Abstract
The purpose of this study was to investigate the role of infliximab on acute lung injury induced by intestinal ischemia/reperfusion (I/R). A total of 30 male Wistar albino rats were divided into three groups: sham, I/R and I/R+ infliximab; each group contain 10 animals. Sham group animals underwent laparotomy without I/R injury. After I/R groups animals underwent laparotomy, 1 h of superior mesenteric artery ligation were followed by 1 h of reperfusion. In the infliximab group, 3 days before I/R, infliximab (3 mg/kg) was administered by intravenously. All animals were sacrificed at the end of reperfusion and lung tissues samples were obtained for biochemical and histopathological investigation in all groups. To date, no more biochemical and histopathological changes on intestinal I/R injury in rats by infliximab treatment have been reported. Infliximab treatment significantly decreased the elevated tissue malondialdehyde levels and increased of reduced superoxide dismutase, and glutathione peroxidase enzyme activities in lung tissues samples. Intestinal I/R caused severe histopathological injury including edema, hemorrhage, increased thickness of the alveolar wall and a great number of inflammatory cells that infiltrated the interstitium and alveoli. Infliximab treatment significantly attenuated the severity of intestinal I/R injury. Furthermore, there is a significant reduction in the activity of inducible nitric oxide synthase and arise in the expression of surfactant protein D in lung tissue of acute lung injury induced by intestinal I/R with infliximab therapy. It was concluded that infliximab treatment might be beneficial in acute lung injury, therefore, shows potential for clinical use. Because of its anti-inflammatory and antioxidant effects, infliximab pretreatment may have protective effects in acute lung injury induced by intestinal I/R.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intestinal ischemia and reperfusion (I/R) injury occurs in the set-ting of various clinical situations, such as necrotizing enterocolitis, midgut volvulus, intussusception, mesenteric ischemia, hemorrhagic and septic shock (Schoenberg and Beger 1993). It is well demonstrated that intestinal I/R can not only cause the injury of intestine itself, but may also cause severe destruction of remote organs (e.g. lung and heart) and even multiple organ dysfunction. This is largely the consequence of systemic inflammatory response resulting from the damaged intestinal mucosal barrier and consequent translocation of bacteria-endotoxin (Deitch 2001; Mitsuoka et al. 2002) and intestinal oxidative stress (Yucel et al. 2011). Of these remote organ injuries, acute lung injury induced by intestinal I/R has been well characterised as an acute inflammation with sequestration of leucocytes and their enzymatic products in lung tissue as well as increases in microvascular permeability, perivascular and interstitial oedema, and pulmonary oedema (Turnage et al. 1994). To date, the mechanisms about intestinal I/R induced acute lung injury has been poorly understood and there have been no good therapeutical treatments for intestinal I/R induced acute lung injury.
Clinical and experimental studies suggest that oxidative stress induced by reactive oxygen species (ROS) is one of the most important mediators in this process (McCord 1985; Parks et al. 1983; Schoenberg and Beger 1993). ROS can cause direct oxidative damage to DNA, proteins, and lipids (Kumamoto et al. 1999; Ravanat et al. 2000; Casini et al. 1997). It has been known that neutrophils attached to the injured tissues produce a large amount of ROS and cause tissue injury (Mayer and Spitzer 1993; Spitzer and Mayer 1993). It has been reported that increased expression of pulmonary inducible nitric oxide synthase (iNOS) protects the lung from injury after intestinal I/R (Terada et al. 1996; Turnage et al. 1995). We have also recently shown that pharmacological preconditioning with doxorubicin, an anticancer drug, protects the acute lung injury induced by intestinal I/R (Ito et al. 2003). In addition, it has been shown that the free radical scavenger methylene blue prevents the development of polymorphonuclear cell-related lung injury after intestinal I/R in rat (Galili et al. 1998).
Infliximab is a chimeric human immunoglobulin G1 (IgG1) with a mouse variable fragment and a high affinity for tumor necrosis factor (TNF)-a (Sartani et al. 1996). Infliximab has been shown to inhibit functional TNF-a activity in a variety of in vitro bioassays using human fibroblasts, endothelial cells, neutrophils, lymphocytes, and epithelial cells (Di Sabatino et al. 2004). In vivo, infliximab is indicated for the treatment of rheumatologic, gastrointestinal, dermatologic, chronic ocular diseases and intestinal I/R injury. It has been reported to scavenge oxygen free radicals and to inhibit inflammation (Pascher et al. 2005; Cury et al. 2008; Pergel et al. 2011). However, to date, no more biochemical and histopathological changes on acute lung injury induced by intestinal I/R in rats by infliximab treatment have been reported. Therefore, the aim of this study was to elucidate the protective effect of infliximab on acute lung injury induced by intestinal I/R.
Materials and methods
Animals
Male albino Wistar albino rats (200–250 g and averaging 12 weeks old) were used in the present study. All the animals were kept under optimum conditions (21 ± 1°C, 40–70% humidity, 12/12 darkness–lightness cycle) at Ondokuz Mayis University’s Laboratory Animal Unit and were fed ad libitum with standard pellet diet and water. The experimental protocol was approved by Ondokuz Mayis University’s Ethic Committee for Animal Research.
Experimental groups
A total of 30 male Wistar albino rats were divided into three groups: sham, I/R and I/R+ infliximab; each group contain 10 rats. Animals were pretreated with infliximab by intravenously (in a dose of 3 mg/kg body weight) for 3 days before intestinal I/R as described for I/R group.
Technique of intestinal I/R
Feeding of the animals was stopped 12 h prior to the start of the intestinal I/R procedure and they received only water. The rats were anesthetized with ketamine (50 mg/kg) and xylazine (5 mg/kg) intraperitoneally (i.p.) and their temperature was regulated by means of a lamp light bulb during the test. Intestinal I/R was induced as follows: The rats were placed in the supine position and secured in the dissection tray. The abdominal region was shaved and cleaned with antiseptic solutions. The intestinal region was reached by means of midline laparotomy. Superior mesenteric artery was subjected with care and occluded with an atraumatic microvascular clamp, thus intestinal ischemia was created in 1 h ischemia was recognized by the existence of pulseless or pale color of the intestine. The abdominal region was then closed. Following ischemia, the clamp was removed and 1 h reperfusion was induced. The return of the pulses and the reestablishment of the pink color were assumed to be the reperfusion of the intestine. At the end of reperfusion, the pulmonary segment was taken out, and the animals were killed by exsanguination.
Biochemical procedures
At the end of the experiment, the harvested lung tissues samples were quickly washed in cold saline and stored at −70°C.
Measurement of tissue malondialdehyde level
Lung tissue samples were frozen at −70°C and irrigated well with a solution of sodium chloride (NaCl) (0.9%). By admixing it with potassium chloride (KCl) (1.5%), homogenization at a ratio of 1:10 was achieved. The DIAX9000 Homogenizer (Heidolph Instruments, Germany) was used to homogenize the tissue samples. The lipid peroxide level in the centrifuged tissue homogenates was measured according to the method described by Ohkawa et al. (1979). The reaction product was assayed spectrophotometrically (Shimadzu UV-1700, Japan) at 532 nm. The lipid peroxide level was expressed as the nanomole (nmol) of MDA per milligram of lung tissue protein. Protein levels were measured according to the method described by Lowry et al. (1951).
Measurement of tissue superoxide dismutase activity
SOD activity was determined according to the method of Sun et al. (1988). This method is based on the inhibition of nitroblue tetrazolium (NBT) reduction by the XO system as a superoxide generator. Activity was assessed in the ethanol phase of the lysate after 1.0 ml ethanol/chloroform mixture (5/3, v/v) was added to the same volume of sample and centrifuged. One unit of SOD was defined as the enzyme amount causing 50% inhibition in the NBT reduction rate. SOD activity was also expressed as units per milligram protein.
Glutathione peroxidase activity
GSH-Px activity was measured by the method of Paglia and Valentine (1967). The enzymatic reaction was initiated in a tube containing the following items: NADPH, reduced glutathione (GSH), sodium azide, and glutathione reductase by addition of H2O2 and the change in absorbance at 340 nm was monitored by a spectrophotometer. Activity was given in units per gram protein. All samples were assayed in duplicate.
Histological analysis
Portions of right lung (anterior lobe, median lobe, posterior lobe, and post caval lobe) and left lung (upper left lobe, and lower left lobe) were individually immersed in 10% neutral-buffered formalin, dehydrated in alcohol, embedded in paraffin, and then cut into 5 μm thick cross-sections through the middle of the lobe so that each section included hilum to periphery. Sections were placed on slides, deparaffinated, and stained with hematoxylin and eosin (H&E) using standard procedures. Six slides were analyzed in a standardized fashion. Each lung lobe sections were divided equally for histopathological and biochemical investigations. Each slide was examined and evaluated in random order under blindfold conditions for immunostaining by a histologist and stained with H&E for histopathologic assessment under a standard light microscopy. The slides were examined for the presence of peribronchial inflammatory cell infiltration (PICI), alveolar septal infiltration (ASI), alveolar edema (AED), alveolar exudate (AEX), and interstitial fibrosis (IF). These changes were scored according to the fourpoint scale used by Takil et al. (2003) (Table 1). A histopathological assessment was performed in at least randomly selected eight microscopic high-power fields from each lung lobe. The final score determined in each category for each individual animal was the mean of the scores from the sections of the lungs examined.
Immunohistochemistry
Immunohistochemical reactions were performed according to the ABC technique described by Hsu et al. (1981). The procedure involved the following steps: (1) endogenous peroxidase activity was inhibited by 3% H2O2 in distilled water for 30 min, (2) the sections were washed in distilled water for 10 min, (3) non-specific binding of antibodies was blocked by incubation with normal goat serum (DAKO X 0907, Carpinteria, CA) with PBS, diluted 1:4, (4) the sections were incubated with specific rabbit polyclonal anti iNOS antibody (Cat. # RB-1605-P, Neomarkers, USA) and specific rabbit polyclonal SP-D antibody (Cat. # AB3434, Chemicon, USA), diluted 1:50 for 1 h, and then at room temperature, (5) the sections were washed in PBS 3 × 3 min, (6) the sections were incubated with biotinylated anti-mouse IgG (DAKO LSAB 2 Kit), (7) the sections were washed in PBS 3 × 3 min, (8) the sections were incubated with ABC complex (DAKO LSAB 2 Kit), (9) the sections were washed in PBS 3 × 3 min, (10) peroxidase was detected with an aminoethylcarbazole substrate kit (AEC kit; Zymed Laboratories), (11) the sections were washed in tap water for 10 min and then dehydrated, (12) the nuclei were stained with hematoxylin, and (13) the sections were mounted in DAKO paramount.
The positive staining of iNOS and SP-D cell numbers were scored in a semiquantitative manner in order to determine the differences between the control group and the experimental groups in the distribution patterns of intensity of immunolabeling of lung tissue. The numbers of the positive staining were recorded as absent (−), a few (±), few (+), medium (++), high (+++), and very high (++++). This analysis was performed in at least randomly selected eight microscopic high-power fields from each lung lobe section, in two sections from each animal at 400× magnification. The final score determined in each category for each individual animal was the average of the scores from the sections of the lungs examined.
Statistical analysis
All statistical analyses were carried out using SPSS statistical software (SPSS for windows, version 11.0). All data were presented in mean (±) standard deviations (SD). Differences in measured parameters among the three groups were analyzed with a nonparametric test (Kruskal–Wallis). Dual comparisons between groups exhibiting significant values were evaluated with a Mann–Whitney U test. These differences were considered significant when probability was less than 0.05.
Results
Biochemical findings
The values of the tissue MDA levels, SOD and GSH-Px activities, and statistical differences of these measurements are shown in Table 2. Intestinal I/R significantly increased the tissue MDA levels (P < 0.001) and decreased the antioxidant enzyme (SOD P < 0.001, GSH-Px P < 0.01) activities (Table 2). Infliximab treatment significantly (P < 0.01) decreased the elevated tissue MDA levels and increased of reduced SOD (P < 0.01), and GSH-Px (P < 0.05) enzyme activities in lung tissues (Table 2).
Histopathologic findings
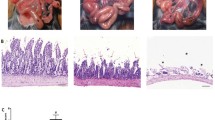
Histopathological examination of H&E stained in lung tissue sections showed that intestinal I/R resulted in the characteristic features of pulmonary injury, whereas rats in the sham group showed normal alveolar architecture (Fig. 1a). In contrast, the lung tissue of the I/R group was obviously damaged with edema, hemorrhage, increased thickness of the alveolar wall and a great number of inflammatory cells that infiltrated the interstitium and alveoli (Fig. 1b). The pathological damage was apparently proved in the infliximab pretreatment group in comparison to the I/R group. The interstitium of the lungs appeared thinner and the number of inflammatory cells apparently reduced (Fig. 1c).
Light microscopy of lung tissues in different groups. H&E: a normal histopathology presented in sham group; b the tissue specimens were performed for the presence of increased thickness of the alveolar wall, peribronchial inflammatory cell infiltration (asteriks), alveolar septal infiltration (thin arrow), hemorrhagic area (arrowhead), alveolar exudate (thick arrow) and oedema (oblique arrow) in I/R group. c Less histopathological parameters were seen in the infliximab treated rats. A Alveol, B Bronchiole. (H&E, scale bar, 100 μm)
Histopathological results of study groups are presented in Table 3. Histopathological parameters including PICI, ASI, AED, AEX and IF were decreased significantly in all treated intestinal I/R with infliximab groups compared to untreated groups (P < 0.001).
Immunohistochemical findings
The number of alveolar cells positive for iNOS was semi-quantitatively higher in intestinal I/R group than sham group (Fig. 2a, b; Table 4). Treatment of infliximab markedly reduced the number of iNOS positive cells (Fig. 2c; Table 4). The number of alveolar type II cells positive for SP-D was lower in I/R group than in sham group as a semi-quantitatively (Fig. 2a, b; Table 4). Infliximab treatment significantly increased the number of SP-D reactivity in alveolar type II cells (Fig. 3c; Table 4).
Immunohistochemical expression of iNOS in lung tissues. a A few immunopositive iNOS cells in sham group; b many iNOS positive cells in I/R group; c decrease iNOS positive cells number in infliximab treated lung tissue. A Alveol, B Bronchiole, arrows positive reactivity (Immunoperoxidase, haematoxylin counterstain, scale bar, 50 μm)
Immunohistochemical expression of SP-D in lung tissues. a Many SP-D immunopositive cells were present in alveolar type II cells of sham group; b few SP-D positive cells in degenerative alveolar type II cells of I/R group; c increase positive cells number of SP-D in alveolar type II cells of infliximab treated lung tissues. A Alveol, B Bronchiole, arrows positive reactivity (Immunoperoxidase, haematoxylin counterstain, scale bar, 50 μm)
Discussion
In this study, we have demonstrated that infliximab treatment improves survival in a rat model of intestinal I/R. We have also identified some of the underlying mechanisms for this protective effect: amelioration of acute lung injury, reduction of MDA level and iNOS expression, and elevation of SOD, GSH-Px activities and SP-D expression in lung tissues. To the best of our knowledge, this is the first study that evaluates the effect of infliximab on survival and acute lung injury after intestinal I/R.
Ischemia and reperfusion may result in not only local, but also distant, organ injury. In particular situations, intestinal ischemia accompanies some surgical procedures, such as thoracoabdominal aortic aneurysm repair, embolectomy for the acute mesenteric arterial occlusion, or small-bowel transplantation. Pulmonary parenchymal damage and respiratory failure as a consequence of I/R-induced remote organ injury may complicate such surgical procedures. This sequence of events has been shown clearly by several experimental studies showing neutrophil accumulation, increased microvascular permeability, perivascular and interstitial edema, pulmonary edema and endothelial cell injury occurring in pulmonary tissue after mesenteric I/R (Koksoy et al. 2000; Frutos-Vivar et al. 2006). In this study, the typical histologic features that occurred in intestinal I/R group were characterized by edema, hemorrhage, increased thickness of the alveolar wall and a great number of inflammatory cells that infiltrated the interstitium and alveoli. Our data are corroborated by previous studies reported by other investigators on intestinal I/R induced acute lung injury damage in animals. However, to date no information about the effects of infliximab on intestinal I/R induced acute lung injury in rats have been reported. Our study showed that infliximab treatment inhibits the inflammatory pulmonary responses reducing significantly peribronchial inflammatory cell infiltration, alveolar septal infiltration, alveolar edema, alveolar exudate, and interstitial fibrosis in intestinal I/R treated rats.
Intestinal I/R injury has been shown not only to cause local damage to the bowel but also to release numerous mediators in the circulation that can cause multiple organ failure including acute lung injury (Hassoun et al. 2001). Among these mediators, ROS play a critical role in the development of acute lung injury. Administration of anti-oxidants has been shown to decrease this injury in various models including hemorrhagic shock, intestine I/R, and sepsis (Fink 2002). Intestinal I/R produces distant organ injury by various mechanisms such as neutrophils, reactive oxygen metabolites, and cytokines. Guzel et al. (2011) showed in a recent study that increased production of ischemia and damage markers such as MDA, an indicator of lipid peroxidation, in the lung tissue, all occurred during I/R injury. By contrast, decreased activity of GSH-Px, and SOD in the lung tissue were observed as a result of I/R injury. During I/R, these endogenous antioxidative defenses are likely to be perturbed as a result of overproduction of oxygen-derived radicals. The pulmonary parenchyma is very vulnerable to free oxygen radicals induced by I/R, which cause oxidative damage to biomembranes, lipids, proteins, and DNA, leading to organ dysfunction and cell death (Shen and Zhang 2003). We found that the MDA content was up-regulated after intestinal I/R injury and remarkably down-regulated after infliximab administration. In addition, another hallmark of intestinal reperfusion injury as determined by SOD and GSH-Px activities were up-regulated after infliximab treatment as well.
The mechanisms of lung injury induced by intestinal I/R are very complex. It is well-established that lipid peroxidation i s one of the major factors causing lung injury (Rossman et al. 1997; Giakoustidis et al. 2006). In addition, evidence showed that overproduction of nitric oxide (NO) generated by inducible nitric oxide synthase (iNOS) not only aggravates oxidative damage (Zhou et al. 2003; Pararajasingam et al. 2000), but leads to pulmonary microvascular dysfunction as well (Turnage et al. 1994). Thus, the therapeutical strategy by removing free radicals and reducing NO overproduction should be potential effective strategies for the protection against lung injury following intestinal I/R. NO derived from iNOS may contribute to intestinal I/R induced acute lung injury. Uchida et al. (2007) reported that inhibition of iNOS ameliorated lung injury after intestinal I/R in rats. Previous studies demonstrated that selective inhibition of iNOS prevented intestinal I/R injury in mice (Barocelli et al. 2006), whereas non-selective inhibition of NOS increased both IL-6 levels in lymph nodes and lung microvascular permeability after intestinal I/R (Breithaupt-Faloppa et al. 2009). The results from the present study were consistent with these previous reports. After intestinal I/R, iNOS expression increased in the lung; infliximab pretreatment downregulated iNOS expression. The reduction of iNOS expression by infliximab makes it a potentially therapeutic anti-inflammatory agent that may protect lung parenchyma in intestinal I/R.
Surfactant protein D (SP-D) is a member of the collectin family of proteins, which play important roles in innate host defense of the lung and regulation of surfactant homeostasis and is synthesized in alveolar type II cells and clara cells of lungs (Leth-Larsen et al. 2003). Alveolar cell damage leads to decreased and impaired synthesis, secretion, function, and composition of SP-D in acute lung injury, (Cheng et al. 2003; Herbein and Wright 2001; Guzel et al. 2008). This study was undertaken to analyze the immunohistochemical expression of SP-D in lung injury due to intestinal I/R. Pursuant to our results, SP-D was strongly expressed in lung injury our non-treated study group. There is a significant reduction in the activity arising in the expression of SP-D in lung tissue of intestinal I/R models with infliximab therapy. Our present study demonstrates that a rise in the expression of SP-D in lung tissue of intestinal I/R treated with infliximab therapy.
In summary, we have revealed for the first time that imfliximab treatment improves survival and attenuates acute lung injury in a rat model of intestinal I/R injury by decreasing pulmonary damage, creating an anti-inflammatory environment and minimizing oxidative damage. We believe that further preclinical research into the utility of infliximab may indicate its usefulness as a potential treatment on acute lung injury after intestinal I/R injury in rats.
References
Barocelli E, Ballabeni V, Ghizzardi P, Cattaruzza F, Bertoni S, Lagrasta CA, Impicciatore M (2006) The selective inhibition of inducible nitric oxide synthase prevents intestinal ischemia-reperfusion injury in mice. Nitric Oxide 14:212–218
Breithaupt-Faloppa AC, Vitoretti LB, Coelho FR, dos Santos Franco AL, Domingos HV, Sudo-Hayashi LS, Oliveira-Filho RM, Tavares de Lima W (2009) Nitric oxide mediates lung vascular permeability and lymph-borne IL-6 after an intestinal ischemic insult. Shock 32:55–61
Casini A, Ceni E, Salzano R, Biondi P, Parola M, Galli A, Foschi M, Caligiuri A, Pinzani M, Surrenti C (1997) Neutrophil-derived superoxide anion induces lipid peroxidation and stimulates collagen synthesis in human hepatic stellate cells: role of nitric oxide. Hepatology 25:361–367
Cheng IW, Ware LB, Greene KE, Nuckton TJ, Eisner MD, Matthay MA (2003) Prognostic value of surfactant proteins A and D in patients with acute lung injury. Crit Care Med 31:20–27
Cury DH, Costa JE, Irika K, Mijji L, Garcez A, Buchiguel C, Silva I, Sipahi A (2008) Protective effect of octreotide and infliximab in an experimental model of indomethacin-induced inflammatory bowel disease. Dig Dis Sci 53:2516–2520
Deitch EA (2001) Role of the gut in multiple system organ failure. Curr Opin Crit Care 7:92–98
Di Sabatino A, Ciccocioppo R, Benazzato L, Sturniolo GC, Corazza GR (2004) Infliximab downregulates basic fibroblast growth factor and vascular endothelial growth factor in Crohn’s disease patients. Aliment Pharmacol Ther 19:1019–1024
Fink MP (2002) Reactive oxygen species as mediators of organ dysfunction caused by sepsis, acute respiratory distress syndrome, or hemorrhagic shock: potential benefits of resuscitation with Ringer’s ethyl pyruvate solution. Curr Opin Clin Nutr Metab Care 5:167–174
Frutos-Vivar F, Ferguson ND, Esteban A (2006) Epidemiology of acute lung injury and acute respiratory distress syndrome. Semin Respir Crit Care Med 27:327
Galili Y, Ben-Abraham R, Weinbroum A, Klausner J, Rabau M, Kluger Y (1998) Methylene blue prevents pulmonary injury following intestinal ischemia reperfusion. J Trauma 45:222–226
Giakoustidis AE, Giakoustidis DE, Iliadis S, Papageorgiou G, Koliakou K, Kontos N, Taitzoglou I, Botsoglou E, Papanikolaou V, Atmatzidis K, Takoudas D, Antoniadis A (2006) Attenuation of intestinal ischemia/reperfusion induced liver and lung injury by intraperitoneal administration of (-)-epigallocatechin-3-gallate. Free Radic Res 40:103–110
Guzel A, Basaran U, Aksu B, Kanter M, Yalcin O, Aktas C, Guzel A, Karasalihoglu S (2008) Protective effects of S-methylisothiourea sulfate on different aspiration materials-induced lung injury in rats. Int J Pediatr Otorhinolaryngol 72(8):1241–1250
Guzel A, Kanter M, Guzel A, Yucel AF, Erboga M (2011) Protective effect of curcumin on acute lung injury induced by intestinal ıschemia/reperfusion. Toxicol Ind Health. doi:10.1177/0748233711430984 [Epub ahead of print]
Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA (2001) Postinjury multiple organ failure: the role of the gut. Shock 15:1–10
Herbein JF, Wright JR (2001) Enhanced clearance of surfactant protein D during LPS-induced acute inflammation in rat lung. Am J Physiol Lung Cell Mol Physiol 281:268–277
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Ito K, Ozasa H, Kojima N, Miura M, Iwai T, Senoo H, Horikawa S (2003) Pharmacological preconditioning protects lung injury induced by intestinal ischemia/reperfusion in rat. Shock 19:462–468
Koksoy C, Kuzu MA, Ergun H, Demirpençe E, Zülfikaroglu B (2000) Intestinal ischaemia and reperfusion impairs vasomotor functions of pulmonary vascular bed. Ann Surg 231:105–111
Kumamoto Y, Suematsu M, Shimazu M, Kato Y, Sano T, Makino N, Hirano KI, Naito M, Wakabayashi G, Ishimura Y, Kitajima M (1999) Kupffer cell-independent acute hepatocellular oxidative stress and decreased bile formation in post-cold-ischemic rat liver. Hepatology 30:1454–1463
Leth-Larsen R, Nordenback C, Tornoe I, Moeller V, Schlosser A, Koch C, Teisner B, Junker P, Holmskov U (2003) Surfactant protein D (SP-D) serum levels in patients with community-acquired pneumonia. Clin Immunol 108:29–37
Lowry OH, Rosenbrough NJ, Farr AC, Randall RJ (1951) Protein measurement with the folin phenol regent. J Biol Chem 193:265–275
Mayer AM, Spitzer JA (1993) Modulation of superoxide anion generation by manoalide, arachidonic acid and staurosporine in liver infiltrated neutrophils in a rat model of endotoxemia. J Pharmacol Exp Ther 267:400–409
McCord JM (1985) Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312:159–163
Mitsuoka H, Kistler EB, Schmid-Schonbein GW (2002) Protease inhibition in the intestinal lumen: attenuation of systemic inflammation and early indicators of multiple organ failure in shock. Shock 17:205–209
Ohkawa H, Oshishi N, Yagi K (1979) Assay of lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem 95:351–358
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterisation of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Pararajasingam R, Weight SC, Bell PR, Nicholson ML, Sayers RD (2000) Pulmonary nitric oxide metabolism following infrarenal aortic cross-clamp-induced ischaemia-reperfusion injury. Eur J Vasc Endovasc Surg 19:41–47
Parks DA, Bulkley GB, Granger DN (1983) Role of oxygen-derived free radicals in digestive tract diseases. Surgery 94:415–422
Pascher A, Klupp J, Langrehr JM, Neuhaus P (2005) Anti-TNF-alpha therapy for acute rejection in intestinal transplantation. Transplant Proc 37:1635–1636
Pergel A, Kanter M, Yucel AF, Aydin I, Erboga M, Guzel A (2011) Anti-inflammatory and anti-oxidant effects of infliximab in a rat model of intestinal ischemia/reperfusion injury. Toxicol Ind Health. doi:10.1177/0748233711427056 [Epub ahead of print]
Ravanat JL, Di Mascio P, Martinez GR, Medeiros MH, Cadet J (2000) Singlet oxygen induces oxidation of cellular DNA. J Biol Chem 275:40601–40604
Rossman JE, Caty MG, Zheng S, Karamanoukian HL, Thusu K, Azizkhan RG, Dandona P (1997) Mucosal protection from intestinal ischemia-reperfusion reduces oxidant injury to the lung. J Surg Res 73:41–46
Sartani G, Silver PB, Rizzo LV, Chan CC, Wiggert B, Mastorakos G, Caspi RR (1996) Anti-tumor necrosis factor alpha therapy suppresses the induction of experimental autoimmune uveoretinitis in mice by inhibiting antigen priming. Invest Ophthalmol Vis Sci 37:2211–2218
Schoenberg MH, Beger HG (1993) Reperfusion injury after intestinal ischemia. Crit Care Med 21:1376–1386
Shen L, Zhang J (2003) Ginsenoside Rg1 increases ischemia-induced cell proliferation and survival in the dentate gyrus of adult gerbils. Neurosci Lett 344:1–4
Spitzer JA, Mayer AM (1993) Hepatic neutrophil influx: eicosanoid and superoxide formation in endotoxemia. J Surg Res 55:60–67
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Takil A, Umuroglu T, Gogus YF, Eti Z, Yildizeli B, Ahiskali R (2003) Histopathologic effects of lipid content of enteral solutions after pulmonary aspiration in rats. Nutrition 19:666–669
Terada LS, Mahr NN, Jacobson ED (1996) Nitric oxide decreases lung injury after intestinal ischemia. J Appl Physiol 81:2456–2460
Turnage RH, Guice KS, Oldham KT (1994) Pulmonary microvascular injury following intestinal reperfusion. New Horiz 2:463–475
Turnage RH, Kadesky KM, Bartula L, Myers SI (1995) Intestinal reperfusion up-regulates inducible nitric oxide synthase activity within the lung. Surgery 118:288–293
Uchida K, Mishima S, Ohta S, Yukioka T (2007) Inhibition of inducible nitric oxide synthase ameliorates lung injury in rats after gut ischemia-reperfusion. J Trauma 63:603–607
Yucel AF, Kanter M, Pergel A, Erboga M, Guzel A (2011) The role of curcumin on intestinal oxidative stress, cell proliferation and apoptosis after ischemia/reperfusion injury in rats. J Mol Histol 42(6):579–587
Zhou JL, Jin GH, Yi YL, Zhang JL, Huang XL (2003) Role of nitric oxide and peroxynitrite anion in lung injury induced by intestinal ischemia-reperfusion in rats. World J Gastroenterol 9:1318–1322
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guzel, A., Kanter, M., Guzel, A. et al. Anti-inflammatory and antioxidant effects of infliximab on acute lung injury in a rat model of intestinal ischemia/reperfusion. J Mol Hist 43, 361–369 (2012). https://doi.org/10.1007/s10735-012-9396-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-012-9396-0