Abstract
Indomethacin administration in animals increases permeability of the small intestine, leading to inflammation that mimics Crohn’s disease. Nonsteroidal anti-inflammatory drugs increase the permeability of the intestinal epithelial barrier and should therefore be used with caution in patients with Crohn’s disease. We analyzed the protective effects of octreotide and the tumor necrosis factor-alpha inhibitor infliximab in a rat model of indomethacin-induced enterocolitis. Male Wistar rats received 20 mg of infliximab or 10 μg of octreotide 24 h prior to injection with indomethacin. Intestinal permeability was analyzed using Cr-51-ethylenediaminetetraacetic acid clearance. No microscopic or macroscopic alterations were observed in the rats receiving infliximab or octreotide, both of which increased permeability (P < 0.001 versus controls). Our macroscopic and microscopic findings might be related to the low specificity of infliximab and suggest that cytokines affect the intestinal epithelial barrier, as evidenced by the protective effect that infliximab had on the permeability parameters evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In recent years, experimental models have been widely used for the study of inflammatory bowel diseases, contributing greatly to the understanding of such diseases and to the delineation of new therapeutic strategies [1, 2].
Nevertheless, no fully satisfactory model capable of simulating inflammatory bowel diseases as they occur in humans has been developed [1].
Since it can produce lesions similar to those caused by Crohn’s disease in humans, indomethacin is the drug most frequently used in experiments designed to study that disease [3].
One of the factors recently implicated in the pathogenesis of Crohn’s disease is increased intestinal permeability [4].
In animals, COX-1 inhibitors such as indomethacin can increase intestinal permeability [5].
The mechanism by which these anti-inflammatory drugs induce intestinal enteropathy is little understood and quite complex. The first type of enteropathy is characterized by biochemical damage occurring in the mitochondria and is known as decoupling oxidative phosphorylation, which leads to increased intestinal permeability. The second type is characterized by the inhibition of COX-1 and COX-2 [6, 7]. Recent studies have shown that the toxic effect of nonsteroidal anti-inflammatory drugs might lead to the release of cytokines such as tumor necrosis factor-alpha (TNF-α), due to the rupture of the intestinal epithelial barrier [8]. Once the functional epithelial barrier is ruptured, lamina propria cells become exposed to the effects of bacteria residing in the lumen and in biliary salts, leading to increased production of cytokines, including TNF-α [9, 10].
Another chemical mediator is octreotide, a potent immunomodulator that has been shown to be a factor in the pathogenesis of this complex disease. In addition, in vitro studies have revealed its capacity to decrease the activity of intestinal lymphocytes and monocytes in the peripheral blood [11].
In experimental models, somatostatin analogs have been shown to reduce damage to the intestinal mucosa [7, 12].
Due to the great importance recently given to the intestinal epithelial barrier in the pathogenic study of Crohn’s disease, various methods of assessing intestinal permeability have been employed, among them the chromium-51-ethylenediaminetetraacetic acid clearance test (Cr-51-EDTA test). The objective of this study was to use the Cr-51-EDTA test, in conjunction with various other tests, to determine whether infliximab and octreotide protect against the intestinal inflammation induced by indomethacin.
Methods
Male Wistar rats, weighing 300–350 mg, were obtained from the animal care facilities of the University of São Paulo. Indomethacin was purchased from Merck, Sharp & Dohme (Rahway, NJ, USA), octreotide (Sandostatin) from Novartis (Basel, Switzerland) and the anti-tumor necrosis factor-alpha (TNF-α) antibody infliximab from Schering Plough (Kenilworth, NJ, USA).
Study Design
Control Group
In the control group animals, enterocolitis was induced through subcutaneous injection of 7.5 mg/kg/day of indomethacin for two consecutive days, in accordance with the Bernardes and Silva model [13, 14].
Vehicle-Only Group
A second group of animals received only the vehicle (NaHCO3), which was administered following the indomethacin model, the objective being to create the same level of stress as caused by the injection of the indomethacin.
Infliximab Group
In the infliximab group animals, infliximab was administered intravenously in a single dose (20 mg/kg). Beginning 24 h after the administration of the infliximab, indomethacin (7.5 mg/kg/day) was given for two consecutive days in order to induce enterocolitis.
Octreotide Group
Each animal in the octreotide group received subcutaneous injections of octreotide (10 μg/8 h) for 24 h (total 30 μg), after which the indomethacin injections were given as described for the infliximab group.
Experimental Procedures
Four days after all of the injections had been given, the animals were submitted to intestinal permeability studies and were subsequently sacrificed.
Intestinal Permeability
The animals received Cr-51-EDTA by gavage, and 5-h urine samples were collected. The samples were subsequently sent to the Nuclear Medicine Department of the University of São Paulo for analysis of permeability, according to the Bernardes and Silva model [13, 14].
Macroscopy
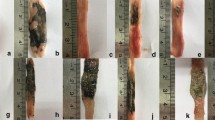
The macroscopic analysis was conducted by opening the abdominal cavity and removing the entire small intestine, which was then opened longitudinally along the mesenteric border. The fragments were placed on individual trays, washed with saline solution, and stained with Evans blue. As shown in Table 1, the samples were scored using the Kucharzik system [13].
Microscopy
For the microscopic analysis, 5 cm of small intestine located 20 cm from the ileocecal valve was removed. The samples were placed in formalin (4%) and stained with hematoxylin and eosin. The slides were read by two different pathologists, who scored them according to the system shown in Table 2.
Statistical Analysis
The analyzed variables were initially checked for Gaussian distribution (normal curve) using the Kolmogorov-Smirnov (K-S distance) and Shapiro-Wilk tests, then classified as parametric or nonparametric. In order to compare the different groups (permeability analysis), the parametric variables were described using analysis of variance for nonrepeated measures with the Student-Newman-Keuls post-test (analysis conditioned using Bartlett’s test) and are expressed as mean ± standard deviation of the sample. Nonparametric variables were described using the Kruskal-Wallis test with the Student-Newman-Keuls post-test and are expressed as medians and interquartile ranges for the comparison between the different groups.
The level of statistical significance was set at an alpha risk of 5% (P < 0.05).
Ethics
The study design was approved by the Ethics Committee for the Analysis of Research Projects, operating under the auspices of the Clinical Board of Directors of the University of São Paulo School of Medicine Hospital das Clínicas (protocol no. 943/01).
Results
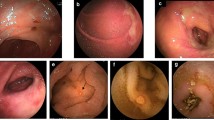
The administration of indomethacin at a dose of 7.5 mg/day for two consecutive days was sufficient to cause intestinal lesions, whereas the administration of vehicle only (control group) caused no such lesions. Administration of indomethacin produced multiple ulcers in the small intestine (Tables 1, 2, and 3).
Macroscopically, the lesions were principally located along the mesenteric border and appeared to be confluent.
Microscopically, the findings ranged from normal mucosa to deep ulcers (Table 1).
Infliximab and octreotide were found to have a clear protective effect, as evidenced by the significantly lower intestinal permeability seen in the infliximab group and octreotide group (P < 0.01 for both; Table 3).
Although the macroscopic and microscopic scores were also lower in the infliximab and octreotide groups than in the control (indomethacin-only) group, the differences were not statistically significant.
Discussion
Although the mechanism by which the administration of indomethacin induces intestinal inflammation remains unclear, previous studies have implicated certain factors, such as diet, local susceptibility, and the toxicity of the medication, all of which are essential for the functional breach of the intestinal barrier [9].
The loss of barrier integrity leads to pronounced efflux of neutrophils from the site of inflammation, as well as to greater exposure to the bacteria residing in the lumen. Therefore, endotoxins or superantigens would produce the initial stimulus for the overproduction of TNF-α [15].
The intestinal epithelial barrier has been described as the pathogenic basis of Crohn’s disease. Defects in the tight junctions, as well as a loss of barrier integrity, lead to increased permeability [16].
When the integrity of the barrier is breached, there is an increase in the synthesis of cytokines, including TNF-α [17].
In the past, TNF-α was implicated only as a trigger of the inflammatory cascade. However, the role it also plays in permeability has recently been investigated, as has its capacity to restore the barrier [10].
It has also been shown that octreotide, a potent chemical mediator, probably plays a role in the pathogenesis of inflammatory bowel disease due to the anti-inflammatory effect that it has on T-cells, together with its ability to decrease the secretion of jejunal/ileal fluids and stimulate greater epithelial cell absorption, thereby regulating the electrolyte balance of the jejunal and ileal fluids [18].
Due to the fact that these agents (octreotide and infliximab) act during different stages of inflammation, our objective was to evaluate their effect on the intestinal epithelial barrier as well to assess on macroscopic and microscopic damage.
In the present study, octreotide proved to be protective against indomethacin-induced inflammatory lesion, as evidenced by the decreased intestinal permeability in the octreotide group (Table 3). However, no significant change was observed in the macroscopic or microscopic parameters (Tables 1 and 2). Nevertheless, in a study involving the prelesion administration of octreotide, Lamrani et al. showed that this mediator was capable of reducing microscopic and macroscopic scores [19]. That finding was probably due to the fact that those authors employed an experimental model in which the response was exclusively of the Th1 type. Reinforcing that possibility is the lack of success achieved by Bergeijk et al., who administered octreotide in humans with retrocolitis, which presents a Th2-type response [11].
Perhaps the model used in the present study did not favor the full activation of octreotide, in which case the indomethacin model might not represent a pure model of the Th1-type response. However, the octreotide-induced reduction in intestinal permeability might be attributable to the fact that octreotide reduces the expression of TNF-α, as has been shown in previous studies [19].
In the present study, prelesion administration of the anti-TNF-α antibody infliximab was also found to reduce intestinal permeability (P < 0.001; Table 1). This suggests that infliximab has an effect on the tight junction, where it likely reduces the expression of TNF-α. This was previously suggested by Suenaert et al. [20], who administered infliximab to patients with Crohn’s disease and observed significant improvement in the intestinal permeability, leading the authors to hypothesize that TNF-α actively participates in the maintenance and repair of the intestinal epithelial barrier [10]. In another study, the same authors analyzed indomethacin-induced hyperresponsiveness of the mucosal barrier in humans. They observed that the administration of infliximab in patients with Crohn’s disease resulted in repair of the barrier and reduced inflammation .
The fact that the mucosal barrier hyperresponsiveness persists might explain the fact that our macroscopic and microscopic findings revealed only differences that were less than significant. This might indicate that infliximab is active at an earlier phase (permeability). However, since the mucosal barrier hyperresponsiveness persists, the tissue damage proceeds, and the time allowed (24 h) for infliximab to act prior to the lesion might not be ideal for the drug to perform fully in terms of protection or repair. Although the administration of infliximab resulted in a reduction in the macroscopic and microscopic aspects of the inflammation, the differences were not statistically significant.
Colpaert et al. studied the participation of T cells in the indomethacin model and observed that the damage resulting from the use of nonsteroidal anti-inflammatory drugs was caused principally by bacterial agents, which could explain the partial activation of anti-TNF-α in the experiment in question [21].
Another issue worthy of mention is that the anti-TNF-α used in the present study is a monoclonal chimeric antibody designed for use in humans and might present lower specificity than that of the specific polyclonal antibody when used in rats.
There is, to date, no fully satisfactory experimental model. However, in the present study, indomethacin proved capable of producing lesions and breaching the intestinal epithelial barrier.
The reduction in permeability resulting from the administration of infliximab indicates that TNF-α has an effect on the intestinal epithelial barrier and that octreotide has an effect on TNF-α.
There were no significant differences between the study and control groups in terms of the macroscopic and microscopic scores, which indicates either that the type of response evoked by indomethacin is in question or that the anti-TNF-α antibody employed in this experimental model presents low specificity.
References
Elson CO, Sartor RB, Tennyson GS, Riddell RH (1995) Experimental models of inflammatory bowel disease. Gastroenterology 109(4):1344–1367
Strober W (1985) Animal models of inflammatory bowel disease—an overview. Dig Dis Sci 30(12 Suppl):3S–10S
Kent TH, Cardelli RM, Stamler FW (1969) Small intestinal ulcers and intestinal flora in rats given indomethacin. Am J Pathol 54(2):237–249
Peeters M, Geypens B, Claus D, Nevens H, Ghoos Y, Verbeke G et al (1997) Clustering of increased small intestinal permeability in families with Crohn’s disease. Gastroenterology 113(3):802–807
Ford J, Martin SW, Houston JB (1995) Assessment of intestinal permeability changes induced by nonsteroidal anti-inflammatory drugs in the rat. J Pharmacol Toxicol Methods 34(1):9–16
Mahmud T, Somasundaram S, Sigthorsson G, Simpson RJ, Rafi S, Foster R et al (1998) Enantiomers of flurbiprofen can distinguish key pathophysiological steps of NSAID enteropathy in the rat. Gut 43(6):775–782
Bjarnason I, Zanelli G, Prouse P, Smethurst P, Smith T, Levi S et al (1987) Blood and protein loss via small-intestinal inflammation induced by non-steroidal anti-inflammatory drugs. Lancet 2(8561):711–714
Sartor RB (1994) Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology 106(2):533–539
Bertrand V, Guimbaud R, Tulliez M, Mauprivez C, Sogni P, Couturier D et al (1998) Increase in tumor necrosis factor-alpha production linked to the toxicity of indomethacin for the rat small intestine. Br J Pharmacol 124(7):1385–1394
Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, Van Assche G et al (2002) Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am J Gastroenterol 97(8):2000–2004
van Bergeijk JD, Wilson JH, Nielsen OH, von Tirpitz C, Karvonen AL, Lygren I et al (2002) Octreotide in patients with active ulcerative colitis treated with high dose corticosteroids (OPUS 1). Eur J Gastroenterol Hepatol 14(3):243–248
Eliakim R, Karmeli F, Okon E, Rachmilewitz D (1993) Octreotide effectively decreases mucosal damage in experimental colitis. Gut 34(2):264–269
Kucharzik T, Lugering A, Lugering N, Rautenberg K, Linnepe M, Cichon C et al (2000) Characterization of M cell development during indomethacin-induced ileitis in rats. Aliment Pharmacol Ther 14(2):247–256
Bernardes-Silva CF, Damiao AO, Sipahi AM, Laurindo FR, Iriya K, Lopasso FP et al (2004) Ursodeoxycholic acid ameliorates experimental ileitis counteracting intestinal barrier dysfunction and oxidative stress. Dig Dis Sci 49(10):1569–1574
Goncalves J, Queiroz G (1996) Purinoceptor modulation of noradrenaline release in rat tail artery: tonic modulation mediated by inhibitory P2Y- and facilitatory A2A-purinoceptors. Br J Pharmacol 117(1):156–160
Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H (1993) Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet 341(8858):1437–1439
Garcia-Lafuente A, Antolin M, Guarner F, Crespo E, Malagelada JR (2001) Modulation of colonic barrier function by the composition of the commensal flora in the rat. Gut 48(4):503–507
Hogenauer C, Aichbichler B, Santa Ana C, Porter J, Fordtran J (2002) Effect of octreotide on fluid absorption and secretion by the normal human jejunum and ileum in vivo. Aliment Pharmacol Ther 16(4):769–777
Lamrani A, Tulliez M, Chauvelot-Moachon L, Chaussade S, Mauprivez C, Hagnere AM et al (1999) Effects of octreotide treatment on early TNF-alpha production and localization in experimental chronic colitis. Aliment Pharmacol Ther 13(5):583–594
Suenaert P, Bulteel V, Vermeire S, Noman M, Van Assche G, Rutgeerts P (2005) Hyperresponsiveness of the mucosal barrier in Crohn’s disease is not tumor necrosis factor-dependent. Inflamm Bowel Dis 11(7):667–673
Colpaert S, Liu Z, De Greef B, Rutgeerts P, Ceuppens JL, Geboes K (2001) Effects of anti-tumour necrosis factor, interleukin-10 and antibiotic therapy in the indometacin-induced bowel inflammation rat model. Aliment Pharmacol Ther 15(11):1827–1836
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cury, D.H.B., Costa, J.E., Irika, K. et al. Protective Effect of Octreotide and Infliximab in an Experimental Model of Indomethacin-Induced Inflammatory Bowel Disease. Dig Dis Sci 53, 2516–2520 (2008). https://doi.org/10.1007/s10620-007-0172-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-007-0172-z