Abstract
Purpose
Osteosarcoma is the most common primary bone cancer in children and young adults. Recent experimental evidence has indicated that Runx2/OPN axis play important roles in the metastasis of osteosarcoma cells. The present study aimed to explore their relationship and prognostic significance in surgically resected osteosarcoma.
Methods
The expression of runt‐related transcription factor2(Runx2) and osteopontin (OPN) in clinical specimens from 105 osteosarcoma patients were detected by immunohistochemistry. The correlations between Runx2, OPN, and clinicopathologic data were analyzed by Chi-square (χ2) tests. The prognostic values were determined by univariate and multivariate survival analysis. The accuracy of oncologic outcome prediction was evaluated by receiver-operating characteristics curves.
Results
The results showed there is a significant positive correlation between Runx2 and OPN expression at protein levels (P = 0.015). Runx2 and OPN were both independent predictors for overall survival and metastasis-free survival. When Runx2 and OPN were taken into consideration together, the predictive range was extended and the sensitivity was improved, and more significant and better biomarkers for osteosarcoma metastasis and survival.
Conclusions
These results suggest that a combined Runx2/OPN expression could be a valuable independent predictor of tumor metastasis and survival in osteosarcoma patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteosarcoma is the most frequent primary bone malignancy in children, adolescents and young adult, with a high propensity to metastasize to the lungs [1]. Despite recent advances in multimodality treatments consisting of chemotherapy and surgical, the overall survival rate since the 1970s has remained unchanged and only approximately 60% [2]. In addition, the prognosis for patients with metastatic osteosarcoma remains dismal with a 5-year survival rate at a range from 10 to 20% [3]. Consequently, it is important to accurately predict the prognosis of osteosarcoma patients to improve their survival rates.
Runt‐related transcription factor 2 (Runx2) is best known as an inducer and regulator of osteoblast differentiation and bone formation [4]. Runx2 is also considered to be a cancer-related transcription factor that promotes the adhesion of endothelial cells and cancer metastasis [5]. Growing evidence have suggested that Runx2 play a pivotal role in metastasis of various malignancies [6,7,8]. Studies of osteosarcoma have revealed that abnormal expression of Runx2 is a key pathological factor in osteosarcoma oncogenesis [9,10,11].
The Runx2 target gene osteopontin (OPN) produced by osteoblasts encodes an extracellular matrix protein that is associated with a poor prognosis, metastasis and therapy failure [12]. OPN can be used as a marker of malignancy in different tumor types including lung cancer, breast cancer, gastrointestinal cancer, hepatocellular cancer and melanoma [13]. Moreover, increased expression of OPN has been shown to contribute to osteosarcoma progression and metastasis in osteosarcoma cell lines [14].
A recent experimental study indicated that Runx2/OPN axis promotes osteosarcoma cells adhesion to pulmonary endothelial cells as a key step of osteosarcoma cells metastasis to the lung [5]. However, study on the correlation between Runx2 and OPN expression and their co-expression with clinical parameters in osteosarcoma still remain unclear. In this study, we evaluated the prognostic significance of Runx2 and OPN expression in osteosarcoma patients following curative resection.
Materials and methods
Patients and samples
Clinical specimens were obtained with primary osteosarcoma undergoing curative resection between 2001 and 2017 at the Second Hospital of Tangshan, China. Only samples from patients without prior treatment, absence of metastasis at diagnosis and the availability of complete clinicopathologic and follow-up data were included in the study(n = 105). All tumors were histologically classified and graded by two pathologists.
All patients underwent neoadjuvant chemotherapy, surgery and postoperative chemotherapy. The chemotherapy protocol was the modified T10, including high-dose methotrexate, adriamycin and cisplatin in each cycle of chemotherapy [15]. Postoperative pathological necrosis of 90% or more indicates good histological response, and necrosis of less than 90% indicates poor response [16]. After the operation, the surgeon and pathologist reviewed the gross specimens and determined the surgical margin according to the indications described by Enneking et al. [17].
Immunohistochemistry
The immunohistochemical examination of Runx2 and OPN were performed with rabbit monoclonal anti-Runx2 antibody (Abcam, clone ab92336, UK) at 1:150 dilution and rabbit monoclonal anti-OPN antibody (Abcam, clone ab218237, UK) at 1:100 dilution. Immunohistochemistry was performed using a two‐step immunoperoxidase technique. Briefly, after deparaffinization, rehydration, and heat-induced antigen retrieval, tissues were incubated with primary antibodies overnight at 4 °C. After incubation for 30 min with secondary antibody, the sections were developed in diaminobenzidine solution under microscopic observation and counterstained with hematoxylin. Negative control slides with the primary antibodies omitted were included in all assays.
Evaluation of immunostaining
Immunohistochemical staining was evaluated by two independent pathologists who were blinded to the patient characteristics, discrepancies were resolved by consensus. Four different areas were randomly selected from each sample were systematically evaluated under a microscope at at × 400 magnification. The staining was determined semi-quantitatively according to the intensity (0, negative; 1, weak; 2, intermediate; 3, strong) and the ratio of positive cells (0: none or < 5%; 1: 5% to 20%; 2: 21% to 40%; 3: > 40%). Scores of 0 to 2 were regarded as negative and scores of 3–6 as positive [18].
Statistical analysis
Overall survival (OS) was defined as the interval between the date of surgery and death or the last follow-up. For the metastasis free survival (MFS) analysis, the duration was measured from resection until the occurrence of metastasis or the last follow-up assessment. The correlations between Runx2, OPN and clinicopathologic data were analyzed by Chi-square (χ2) tests or Fisher’s exact test. OS and MFS were analyzed using the univariate Kaplan–Meier method and multivariate Cox proportional hazard model analysis. A two-sided P value < 0.05 was considered statistically significant. All statistical analyses were carried out using SPSS, version 18.0 (Chicago, USA) statistical programs.
Results
Patient characteristics
A retrospective series of 105 osteosarcoma patients, who were a median age of 19 years (range 9–71 years), was retrieved from the original files of the Second Hospital of Tangshan, China. Follow-up data were obtained by a combination of hospital records, outpatient visits, and telephone calls. The clinical and histopathologic details of the 105 cases are shown in Tables 1 and 2.
Clinicopathologic correlation of Runx2 and OPN expression in osteosarcoma
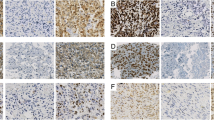
Runx2 expression was observed at the nuclear and OPN was mainly stained in cytoplasm of the tumor cells, especially in areas of high vascular density (Fig. 1). The positive expression rate of Runx2 and OPN was 52.4% and 50.5%, respectively. Interestingly, there was a significant correlation between the expression of Runx2 and OPN (P = 0.015) in these osteosarcoma tissues (Table 3).
Representative Runx2 staining samples at magnification of 200 at levels of negative (A), weak (B), intermediate (C), strong intensity (D) and OPN staining negative (E), weak (F), intermediate (G), strong intensity (H). Runx2 runt‐related transcription factor 2, OPN osteopontin, − negative, + positive
To clarify the biologic significance, we investigated the correlation between clinicopathological features and Runx2/ OPN expression. As shown in Table 1, Runx2 and OPN expression were not associated with age, gender, tumor location, histological classification or surgical margin by Chi-square test analyses. However, more importantly, significant difference was observed between the expression of Runx2, OPN and lung metastasis (P < 0.001). Moreover, Runx2 expression was correlated with tumor size and histological response (P = 0.027 and P = 0.012, respectively).
Prognostic effect of Runx2 and OPN expression in osteosarcoma
The 3-year OS and MFS rates were 68.6 and 60.2% for the entire study population, respectively. The 3-year OS and MFS rates in the Runx2 + group were significantly lower than those in the Runx2- group (54.5 vs 84.0%, P < 0.001; 40.4 vs 81.8%, P < 0.001; Fig. 2a, b). Interestingly, the OPN + group had significantly lower 3-year OS rate (56.5 vs 80.5%, p < 0.001; Fig. 2c) and MFS rate (42.0 vs 78.5%, p < 0.001; Fig. 2d) than those of the OPN-group.
Prognostic significance of assessed using Kaplan–Meier survival estimates and log-rank tests. Comparisons of OS and MFS by Runx2 (a, b), OPN (c, d), combined Runx2 and OPN (e, f). OS overall survival, MFS metastasis-free survival, Runx2 runt‐related transcription factor 2, OPN osteopontin, − negative, + positive
When further stratification was conducted, 31(29.5%) cases Runx2 and OPN were both negative, 74(70.5%) cases either Runx2 or OPN positive, of which 34 (32.4%) cases were Runx2 + and OPN + simultaneously. The 3-year OS (90.2%) and MFS (86.5%) rates in double negative group for Runx2 and OPN was the best (P < 0.001, Fig. 2e, f). The prognosis of Runx2 + OPN- or Runx2- OPN + group dropped dramatically with 3-year OS and MFS rates at 68.3 and 61.7%, yet 3-year OS (48.5%) and MFS (22.1%) rates in the group Runx2 + and OPN + were further decreased, indicating that Runx2 overexpression and OPN may play an additive role in prognosis.
As analyzed by univariate analysis indicated that lung metastasis, the expression of Runx2, OPN, or both were significantly associated with OS and MFS (P < 0.001, Table 2). Cox proportional hazard model multivariate analysis demonstrated that co-expression of Runx2 and OPN (OS, HR 4.332, 95% CI 1.582–11.861; MFS, HR 3.314, 95% CI 1.272–8.636; P < 0.001; Table 4) remained the most significant and independent prognostic factors for survival, independent of metastasis.
ROC curve of Runx2 and OPN in predicting tumor metastasis
To evaluate the predictive ability of Runx2 and OPN expression for tumor metastasis, we calculated the area under the Receiver Operating Characteristic curve (ROC). The positive rates of Runx2 and OPN were significantly higher in the lung metastasis group than in the non-metastasis group (both P < 0.001, Table1). Both Runx2 and OPN could be potential biomarkers for predicting lung metastasis of osteosarcoma, with an area of 0.726 and 0.722 under the ROC curve, respectively. Furthermore, Runx2 combined with OPN ROC analyses revealed an area under the curve(AUC) of 0.805, indicating that the predictive value of the two genes had additive effect (Table 5).
Discussion
Metastasis is still the main reason of the cancer-related death in osteosarcoma patients with a 5-year survival rate at only 10 to 20% [3], despite improvements in surveillance and clinical treatment strategies. Therefore, it is highly desirable to identify patients with a high probability of tumor metastasis by reliable novel targets and to provide timely personalized therapy.
Runx2 is considered to be a crucial factor affecting osteoblast differentiation, maturation and bone development [4], which is supported by expression and secretion of several bone extracellular matrix proteins including OPN [19]. When the p53-Mdm2 pathway is genetically disrupted, Runx2 dependent osteoblastic differentiation is inhibited, and loss of p53 function increases Runx2 differentiation-related accumulation [20]. Accumulating evidence suggests that Runx2 is associated with tumor formation, invasion, metastasis, and poor prognosis in multiple malignancies including osteosarcoma [21]. Runx2 gene amplification and overexpression at the protein level to be common in osteosarcoma tissues [9, 22]. Its protein elevation is likely driven by gene amplification at the DNA level, so it may be an early event in osteosarcoma pathogenesis [22]. Furthermore, Runx2 overexpression was significantly related to metastasis and indicated a trend of worse survival in osteosarcoma patients [23]. The above studies suggest that Runx2 expression is a key pathological factor in osteosarcoma oncogenesis.
OPN was first identified as a marker of transformation of epithelial cells [24], and subsequently found to be frequently overexpressed in many malignant tumors that is associated with progression, metastasis, treatment response and a poor prognosis [12], suggesting OPN may have marked clinical value in the treatment of cancer. The decreased expression of OPN in osteosarcoma cells indicated that the majority of cells were unable to undergo terminal osteogenic differentiation, thus promoting the growth of osteosarcoma [25]. Furthermore, it has been reported that elevated OPN levels in tumor or stromal cells could improve the metastatic ability of osteosarcoma [26]. Recently, Runx2/OPN axis has been shown to contribute to osteosarcoma lung metastasis by promoting cell adhesion to pulmonary endothelial cells [5]. However, in human osteosarcoma, the relationship between the co-expression of Runx2 and OPN and clinicopathological features are still remain unknown.
It is the important finding that the expression of Runx2 and OPN positively correlated with each other and they may have a collaborative effect in predicting the prognosis of osteosarcoma patients. Runx2 combined with OPN, as a prognostic factor, can help to make a new stratification, including Runx2 + /OPN + (31.4%), Runx2-, OPN + /Runx2 + , OPN- (39.0%), and Runx2-/OPN- (29.5%) groups.
In this study, both Runx2 and OPN expression were found to be associated with lung metastasis, and either Runx2 or OPN could be potential prognostic biomarker for predicting osteosarcoma metastasis, with an area of 0.726 and 0.722 under the ROC curve, respectively. More interestingly, Runx2 combined with OPN ROC analyses revealed an AUC of 0.805, indicating that the predictive value of these two genes has an additive effect. In addition, both Runx2 and OPN have been demonstrated to be independent prognostic factors for OS and MFS by multivariate Cox proportional hazard model analysis in osteosarcoma patients. Patients with both Runx2 + and OPN + would be more prone to lung metastasis and have the worst survival following curative resection at the 3-year OS rate (48.5%) and the 3-year MFS rate (22.1%). The 3-year OS (90.2%) and MFS (86.5%) rates in the Runx2—and OPN—group was the best (P < 0.001, Fig. 2e, f). These data suggested that Runx2 combined with OPN could be useful as the predictors of potential metastatic development and survival in osteosarcoma patients.
In conclusion, we found for the first time that the prediction range was expanded and the sensitivity was improved when considering Runx2 and OPN together. These findings strongly suggested that Runx2 plus OPN could be a more accurate prognostic value as the predictors of potential metastatic development and survival in patients with osteosarcoma. Thus, an early initiation of appropriate therapy in Runx2 + and/or OPN + patients may reduce tumor metastasis and extended survival time.
Data availability
The data used in this study are available from the corresponding author on reasonable request.
References
Ritter J, Bielack SS (2010) Osteosarcoma. Ann Oncol 21(7):vii320-325. https://doi.org/10.1093/annonc/mdq276
Maugg D, Rothenaigner I, Schorpp K et al (2015) New small molecules targeting apoptosis and cell viability in osteosarcoma. PLoS One 10(6):e0129058. https://doi.org/10.1371/journal.pone.0129058
Ferguson WS, Goorin AM (2001) Current treatment of osteosarcoma. Cancer Invest 19(3):292–315. https://doi.org/10.1081/cnv-100102557
Ducy P, Zhang R, Geoffroy V et al (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89(5):747–754. https://doi.org/10.1016/s0092-8674(00)80257-3
Villanueva F, Araya H, Briceño P et al (2019) The cancer-related transcription factor RUNX2 modulates expression and secretion of the matricellular protein osteopontin in osteosarcoma cells to promote adhesion to endothelial pulmonary cells and lung metastasis. J Cell Physiol 234(8):13659–13679. https://doi.org/10.1002/jcp.28046
Wai PY, Mi Z, Gao C et al (2006) Ets-1 and runx2 regulate transcription of a metastatic gene, osteopontin, in murine colorectal cancer cells. J Biol Chem 281(28):18973–18982. https://doi.org/10.1074/jbc.M511962200
Onodera Y, Miki Y, Suzuki T et al (2010) Runx2 in human breast carcinoma: its potential roles in cancer progression. Cancer Sci 101(12):2670–2675. https://doi.org/10.1111/j.1349-7006.2010.01742.x
Sase T, Suzuki T, Miura K et al (2012) Runt-related transcription factor 2 in human colon carcinoma: a potent prognostic factor associated with estrogen receptor. Int J Cancer 131(10):2284–2293. https://doi.org/10.1002/ijc.27525
Yang J, Zhao L, Tian W et al (2013) Correlation of WWOX, RUNX2 and VEGFA protein expression in human osteosarcoma. BMC Med Genomics 6:56. https://doi.org/10.1186/1755-8794-6-56
Gupta S, Ito T, Alex D et al (2019) RUNX2 (6p21.1) amplification in osteosarcoma. Hum Pathol 94:23–28. https://doi.org/10.1016/j.humpath.2019.09.010
Lucero CM, Vega OA, Osorio MM et al (2013) The cancer-related transcription factor Runx2 modulates cell proliferation in human osteosarcoma cell lines. J Cell Physiol 228(4):714–723. https://doi.org/10.1002/jcp.24218
Wei R, Wong JPC, Kwok HF (2017) Osteopontin–a promising biomarker for cancer therapy. J Cancer 8(12):2173–2183. https://doi.org/10.7150/jca.20480
Han X, Wang W, He J et al (2019) Osteopontin as a biomarker for osteosarcoma therapy and prognosis. Oncol Lett 17(3):2592–2598. https://doi.org/10.3892/ol.2019.9905
Liu SJ, Hu GF, Liu YJ et al (2004) Effect of human osteopontin on proliferation, transmigration and expression of MMP-2 and MMP-9 in osteosarcoma cells. Chin Med J (Engl) 117(2):235–240
Meyers PA, Heller G, Healey J et al (1992) Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol 10(1):5–15. https://doi.org/10.1200/jco.1992.10.1.5
Bacci G, Bertoni F, Longhi A et al (2003) Neoadjuvant chemotherapy for high-grade central osteosarcoma of the extremity. Histologic response to preoperative chemotherapy correlates with histologic subtype of the tumor. Cancer 97(12):3068–3075. https://doi.org/10.1002/cncr.11456
Enneking WF, Spanier SS, Goodman MA (1980) A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 153:106–120
Ren Z, Liang S, Yang J et al (2016) Coexpression of CXCR4 and MMP9 predicts lung metastasis and poor prognosis in resected osteosarcoma. Tumour Biol 37(4):5089–5096. https://doi.org/10.1007/s13277-015-4352-8
Stein GS, Lian JB, van Wijnen AJ et al (2004) Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene 23(24):4315–4329. https://doi.org/10.1038/sj.onc.1207676
Lengner CJ, Steinman HA, Gagnon J et al (2006) Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling. J Cell Biol 172(6):909–921. https://doi.org/10.1083/jcb.200508130
Martin JW, Zielenska M, Stein GS et al (2011) The role of RUNX2 in osteosarcoma oncogenesis. Sarcoma 2011:282745. https://doi.org/10.1155/2011/282745
Sadikovic B, Yoshimoto M, Chilton-MacNeill S et al (2009) Identification of interactive networks of gene expression associated with osteosarcoma oncogenesis by integrated molecular profiling. Hum Mol Genet 18(11):1962–1975. https://doi.org/10.1093/hmg/ddp117
Won KY, Park HR, Park YK (2009) Prognostic implication of immunohistochemical Runx2 expression in osteosarcoma. Tumori 95(3):311–316
Senger DR, Wirth DF, Hynes RO (1979) Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell 16(4):885–893. https://doi.org/10.1016/0092-8674(79)90103-x
Luo X, Chen J, Song WX et al (2008) Osteogenic BMPs promote tumor growth of human osteosarcomas that harbor differentiation defects. Lab Invest 88(12):1264–1277. https://doi.org/10.1038/labinvest.2008.98
Velupillai P, Sung CK, Tian Y et al (2010) Polyoma virus-induced osteosarcomas in inbred strains of mice: host determinants of metastasis. PLoS Pathog 6(1):e1000733. https://doi.org/10.1371/journal.ppat.1000733
Funding
No funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Liang, S., Li, Y. & Wang, B. The cancer‐related transcription factor Runx2 combined with osteopontin: a novel prognostic biomarker in resected osteosarcoma. Int J Clin Oncol 26, 2347–2354 (2021). https://doi.org/10.1007/s10147-021-02025-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-02025-4