explain raised dermal scar formation and differentiation. It has been proposed that the development and progression of raised dermal scars comprises the activation of several molecular pathways and cell defence mechanisms that can lead to elevated extracellular matrix (ECM) component synthesis, delayed apoptosis, altered migration and differentiation [1, 25, 50, 51, 71]. The proteins and signalling pathways activated during pathologic scarring processes include neuropeptides, chaperones, cytokines and chemokines among other proteins [13, 25, 29, 31–34, 63].
The aim of the present study was to characterise the expression of target biomarkers involved in the differentiation of HS and KS. To this end, potential biomarkers (genes and proteins) were identified according to the following criteria. Firstly, a correlation between different microarray studies available to date was performed, and the up-regulated biomarkers in HS or KS with respect to normal tissue were selected and summarised in Tables 1 and 2, respectively; whereas Table 3 summarises all those genes that were found commonly up-regulated in both scar types after microarray comparisons performed by Shih et al. [60] and Huang et al. [32]. These three previously mentioned lists of biomarkers were compared with our own unpublished microarray data which was derived from KS tissue. The biomarkers that were highly statistically significant and which were shared among these datasets were included. The second criteria employed for the biomarker selection was based on previous studies on both scar types which highlighted genes, and molecular pathways (immunological dysregulation, genetic predisposition, neurogenic inflammation and mechano-transduction among others) [2, 21, 26, 35, 36, 43, 49, 55, 61] in HS or KS found in at least three independent studies. The final selection criteria employed was based on the biological relationship between potential target biomarkers identified from the above two approaches which were linked to both fibrosis and apoptosis. Candidate biomarkers (n = 21) were selected for evaluation in both tissue and cells extracted from HS and KS.
Methods
The study was undertaken in three parts: (1) histological analysis of tissue specimens taken from normal skin (NS), KS and HS; (2) gene expression screening in NS, KS and HS tissue samples; and (3) gene and protein expression screening in primary fibroblast cultures established from NS, KS and HS biopsies. All samples employed in the study were obtained from biopsies taken from different anatomical locations. All HS and KS samples were confirmed to have clinical and pathological evidence of raised dermal scarring in the form of HS and KS as previously described by Syed et al. [65]. Haematoxylin and eosin staining was employed to enable histological comparison of the different skin scar tissue specimens. Gene expression was evaluated in the tissue samples using quantitative reverse transcription-polymerase chain reaction (qRT-PCR). Primary normal skin, keloid, hypertrophic fibroblast cultures form passages 0–4 were established from tissue samples collected and employed to evaluate gene and protein expressions by qRT-PCR and in-cell western blotting, respectively.
Biomarkers (genes or proteins) were identified for inclusion in our study based on a number of criteria. Firstly, candidate biomarkers that had demonstrated high expression in at least three previous microarray studies in humans and provided detailed results of the gene expression evaluation, in addition to unpublished in-house microarray performed in KS were included [7, 15, 20, 25, 30, 40, 45, 51, 54, 57, 64, 67, 71]. Secondly, genes that had been identified as being associated with wound healing processes were chosen. Studies concerning key molecules and pathways investigated in both KS and HS were also included if evidence of up-regulation of the aforementioned molecules was found in more than three independent studies. Finally, candidate genes implicated in fibrotic and apoptotic processes were selected [5, 7, 15, 42, 63]. Use of the above strict criteria resulted in the generation of a list of 21 biomarkers which included neuropeptides, tension-related (Hsps, MMPs and MCPs), ECM-related and cytokines genes. A list of the 21 biomarkers investigated is given in Table 4. In addition, the experimental design of the study is depicted in Fig. 1.
Patient data and tissue collection
Normal skin (NS) (n = 14), keloid (KS) (n = 14) and hypertrophic skin (HS) (n = 14) samples were employed in this study. Samples were taken from a number of different sites including the sternum, ear, pubis and scalp (see Table 5). Samples were obtained following informed consent from all patients (full ethical approval was obtained from the local hospital, University and regional NHS Ethics Committee in England, UK) prior to surgery. At the time of surgical excision, tissue biopsies from each lesional site were collected and processed as previously described by Syed et al. [65]. The samples were processed for cell culturing within 12 h.
Primary fibroblast culture establishment
The tissue was collected in Dulbecco’s modified Eagle medium (Sigma-Aldrich, Dorset, UK) and fibroblast cultures were extracted using collagenase type I solution, 0.5 mg mL−1 (Roche Diagnostics, West Sussex, UK) and grown as described previously in Syed et al. [65] and Suarez et al. [63]. Fibroblasts from passages 0–4 were employed for the experiments.
Haematoxylin and eosin staining (H&E)
Sections of 5 μm thickness from tissue specimens, which had previously been embedded in paraffin blocks and fixed in formaldehyde, were stained with haematoxylin and eosin (H&E) (Surgipath, Peterborough, UK) for histological evaluation. Tissue samples were first stained for nuclei with iron haematoxylin (Sigma-Aldrich, Dorset, UK) for 10 min. After incubation, three washes with top water were performed. A subsequent staining employing van Gieson (Sigma-Aldrich, Dorset, UK) was then performed for 10 min. Tissue sections were washed in 1 % acetic acid for 1 min and dehydrated in graded ethanol (95 and 100 %), treated with xylene for clearing and mounted. More details about the procedure can be found at Syed et al. [65].
RNA extraction, cDNA synthesis and quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
RNA was extracted from cells and tissue biopsies and cDNA synthesis and qRT-PCR were performed as previously described in Shih et al. [60] and Syed et al. [65]. The primer list employed can be found in Suarez et al. [63]. Each reaction was performed in triplicate. The gene expression levels were normalised with respect to a reference gene, the L32 ribosomal protein gene (RPL32).
In-cell western blotting (ICW)
p0–p3 fibroblasts were grown to 95–100 % confluence in T25 flasks. Samples were processed as described previously in Syed et al. [65]. The panel of antibodies used is given in Table 6. Data were acquired using the Odyssey software package 2.1 software (LI-COR Biosciences, Cambridge, UK), then exported and analysed using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA).
Statistical analysis
Data were expressed as the mean ± SEM of at least three independent experiments. To determinate statistical differences, a one-way nonparametric ANOVA analysis was performed followed by a Tukey multiple comparison post-test using GraphPad Prism 5 software (GraphPad Software, La Jolla, USA). The difference between the means for all conditions was considered statistically significant at p < 0.05.
Results
Histological comparison of normal skin with keloid and hypertrophic scars
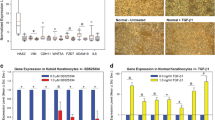
Haematoxylin and eosin (H&E) stained tissue samples (Fig. 2) revealed that in the normal skin samples, the epidermal layer is well defined and relatively narrow and uniform in thickness compared to the scar tissue samples, which were found to have thicker, less well defined and non-uniform epidermal layers. Of the two scar tissue types analysed, the hypertrophic samples displayed the thickest epidermal layer. In normal skin, the characteristic random orientation and bundle formation of collagen fibres was observed (Fig. 2a). The KS samples exhibited normal epidermis thickness with regular basal cell organisation, an increased number of thick collagen fibres arranged in bundles in the reticular dermis region compared to both NS and HS samples. Collagen fibres were found horizontally arranged to the epidermal layer in most KS cases (Fig. 2b). The analysis of the hypertrophic tissue revealed disarray of basal epidermal cells, and thinner collagen fibres in the dermis. The collagen fibres were arranged randomly with respect to the epidermis and showed highly cellular zones in the reticular dermis (Fig. 2c) in HS tissue. The above are in keeping with similar findings reported previously in other studies [39, 68].
Histology of normal skin sample compared hypertrophic and keloid scars. A histological comparison of a normal skin, b keloid scar and c hypertrophic scars biopsies employing haematoxylin and eosin (H&E) staining. Significant difference in the collagen fibres arrangement can be observed among the different tissue sections as well as highly cellular populated zones in both keloid and hypertrophic scars in the reticular dermis areas (black arrows). Normal skin presented random and relaxed collagen bundles arrangement whereas, keloid and hypertrophic tissue present more stretched and thicker collagen bundles. Abundant cellular islands were found in both the papillary and the reticular dermis of hypertrophic scar tissue. K keratin layer, EP epidermis, PD papillary dermis, RD reticular dermis
Gene expression screening in normal, keloid and hypertrophic scar tissue
For analysis purposes, target biomarkers were classified into the following four categories:
-
(a)
Neuropeptides, CGRP (calcitonin-related polypeptide alpha), NPY (neuropeptide Y), SP (substance-P tachykinin, precursor 1), VIP (vasoactive intestinal peptide);
-
(b)
Tension-related, PAI-2 [serpin peptidase inhibitor, clade B (ovalbumin), member 2]; Hsps, [Hsp27 (heat shock protein 27), Hsp47 (heat shock protein 47), Hsp60 (heat shock protein 60), Hsp70 (heat shock protein 70), Hsp90 (heat shock protein 90)]; MMPs, MMP-3 (matrix metallopeptidase 3), MMP-13 (matrix metallopeptidase 13), MMP19 (matrix metallopeptidase 19) and MCPs, MCP-1 (monocyte chemotactic protein-1), MCP-3 (monocyte chemotactic protein-3);
-
(c)
ECM-related, ITGA2 (α2β1-Integrin), Cyr61 (cysteine-rich, angiogenic inducer, 61) and TNXB (tenascin XB) and
-
(d)
Cytokines, TGF-β1 (transforming growth factor, beta 1), TGF-β2 (transforming growth factor, beta 2) and TGF-β3 (transforming growth factor, beta 3).
Neuropeptides gene expression in tissue biopsies and fibroblasts from keloid and hypertrophic scars
Total RNA was extracted from tissue biopsies and the expression of the neuropeptides SP, CGRP, NPY and VIP was evaluated using qRT-PCR. mRNA levels of neuropeptides detected were significantly different among the scar tissue types tested (Fig. 3). SP expression was significantly higher in HS compared to NS and KS (p < 0.05), whereas CGRP mRNA level was notably up-regulated in KS compared to NS and HS (p < 0.05). Moreover, mRNA levels were notably down-regulated for the NPY and VIP biomarkers in KS compared to NS and HS samples (p < 0.05). To evaluate the neuropeptide expression in fibroblasts, total mRNA was extracted from primary fibroblasts established from NS, KS and HS biopsies. qRT-PCR analysis of the samples (Fig. 4) revealed that of the four biomarkers tested, only CGRP was significantly up-regulated in KS and HS fibroblasts compared to NS fibroblasts (p < 0.001). No expression of the gene VIP was detected among the samples. Interestingly, the expression of SP and NPY was significantly higher in KS fibroblasts compared to NS (p < 0.05).
Tissue mRNA expression of neuropeptides seen in keloid and hypertrophic scar compared to normal skin evaluated by qRT-PCR. The expression of neuropeptides including SP, CGRP, NPY and VIP was normalised to an internal reference gene (RPL32). The results are expressed as mean ± SEM of triplicates of independent experiments (n = 14). *p < 0.05 indicates a significantly increased difference between the mRNA expression in tissue biopsy specimens from normal skin (n = 14); whereas # p > 0.05 expresses significant reduction on the mRNA expression extracted from raised dermal scar biopsies versus normal skin
Cellular mRNA expression of neuropeptides in keloid and hypertrophic scar compared to normal skin evaluated by qRT-PCR. Fibroblasts were cultured from passage 0 (p0) to passage 4 (p4). The expression of neuropeptides including SP, CGRP, NPY and VIP was normalised to an internal reference gene (RPL32). The results are expressed as mean ± SEM of triplicates of independent experiments (n = 14). *p < 0.05 indicates a significantly increased difference between the mRNA expression in tissue biopsy specimens
Characterisation of the mRNA levels of tension-related biomarkers in keloid and hypertrophic scar tissue biopsies and fibroblasts
Figure 5 shows the results of the tension-related gene expression characterisation of tissue biopsies for 11 of the previously identified 21 genes. Of the 11 biomarkers tested in this group, 5 showed significant over-expression at mRNA levels (p < 0.05) in both KS and HS when compared to NS samples, these were Hsp47, Hsp60, MCP-3, MCP-1 and MMP-19. In contrast, three genes, Hsp90, MMP-3 and MMP-13, demonstrated lower expression at mRNA levels in both scar types compared to NS. No significant difference was detected in Hsp70 expression among the samples. Hsp27 and PAI-2 were found to be over-expressed in KS (p < 0.05). The expression of tension-related biomarkers was also evaluated in primary fibroblast cultures; the results are shown in Fig. 6. At mRNA level, significant difference was found between the raised dermal scar cell samples and NS fibroblasts for the Hsp27, Hsp90, MMP-13 and MMP-3 biomarkers (p < 0.05), whereas no significant difference was found among the samples for the Hsp70 and MMP-19 genes. The expression of the MCP-3 and PAI-2 was significantly higher in KS fibroblasts compared to the other samples (p < 0.05 and p < 0.01, respectively), but the Hsp47 gene exhibited a lower expression in KS fibroblasts. In HS fibroblasts samples the expression of Hsp60 was significantly higher compared to NS fibroblasts (p < 0.05), but the expression was lower for the MCP-1 gene.
Tissue expression of mRNA of tension-related biomarkers in keloid and hypertrophic scar compared to normal skin evaluated by qRT-PCR. Total RNA extracted from tissue biopsies as described in “Methods” section was employed to evaluate the expression of tension-related proteins and normalised to an internal reference gene (RPL32). The results are expressed as mean ± SEM of triplicates of independent experiments (n = 14). *p < 0.05 indicates a significantly increased difference between the mRNA expression in tissue biopsy specimens from normal skin (n = 14); whereas # p > 0.05 expresses significant reduction on the mRNA expression extracted from dermal scar biopsies versus normal skin
Cellular mRNA expression for tension-related biomarkers in keloid and hypertrophic scar compared to normal skin evaluated by qRT-PCR. Fibroblasts were cultured from p0 to p4. The expression of tension-related genes was normalised to an internal reference gene (RPL32). The results are expressed as mean ± SEM of triplicates of independent experiments (n = 14). *p < 0.05 indicates a significantly increased difference between the mRNA expression in tissue biopsy specimens from normal skin (n = 14); whereas # p > 0.05 expresses significant reduction on the mRNA expression extracted from dermal scar biopsies versus normal skin
Expression profile of ECM-related biomarkers in keloid and hypertrophic scar tissue and fibroblasts
The following ECM-related biomarkers, tenascin, α2β1-integrin and Cyr61, were evaluated for their association with KS and HS. The analysis of the mRNA expression of these biomarkers was performed using NS, KS and HS samples. The qRT-PCR results presented in Fig. 7 show a clear over-expression of tenascin and α2β1-integrin in both KS and HS compared to NS (p < 0.05), whereas Cyr61 showed lower mRNA levels in both raised dermal scar types (p < 0.05).When we assessed the expression of ECM-related biomarkers in fibroblasts by qRT-PCR, the results showed significantly higher expression levels of α2β1-integrin in both KS and HS fibroblasts (p < 0.01) (Fig. 8), whereas no significant difference was found among the samples when tenascin where evaluated. The expression of Cyr61 was found to be significantly up-regulated in KS compared to NS fibroblasts (p < 0.05).
Tissue mRNA expression of ECM-related seen in keloid and hypertrophic scar compared to normal skin evaluated by qRT-PCR. The expression of neuropeptides including SP, CGRP, NPY and VIP was normalised to an internal reference gene (RPL32). The results are expressed as mean ± SEM of triplicates of independent experiments (n = 14). *p < 0.05 indicates a significantly increased difference between the mRNA expression in tissue biopsy specimens from normal skin (n = 14); whereas # p > 0.05 expresses significant reduction on the mRNA expression extracted from dermal scar biopsies versus normal skin
Cellular mRNA expression for ECM-related biomarkers in keloid and hypertrophic scar compared to normal skin evaluated by qRT-PCR. Fibroblasts were cultured from p0 to p4. The expression of tension-related genes was normalised to an internal reference gene (RPL32). The results are expressed as mean ± SEM of triplicates of independent experiments (n = 14). *p < 0.05 indicates a significantly increased difference between the mRNA expression in tissue biopsy specimens from normal skin (n = 14); whereas # p > 0.05 expresses significant reduction on the mRNA expression extracted from dermal scar biopsies versus normal skin
Differential expression of cytokines in keloid and hypertrophic scar tissue samples and fibroblasts
The expression of the 3 TGF-β isoforms (TGF-β1, TGF-β2 and TGF-β3) were evaluated in raised dermal raised scars at mRNA level, using qRT-PCR. The results of the analysis are shown in Fig. 9 where it can be seen that that the expression of TGF-β1 and TGF-β2 is significantly lower in KS and HS when compared to NS (p < 0.05). However, no statistical difference was found in the expression of TGF-β3 among the study samples, whereas, the expressions of the TGF-β1 and TGF-β2 isoforms were found to be significantly up-regulated in HS scars when compared to NS fibroblasts (p < 0.05) (Fig. 10). No significant difference was found in the expression of TGF-β3 among the samples considered.
Tissue mRNA expression of cytokines seen in keloid and hypertrophic scar compared to normal skin evaluated by qRT-PCR. The expression of neuropeptides including SP, CGRP, NPY and VIP was normalised to an internal reference gene (RPL32). The results are expressed as mean ± SEM of triplicates of independent experiments (n = 14). *p < 0.05 indicates a significantly increased difference between the mRNA expression in tissue biopsy specimens from normal skin (n = 14); whereas # p > 0.05 expresses significant reduction on the mRNA expression extracted from dermal scar biopsies versus normal skin
Cellular mRNA expression for ECM-related biomarkers in keloid and hypertrophic scar compared to normal skin evaluated by qRT-PCR. Fibroblasts were cultured from p0 to p4. The expression of tension-related genes was normalised to an internal reference gene (RPL32). The results are expressed as mean ± SEM of triplicates of independent experiments (n = 14). *p < 0.05 indicates a significantly increased difference between the mRNA expression in tissue biopsy specimens from normal skin (n = 14); whereas # p > 0.05 expresses significant reduction on the mRNA expression extracted from dermal scar biopsies versus normal skin
Characterisation of neuropeptide protein expression in keloid and hypertrophic scar fibroblasts
To further investigate our aim of identifying target biomarkers involved in the regulation of raised dermal scar development and differentiation, the protein expression of the four neuropeptides, SP, CGRP, NPY and VIP, was evaluated in primary fibroblast cultures by In-Cell Western Blotting. The results of the analysis are shown in Fig. 11, where upon inspection, it can be seen that SP and CGRP were significantly expressed higher in KS fibroblasts compared to NS fibroblasts (p < 0.05), whereas the NYP protein expression was significantly higher in HS compared to NS fibroblast (p < 0.05). In addition, the protein levels of VIP were significantly lower in HS fibroblasts (p < 0.05).
Cellular protein levels of neuropeptides in keloid and hypertrophic scar compared to normal skin evaluated by in-cell western blotting. Fibroblasts were cultured from p0 to p4. A representative output infrared image of keloid and hypertrophic fibroblasts for neuropeptides expression (green) from 96-well plates is shown in a. b The bar graphs represent the quantification of the expression of the analysed proteins normalised to the β-actin loading control; the result is expressed as mean ± SEM of three independent experiments. *p < 0.05 indicates significant up-regulation of the neuropeptides expression when compared to normal skin fibroblasts, while # p < 0.05 can be appreciated as significant down-regulation of the neuropeptides expression from dermal scar cells versus normal skin fibroblasts (n = 14)
Characterisation of the protein expression of tension-related biomarkers in keloid and hypertrophic scar fibroblasts
Based on the results of our qRT-PCR analysis in keloid and hypertrophic fibroblasts, 4 of the 11 biomarkers included in this group, Hsp27, Hsp47, MMP-19 and PAI-2, representative of the range of results reported previously, were chosen for protein expression evaluation in primary fibroblast cultures. The results are shown in Fig. 12 and indicate that Hsp27, MMP-19 and PAI-2 protein levels were significantly higher in KS compared to NS and HS fibroblasts (p < 0.05), whereas Hsp47 protein levels were significantly higher in HS fibroblasts compared to NS fibroblasts (p < 0.05).
Cellular protein levels of tension-related in keloid and hypertrophic scar compared to normal skin evaluated by in-cell western blotting. Fibroblasts were cultured from p0 to p4. A representative output infrared image of keloid and hypertrophic fibroblasts for neuropeptides expression (green) from 96-well plates is shown in a. b The bar graphs represent the quantification of the expression of the analysed proteins normalised to the β-actin loading control; the result is expressed as mean ± SEM of three independent experiments. *p < 0.05 indicates significant up-regulation of the tension-related biomarkers expression when compared to normal skin fibroblasts, while # p < 0.05 can be appreciated as significant down-regulation of the tension-related biomarkers from dermal scar cells versus normal skin fibroblasts (n = 14)
Protein expression of ECM-related proteins in primary fibroblast from keloid and hypertrophic scars
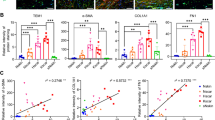
Figure 13 shows the differences in the protein levels among the cell samples. Fibronectin, α-SMA and vinculin protein expression levels were found to be significantly higher in both raised dermal scar cell types (p < 0.05). α2β1-integrin and collagen I were strongly expressed in KS fibroblasts compared to NS cells (p < 0.05).
Cellular protein levels of ECM-related in keloid and hypertrophic scar compared to normal skin evaluated by in-cell western blotting. Fibroblasts were cultured from p0 to p4. A representative output infrared image of keloid and hypertrophic fibroblasts for neuropeptides expression (green) from 96-well plates is shown in a. b The bar graphs represent the quantification of the expression of the analysed proteins normalised to the β-actin loading control; the result is expressed as mean ± SEM of three independent experiments. *p < 0.05 indicates significant up-regulation of the ECM-related biomarkers expression when compared to normal skin fibroblasts, while # p < 0.05 can be appreciated as significant down-regulation of the ECM-related biomarkers from dermal scar cells versus normal skin fibroblasts (n = 14)
Characterisation of cytokines protein expression in keloid and hypertrophic scar fibroblasts
As can be seen from the results of the in-cell western blotting assay shown in Fig. 14, TGF-β2 protein levels were significantly higher in both KS and HS fibroblasts compared to NS cells (p < 0.05). Furthermore, TGF-β1 was up-regulated in KS fibroblasts compared to the rest of the samples (p < 0.05), whereas TGF-β3 expression was lower in HS fibroblasts (p < 0.05).
Cellular protein levels of cytokines in keloid and hypertrophic scar compared to normal skin evaluated by in-cell western blotting. Fibroblasts were cultured from p0 to p4. A representative output infrared image of keloid and hypertrophic fibroblasts for neuropeptides expression (green) from 96-well plates is shown in a. b The bar graphs represent the quantification of the expression of the analysed proteins normalised to the β-actin loading control; the result is expressed as mean ± SEM of three independent experiments. *p < 0.05 indicates significant up-regulation of the cytokines expression when compared to normal skin fibroblasts, while # p < 0.05 can be appreciated as significant down-regulation of the cytokines from dermal scar cells versus normal skin fibroblasts (n = 14)
Gene expression in keloid scar tissue samples and fibroblasts analysed by anatomical location
Figure 15 shows the gene expression for five molecules, α2β1-integrin, Hsp27, PAI-2, MMP-19 and CGRP, which our analysis showed were significantly up-regulated in KS tissue and fibroblasts. The results are presented based on the location from which the scar tissue samples and cells were collected. It can be seen upon inspection of Fig. 15 that the highest expression for the five biomarkers shown in both tissue and cells was found to be in the samples taken from the sternum (p < 0.05).
Tissue and cellular mRNA expression for the top 5 up-regulated biomarkers in keloid scar compared to normal skin evaluated by qRT-PCR. The bar graphs represent the quantification of the total mRNA expression of the top 5 up-regulated biomarkers extracted from tissue biopsies and fibroblasts from keloid samples and normalised to an internal reference gene (RPL32). The results are expressed as mean ± SEM of triplicates of independent experiments (n = 14). *p < 0.05 indicates a significantly increased difference between the mRNA expression among the scars collected from specific anatomical locations (n = 14)
Discussion
In the present study, a list of 21 candidate biomarkers was selected following an extensive literature review in addition to using our own unpublished microarray data. We analysed for the first time, mRNA levels in tissue and primary fibroblasts as well as protein levels in primary fibroblasts obtained from KS and HS. The findings, summarised in Table 7, provide a list of candidate biomarkers that may be involved in the development and differentiation of HS and KS.
We identified five potential biomarkers including CGRP, Hsp27, MMP-19, PAI-2 and α2β1-integrin that were selected on the basis of consistent up-regulation at both mRNA (tissue and cells) and protein (cells) levels in KS compared to HS and NS. However, there was a lack of consistency in expression levels (mRNA and protein) in tissue and cells from hypertrophic scar tissue samples. Several discrepancies in the patterns of expression of proposed biomarkers were found among the samples used in this study, although this was not totally unexpected as this phenomenon had been identified and discussed by other investigators [6, 49, 69, 70]. Indeed, Shih et al. [60], demonstrated that transcriptomic data obtained from primary fibroblast cultures do not always correlate exactly to their respective tissue biopsy sample data. It has also been suggested that culturing conditions exert a profound impact on gene expression levels [24]; and the passage number during culturing could significantly affect gene and protein expression levels in primary fibroblast cultures [10, 62, 65]. The five biomarkers found to be consistently up-regulated in KS are strongly associated with mechanical tension [2, 11, 12, 22].
In addition, our results obtained at mRNA level in both tissue and fibroblasts from KS were analysed by scar anatomical location. This was carried out in order to better define gene expression differences seen in specific anatomical sites. The expression of the five target biomarkers was highest in the samples taken from the sternum, which, correlates with other studies previously undertaken in relation to anatomical site and mechanical tension [1, 6, 47, 48].
The mechanisms leading to the development of both KS and HS are thought to involve the interaction of many biomarkers, several signalling pathways as well as environmental influences [4, 14]. The aim of this study was to identify potential biomarkers that could be used to distinguish between KS and HS. For analysis purposes, target biomarkers were classified into the following four categories: (a) neuropeptides, (b) tension-related, (c) ECM-related and (d) cytokines. To this end, we included neuropeptides in the study, in order to evaluate the potential role of constant stimulation of the sensory skin nerve fibres produced by mechanical stress that may affect the transmission of signals from the sensory nerves resulting in release of specific neuropeptides in the skin [23, 56]. Neuropeptides binding to their respective receptors located on skin cells’ surface could induce vasodilatation and vessel permeabilisation evoking inflammatory responses [2, 56, 72]. This may then lead to the development of neurogenic inflammation followed by an exaggerated immune response triggering the release of proteins, such as Hsps, MMPs and MCPs [1, 23, 27, 37, 44, 50, 57, 70]. We also decided to study the proposed correlation existing between the expression of several Hsps and the increased synthesis of collagen I, as well as Hsps participation as mediators in keratinocyte proliferation and differentiation [11]. Hsps also regulate the proliferative phase during wound healing and promote new tissue formation. Hsps act as cellular chaperones that modulate cell death signals such as the FAS-mediated apoptotic pathway, allowing to cells adapt to gradual changes in their environment and to survive in otherwise lethal conditions [16, 18, 38, 44, 52]. In addition, MMPs have been implicated in angiogenesis, scar resorption, inflammation, re-epithelialisation and remodelling phases of wound healing [63, 66]. MCPs, transcription factors and cytokines are also linked with the regulation of inflammatory processes and cell recruitment in normal wound healing [27].
Interestingly, a correlation between neuropeptides, HSPs, MMPs and MCPs and the skin mechanical tension has been proposed in that this may promote the development and progression of raised dermal scars such as KS and HS [70], as they are prone to develop frequently at highly tensioned anatomical locations [28]. Several researchers have suggested that the site of injury has been found to be substantially influenced by scar formation and wound closure [50] with raised dermal scars tending to occur more frequently in body areas subjected to greater mechanical forces [2, 46]. In this context, the sternum, shoulders and suprapubic region are body zones considered as strong candidates for the development of raised dermal scarring [2, 13, 29, 53, 60]. Furthermore, the development of pathologic healing processes have been linked to mechano-signal transduction [47], whereby mechanical stress signals are transduced into biomechanical signals resulting in cellular responses that may promote raised dermal scar development [31, 33, 63]. Despite this knowledge, target molecules that promote and differentiate abnormal scar types have not been clearly identified to date.
Based on our results, we propose that the regulation of both raised dermal scaring processes are closely related to pathways that regulate mechanisms including proliferation and migration, angiogenesis, ECM degradation, inflammation, communication and cell survival among others [2, 3, 17, 19, 23, 41, 46] and also, that the expression of these target molecules is closely related to highly tensioned body areas. The limitations of this study include sample size as well as the lack of an in-house microarray dataset in HS samples. Despite this, we were able to identify five potential biomarkers that may be used in evaluation of HS and KS but would certainly require further validation in larger studies with different cohort of samples including both varieties of raised dermal scars. These potential biomarkers may be used in the diagnostic and prognostic evaluation of both scar types. In addition to repeat validation, further studies are required to fully explore the mechanisms involving these biomarkers in KS and HS pathogenesis.
References
Akaishi S, Akimoto M, Ogawa R, Hyakusoku H (2008) The relationship between keloid growth pattern and stretching tension: visual analysis using the finite element method. Ann Plast Surg 604:445–451
Akaishi S, Ogawa R, Hyakusoku H (2008) Keloid and hypertrophic scar: neurogenic inflammation hypotheses. Med Hypotheses 711:32–38
Altun V, Hakvoort TE, Van Zuijlen PPM, Van Der Kwast TH, Prens EP (2001) Nerve outgrowth and neuropeptide expression during the remodeling of human burn wound scars: a 7-month follow-up study of 22 patients. Burns 277:717–722
Atiyeh BS, Costagliola M, Hayek SN (2005) Keloid or hypertrophic scar: the controversy: review of the literature. Ann Plast Surg 546:676–680
Bayat A, Arscott G, Ollier WE, McGrouther DA, Ferguson MW (2005) Keloid disease: clinical relevance of single versus multiple site scars. Br J Plast Surg 581:28–37
Bayat A, Arscott G, Ollier WER, Ferguson MWJ, Mc Grouther DA (2004) Description of site-specific morphology of keloid phenotypes in an Afrocaribbean population. Br J Plast Surg 572:122–133
Bayat A, Bock O, Mrowietz U, Ollier WE, Ferguson MW (2003) Genetic susceptibility to keloid disease and hypertrophic scarring: transforming growth factor beta1 common polymorphisms and plasma levels. Plast Reconstr Surg 1112:535–543
Bayat A, McGrouther DA, Ferguson MWJ (2003) Skin scarring. Br Med J 3267380:88–92
Berry DP, Harding KG, Stanton MR, Jasani B, Ehrlich HP (1998) Human wound contraction: collagen organization, fibroblasts, and myofibroblasts. Plast Reconstr Surg 1021:124–131
Bignotti E, Tassi RA, Calza S, Ravaggi A, Romani C, Rossi E, Falchetti M, Odicino FE, Pecorelli S, Santin AD (2006) Differential gene expression profiles between tumor biopsies and short-term primary cultures of ovarian serous carcinomas: identification of novel molecular biomarkers for early diagnosis and therapy. Gynecol Oncol 1032:405–416
Bitar KN, Kaminski MS, Hailat N, Cease KB, Strahler JR (1991) Hsp27 is a mediator of sustained smooth muscle contraction in response to bombesin. Biochem Biophys Res Commun 1813:1192–1200
Blake MJ, Gershon D, Fargnoli J, Holbrook NJ (1990) Discordant expression of heat shock protein mRNAs in tissues of heat-stressed rats. J Biol Chem 26525:15275–15279
Brissett AE, Sherris DA (2001) Scar contractures, hypertrophic scars, and keloids. Facial Plast Surg 174:263–271
Brown JJ, Bayat A (2009) Genetic susceptibility to raised dermal scarring. Br J Dermatol 1611:8–18
Butler PD, Longaker MT, Yang GP (2008) Current progress in keloid research and treatment. J Am Coll Surg 2064:731–741
Charette SJ, Lavoie JN, Lambert H, Landry J (2000) Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol 2020:7602–7612
Chen G, Gharib TG, Huang CC, Taylor JM, Misek DE, Kardia SL, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, Beer DG (2002) Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics 14:304–313
Chen J-J, Jin P-S, Zhao S, Cen Y, Liu Y, Xu X-W, Duan W-Q, Wang H-S (2011) Effect of heat shock protein 47 on collagen synthesis of keloid in vivo. ANZ J Surg 816:425–430
Chen J, Wang JH, Zhuang HX (2006) Influence of substance P on the proliferation and apoptosis of fibroblasts of pathological scars. Chin J Burns 224:277–280
Chen W, Fu X, Sun X, Sun T, Zhao Z, Sheng Z (2003) Analysis of differentially expressed genes in keloids and normal skin with cDNA microarray. J Surg Res 1132:208–216
Chike-Obi CJ, Cole PD, Brissett AE (2009) Keloids: pathogenesis, clinical features, and management. Semin Plast Surg 233:178–184
Croucher DR, Saunders DN, Lobov S, Ranson M (2008) Revisiting the biological roles of PAI2 (SERPINB2) in cancer. Nat Rev Cancer 87:535–545
Crowe R, Parkhouse N, McGrouther D, Burnstock G (1994) Neuropeptide-containing nerves in painful hypertrophic human scar tissue. Br J Dermatol 1304:444–452
Dangles V, Lazar V, Validire P, Richon S, Wertheimer M, Laville V, Janneau JL, Barrois M, Bovin C, Poynard T, Vallancien G, Bellet D (2002) Gene expression profiles of bladder cancers: evidence for a striking effect of in vitro cell models on gene patterns. Br J Cancer 868:1283–1289
Dasu MR, Hawkins HK, Barrow RE, Xue H, Herndon DN (2004) Gene expression profiles from hypertrophic scar fibroblasts before and after IL-6 stimulation. J Pathol 2024:476–485
Davidson S, Aziz N, Rashid RM, Khachemoune A (2009) A primary care perspective on keloids. Med Gen Med Medscape Gen Med 11:18
Dipietro LA, Reintjes MG, Low QEH, Levi B, Gamelli RL (2001) Modulation of macrophage recruitment into wounds by monocyte chemoattractant protein-1. Wound Repair Regen 91:28–33
Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG (2011) Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med 171–2:113–125
Gu LH, Coulombe PA (2007) Keratin function in skin epithelia: a broadening palette with surprising shades. Curr Opin Cell Biol 191:13–23
Hu ZF, Gao JH, Li W, Song YB, Li CL (2006) Differential gene expression profile of keloids: a study with cDNA microarray. Nan Fang Yi Ke Da Xue Xue Bao 263:308–312
Huang C, Akaishi S, Ogawa R (2012) Mechanosignaling pathways in cutaneous scarring. Arch Dermatol Res 3048:589–597
Huang C, Nie F, Qin Z, Li B, Zhao X (2013) A snapshot of gene expression signatures generated using microarray datasets associated with excessive scarring. Am J Dermatopathol 351:64–73
Huang H, Kamm RD, Lee RT (2004) Cell mechanics and mechanotransduction: pathways, probes, and physiology. Am J Physiol Cell Physiol 2871:C1–C11
Ingber D (1991) Integrins as mechanochemical transducers. Curr Opin Cell Biol 35:841–848
Kazeem AA (1988) The immunological aspects of keloid tumor formation. J Surg Oncol 381:16–18
Ketchum LD, Cohen IK, Masters FW (1974) Hypertrophic scars and keloids. A collective review. Plast Reconstr Surg 532:140–154
Kuwano K, Araya J, Hara H (2007) Molecular targets in idiopathic pulmonary fibrosis. Respir Circ 5810:979–985
Laplante AF, Moulin V, Auger FA, Landry J, Li H, Morrow G, Tanguay RM, Germain L (1998) Expression of heat shock proteins in mouse skin during wound healing. J Histochem Cytochem 4611:1291–1301
Lee JY, Yang CC, Chao SC, Wong TW (2004) Histopathological differential diagnosis of keloid and hypertrophic scar. Am J Dermatopathol 265:379–384
Low SQ, Moy RL (1992) Scar wars strategies: target collagen. J Dermatol Surg Oncol 1811:981–986
Luger TA, Lotti T (1998) Neuropeptides: role in inflammatory skin diseases. J Eur Acad Dermatol Venereol 103:207–211
Luo S, Benathan M, Raffoul W, Panizzon RG, Egloff DV (2001) Abnormal balance between proliferation and apoptotic cell death in fibroblasts derived from keloid lesions. Plast Reconstr Surg 1071:87–96
Marneros AG, Krieg T (2004) Keloids—clinical diagnosis, pathogenesis, and treatment options. J Dtsch Dermatol Ges 211:905–913
Morris SD (2002) Heat shock proteins and the skin. Clin Exp Dermatol 273:220–224
Naitoh M, Kubota H, Ikeda M, Tanaka T, Shirane H, Suzuki S, Nagata K (2005) Gene expression in human keloids is altered from dermal to chondrocytic and osteogenic lineage. Genes Cells 1011:1081–1091
Ogawa R (2008) Keloid and hypertrophic scarring may result from a mechanoreceptor or mechanosensitive nociceptor disorder. Med Hypotheses 714:493–500
Ogawa R (2011) Mechanobiology of scarring. Wound Repair Regen 19:s2–s9
Ogawa R, Akaishi S, Huang C, Dohi T, Aoki M, Omori Y, Koike S, Kobe K, Akimoto M, Hyakusoku H (2011) Clinical applications of basic research that shows reducing skin tension could prevent and treat abnormal scarring: the importance of fascial/subcutaneous tensile reduction sutures and flap surgery for keloid and hypertrophic scar reconstruction. J Nippon Med School 782:68–76
Ogawa R, Hsu C-K (2013) Mechanobiological dysregulation of the epidermis and dermis in skin disorders and in degeneration. J Cell Mol Med 177:817–822
Ogawa R, Okai K, Tokumura F, Mori K, Ohmori Y, Huang C, Hyakusoku H, Akaishi S (2012) The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen 202:149–157
Paddock HN, Schultz GS, Baker HV, Varela JC, Beierle EA, Moldawer LL, Mozingo DW (2003) Analysis of gene expression patterns in human postburn hypertrophic scars. J Burn Care Rehabil 246:371–377
Parcellier A, Gurbuxani S, Schmitt E, Solary E, Garrido C (2003) Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem Biophys Res Commun 3043:505–512
Rockwell WB (1997) Elevated levels of testosterone receptors in keloid tissue: an experimental investigation. Plast Reconstr Surg 1002:396
Satish L, Lyons-Weiler J, Hebda PA, Wells A (2006) Gene expression patterns in isolated keloid fibroblasts. Wound Repair Regen 144:463–470
Schierle HP, Scholz D, Lemperle G (1997) Elevated levels of testosterone receptors in keloid tissue: an experimental investigation. Plast Reconstr Surg 1002:390–395
Scholzen T, Armstrong CA, Bunnett NW, Luger TA, Olerud JC, Ansel JE (1998) Neuropeptides in the skin: interactions between the neuroendocrine and the skin immune systems. Exp Dermatol 72–73:81–96
Seifert O, Bayat A, Geffers R, Dienus K, Buer J, Löfgren S, Matussek A (2008) Identification of unique gene expression patterns within different lesional sites of keloids. Wound Repair Regen 162:254–265
Seifert O, Mrowietz U (2009) Keloid scarring: bench and bedside. Arch Dermatol Res 3014:259–272
Shih B, Bayat A (2010) Genetics of keloid scarring. Arch Dermatol Res 3025:319–339
Shih B, McGrouther DA, Bayat A (2010) Identification of novel keloid biomarkers through profiling of tissue biopsies versus cell cultures in keloid margin specimens compared to adjacent normal skin. Eplasty 7(10):e24
Slemp AE, Kirschner RE (2006) Keloids and scars: a review of keloids and scars, their pathogenesis, risk factors, and management. Curr Opin Pediatr 184:396–402
Smith JC, Boone BE, Opalenik SR, Williams SM, Russell SB (2007) Gene profiling of keloid fibroblasts shows altered expression in multiple fibrosis-associated pathways. J Invest Dermatol 1285:1298–1310
Suarez E, Syed F, Alonso-Rasgado T, Mandal P, Bayat A (2013) Up-regulation of tension-related proteins in keloids: knockdown of hsp27, α2β1-integrin, and pai-2 shows convincing reduction of extracellular matrix production. Plast Reconstr Surg 1312:158e–173e
Swamy SMK, Tan P, Zhu YZ, Lu J, Achuth HN, Moochhala S (2004) Role of phenytoin in wound healing: microarray analysis of early transcriptional responses in human dermal fibroblasts. Biochem Biophys Res Commun 3143:661–666
Syed F, Ahmadi E, Iqbal SA, Singh S, McGrouther DA, Bayat A (2011) Fibroblasts from the growing margin of keloid scars produce higher levels of collagen I and III compared with intralesional and extralesional sites: clinical implications for lesional site-directed therapy. Br J Dermatol 1641:83–96
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano: regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 35:349–363
Tsou R, Cole JK, Nathens AB, Isik FF, Heimbach DM, Engrav LH, Gibran NS (2000) Analysis of hypertrophic and normal scar gene expression with cDNA microarrays. J Burn Care Rehabil 216:541–550
Verhaegen PD, van Zuijlen PP, Pennings NM, van Marle J, Niessen FB, van der Horst CM, Middelkoop E (2009) Differences in collagen architecture between keloid, hypertrophic scar, normotrophic scar, and normal skin: an objective histopathological analysis. Wound Repair Regen 175:649–656
Wong TW, Chiu HC, Chen JS, Lin LJ, Chang CC (1995) Symptomatic keloids in two children: dramatic improvement with silicone cream occlusive dressing. Arch Dermatol 1317:775–777
Wong VW, Akaishi S, Longaker MT, Gurtner GC (2011) Pushing back: wound mechanotransduction in repair and regeneration. J Invest Dermatol 13111:2186–2196
Wu J, Ma B, Yi S, Wang Z, He W, Luo G, Chen X, Wang X, Chen A, Barisoni D (2004) Gene expression of early hypertrophic scar tissue screened by means of cDNA microarrays. J Trauma 576:1276–1286
Yamaoka J, Di ZH, Sun W, Kawana S (2007) Erratum to “Changes in cutaneous sensory nerve fibers induced by skin-scratching in mice” [J. Dermatol. Sci. 46 (2007) 41–51]. J Dermatol Sci 472:172–182
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suarez, E., Syed, F., Alonso-Rasgado, T. et al. Identification of biomarkers involved in differential profiling of hypertrophic and keloid scars versus normal skin. Arch Dermatol Res 307, 115–133 (2015). https://doi.org/10.1007/s00403-014-1512-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-014-1512-4