Abstract
It has been implicated noncollagenous proteins act as important regulators during odontogenesis. To test the hypothesis that the roles of Dspp, Mepe, Versican and Mimecan in the regulation of odontogenesis may be complementary, comparative investigations on the localization of four proteins were performed by immunohistochemical staining using mouse first molar at different developmental stages as a model. In postnatal 1- day-old mice, all the proteins, excluding Mepe, showed co-expression in young odontoblasts. At postnatal 3, strong immunoreactions for all proteins were detected in odontoblasts. Interestingly, Mepe was present within both cytoplasm and nucleus in odontoblasts. In mice older than 5 days, the expression of Dspp, Mimecan and Versican accumulated in subodontoblastic layer of the coronal pulp at high levels while the co-expression of Mepe and Mimecan significantly existed in predentin. The temporal-spatial specific pattern and unique co-localization of Dspp, Mepe, Mimecan and Versican suggest they play complementary roles during odontogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tooth development involves sequential and reciprocal interactions between epithelial-mesenchymal cells and proceeds through a series of cytodifferentiations leading to the terminal differentiation of matrix-producing cells. Odontoblast and dental pulp cells synthesize and secrete several collagenous and non-collagenous proteins (NCPs) to form a unique dentin extracellular matrix to constitute the extracellular environment of developmenting teeth.
Among NCPs, Dentin sialophosphoprotein (Dspp), representing a major component of extracellular matrices, is involved in tooth development and essential for dentinogenesis (Butler and Ritchie 1995; Jianjun et al. 2009). In situ hybridization and other experimental analyses have shown that Dspp transcript is expressed predominantly in odontoblasts, transiently in preameloblasts, significantly in periodontium (D’Souza et al. 1997; Baba et al. 2004; Chen et al. 2008a). Defects in human Dspp are associated with various types of hereditary dentin disorders II (Rajpar et al., 2002; Kim et al. 2004; dong et al. 2005; Kim and Simmer 2007; McKnight et al. 2008; Song et al. 2008). Further study showed that teeth of Dspp knockout mice are similar to human dentinogenesis imperfecta type III (DGI-III) (Sreenath et al. 2003). These date indicate that Dspp plays an important role in tooth formation and mineralization.
Matrix extracellular phosphoglycoprotein (Mepe) is mainly expressed in mineralizing tissues, including the dental pulp (Boukpessi et al. 2006; Wang et al. 2010). Mepe is considered to be crucially involved in mineralization. However, its exact role as a regulator of mineralization and the mechanism underlying this function are still controversial.
Mimecan, a proteoglycan, mainly expressed in connective tissues, was originally named osteoinductive factor. Subsequently, after the determination that co-purifying bone morphogenic proteins 2 and 3 are the source of its growth stimulatory activity, the protein was renamed osteoglycin (Dash et al. 1993). A novel finding of the previous study showed that Mimecan (osteoglycin) was detected at high levels in dentin, what is more, the immunoreactivity of Mimecan in human dentin was more intense than that in human mandibular bone (Park et al. 2009).
Versican is a large chondroitin sulfate proteoglycan that is present in a variety of connective tissue (Wight 2002). Versican is known to be involved in tissue formation as a temporary scaffold in the capture of new space for succeeding tissues/cells in embryonic tissue, cell adhesion, cell proliferation and cell migration (Shibata et al. 2007; Wight 2002), and in the processes such as tooth eruption and early dentinogenesis (Waddington et al. 2003; Sone et al. 2005). Previous studies reported that Versican expression occurred in dental pulp (Harlamb and Messer 1996; Yamauchi et al. 1997; Shibata et al. 2007), dentin (Waddington et al. 2003), cementum (Cheng et al. 1999) and periodontal ligaments (Larjava et al. 1992; Sato et al. 2002). In addition to primary existence in mesenchymal tissue described above, Jiang et al. (2010) concluded that the dental epithelium of enamel organ of developing tooth germ was able to synthesize significant amounts of Versican. The specific expression pattern of Versican implies its involvement in the epithelial-mesenchymal interactions.
Previous study reported the co-expression of Dspp, Mepe, Versican or Mimecan in dental matrix-producing cells. Using immunohistochemical and ultrastructural study, Shibata et al. (2007) detected the co-localization of the pericellular matrix such as Versican, type I collagen, DMP-1 and Mepe in the osteocyte lacunae of lower region of mandibular condyle in aged c-src-deficient mice. Chen et al. (2008a) showed that all of the five SIBLING (small integrin binding ligand, N-linked glycoprotein) family members including Mepe, Dspp were expressed within the cytoplasm and cellular processes in the mouse odontoblastic cell lines by Immunohistochemistry study. What is more, mRNA levels of Mepe, Dspp were increased in a time-dependent manner in human dental pulp cells undergoing odontoblastic differentiation (Wei et al. 2009). Additionally, the proteins identified in human dentin using liquid chromatography-tandem mass spectroscopy (LC–MS/MS) proteomic approaches included Dspp and Mimecan (Park et al. 2009). All the date indicate the correlation among those mineralization related proteins in tooth germ development.
Despite this large body of information on the NCPs during odontogenesis, however, a systematic study correlating protein localization along with the relationships among Dspp, Mepe, Mimecan and Versican in tooth development have not yet been studied. Since mice is a comprehensive model for the study of tooth development and formation. Hence, the present investigation was performed to compare the localization and expression of Dspp, Mepe, Mimecan and Versican during the formation of mouse first molar at various stages of development.
Materials and methods
Animals and study design
Ninety postnatal BALB/c mice from different mothers and periods of post-natal day (PN 1, PN 3, PN 5, PN 9, PN 14 and PN 19) were maintained in specific pathogen-free facilities for experiment. Each group of different periods contains about fifteen mice. The noon hour of the day of birth was taken as post-natal day 0.5 (PN 0.5). BALB/c mice were sacrificed under anesthesia. This study was conducted in conformity with the Animal Care and Use Committee of Shandong University, Jinan, Shandong Province, China.
Tissue preparation
The mandibles of postnatal (PN 1, PN 3, PN 5, PN 9, PN 14 and PN 19) mice were dissected and fixed 24 h in buffered 4% paraformaldehyde, pH 7.2, at 4°C. The mandibles (PN 1, PN 3, PN 5, PN 9, PN 14 and PN 19) were then decalcified in 10% ethylene diaminetetraacetic acid/phosphate-buffered saline (PBS) solution, dehydrated through a graded alcohol series and embedded in paraffin. Sagittal serial sections for mandibles at 5 μm-thick were then prepared.
Immunohistochemical processes
The serial sections were deparaffinized in xylene, hydrated through a graded alcohol series and washed with tap water. Antigen retrieval for PN 1, PN 3, PN 5, PN 9, PN 14 and PN 19 sections was performed by treating with 0.1% (w/v) trypsin (Sigma, St. Louis, USA) dissolved in 0.01 M PBS, pH 7.2, 37°C for 15 min. The activity of endogenous tissue peroxidase was blocked with 3% H2O2 for 30 min. After pretreatment with normal goat serum for 30 min to block non-specific binding, the sections were incubated with antibodies specific for Dspp, Mepe, Mimecan and Versican, respectively (provided by Dr. Larry Fisher, National Institute of Dental and Craniofacial Research, Maryland, USA) at 4°C overnight. Biotinylated goat anti-rabbit immunoglobulin G (IgG) was applied as secondary antibody for 15 min at 37°C. Sections were exposed to streptavidin-peroxidase conjugate for 10 min at 37, and then visualized by the application of diaminobenzidine solution (Vector Laboratories, Burlingame, CA) for 1 min. Finally, the sections were lightly counterstained with hematoxylin. Six serial sections were used in per animal per stain analyzed. A negative control of mouse IgG was purchased from Dakocytomation (Carpinteria, CA, USA). Images of Alexa Fluo ®488 staining was obtained at the Core Optical Imaging Facility, under the same parameters, with a Nikon inverted microscope.
Results
Expression patterns of Dspp, Mepe, Mimecan and Versican in developing teeth
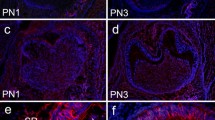
At postnatal day 1 (PN 1), no immunopositive staining for Dspp, Mepe, Mimecan and Versican was detected in tooth germ (Figs. 1a, b, 2a, b, 3a, b, 4a, b).
Immunostaining for Dspp at various stages of tooth development in postnatal mouse, the localization of Dspp is stained brown. ab ameloblasts, d dentin, e enamel, dp dental pulp tissue, ob odontoblasts, sr stellate reticulum, si stratum intermedium, iee inner enamel epithelium, oee outer enamel epithelium. a, d, f, h at low magnification. b, c, e, g, i at higher magnification. At postnatal day 1 (PN 1), no immunopositive staining was detected in tooth germ (a, b). Dspp was first expressed by odontoblasts at late bell stage and continued at the later stages, whereas no transient expression was detected in mature ameloblasts as well as in preameloblasts (c, d, e). At PN 5, immunolabelling signal for Dspp was observed in odontoblasts and subodontoblastic cell layers (f, g). After PN 9, especially when reduced enamel epithelium had formed, the expression of Dspp was weakened in odontoblasts (h, i)
Immunostaining for Mepe at various stages of tooth development in postnatal mouse, the localization of Mepe is stained brown. ab ameloblasts, d dentin, pd predentin, e enamel, AL alveolar bone, dp dental pulp, ob odontoblasts, sr stellate reticulum, si stratum intermedium, iee inner enamel epithelium, oee outer enamel epithelium, pdl periodontal ligament. a, c, e, g at low magnification. b, d, f, h at higher magnification. There was no positive staining in the epithelial tissues or mesenchymal tissues in tooth germ (a, b). Differentiating odontoblasts and ameloblast showed strongly and equally distributed expression of Mepe protein at late bell stage (c, d); besides, it is obvious to note that its expression was detectable in both the cytoplasm and nucleus. After postnatal 5 (PN 5), Mepe expression obviously increased in predentin (e, f). At PN 19, after completion of tooth development, Mepe had a significant expression in osteocytes and osteoblasts of the alveolar bone as well as in the pericellular bone matrix of the bone-embedded osteocytes (g, h)
Immunostaining for Mimecan at various stages of tooth development in postnatal mouse, the localization of Mimecan is stained brown. ab ameloblasts, pd predentin, e enamel, dp dental pulp tissue, ob odontoblasts, sr stellate reticulum, si stratum intermedium, iee inner enamel epithelium, oee outer enamel epithelium. a, c, e at low magnification. b, d, f at higher magnification. At postnatal day 1 (PN 1), no immunopositive staining was detected in tooth germ (a, b). In the secretory stage of the tooth germ, Mimecan was first found in differentiated odontoblasts at late bell stage and persisted in dental papilla cells by strong and equal distribution until completion of crown formation (c, d). After postnatal (PN 5), the expression also converged strongly in the predentin (e, f)
Immunostaining for Versican at various stages of tooth development in postnatal mouse, the localization of Versican is stained brown. ab ameloblasts, d = dentin, e enamel, dp dental pulp tissue, ob odontoblasts, sr stellate reticulum, si stratum intermedium, iee inner enamel epithelium, oee outer enamel epithelium. a, c, e at low magnification. b, d, f at higher magnification. At postnatal l (PN 1), Versican expression was negative in tooth germ (a, b). The expression intensity of Versican varied in accordance with different stages of the tooth development. Versican was first detected in differentiating odontoblast at late bell stage(c, d) and were intensely expressed in the area where proliferation cells located such as differentiating odontoblast, Hertwig’s epithelial root sheath (HERS), the proliferational layer in the dental pulp cell until completion of crown development(e, f). No expression of Versican was observed in the dentin and predentin, as well
At postnatal day 3 (PN 3), Dspp, Mepe, Mimecan and Versican were detected co-expression in young odontoblasts at middle or late bell stage before the start of mineralization. (Figs. 1c–e, 2c, d, 3c, d, 4c, d). In this stage, differentiating odontoblasts and ameloblast showed strongly and equally distributed expression of Mepe protein during the initial stage of tooth development. What is more, it is interesting to note that the expression of Mepe was detectable in both the cytoplasm and nucleus.
At postnatal day 5 (PN 5), Dspp, Mimecan, and Versican persisted in dental papilla cells at high levels until completion of crown formation, (Figs. 1f, g, 3e, f, 4e, f). However, Mepe expression was sharply down-regulated in the above mentioned cells, but obviously increased in predentin at the epithelial–mesenchymal interface (Fig. 2e, f). Interestingly, Mepe and Mimecan showed co-expression significantly in predentin at same time (Figs. 2e, f, 3e, f). No expression of Dspp and Versican was observed in the dentin and predentin, both were expressed strongly in the subodontoblastic cell layers (Figs. 1f, g, 4e, f). Whereas, the distribution of Dspp included differentiated odontoblast, the latter of Versican excluded differentiated odontoblast.
After postnatal day 9 (PN 9), the expression of Dspp, Mimecan and Versican subsided gradually in the dental pulp with the maturation of root development. While Mepe staining still converged in predentin until completion of tooth development.
Negative control for the proteins examined above demonstrated no positive staining by immunohistochemistry (data not shown).
Discussion
We first detected the co-expression of these proteins in young odontoblasts at middle or late bell stage before the start of mineralization. These findings suggest that they, perhaps as an initiator, all involved in the differentiation of odontoblast at early stages of tooth development.
To assess Mepe expression and processing, it was observed only in immature odontoblasts, and was down-regulated during odontoblast differentiation (MacDougall et al. 2002; H Liu et al. 2005), which were consistent with our observation. Wang et al. (2010) reported that stable Mepe-overexpressing and Mepe-knockdown odontoblast-lineage cell lines, showed lower and higher differentiation capabilities, respectively. Mepe may play important roles in pulpal homeostasis by keeping the odontoblasts in immature condition.
However, at the same time, the expression of the Dspp predominantly persisted in functional and/or physiologically differentiated odontoblasts at high levels throughout all phases of primary dentinogenesis (Fig. 1), which was different from Mepe. Based on our observation and contrast between Mepe and Dspp initial expression, we speculate the well-marked down-regulation of Mepe in more mature odontoblasts may act as an initiator or as a negative regulator of mineralization in initial stages of dentinogenesis, whereas the latter would be involved in subsequent later steps.
After PN 5, Mepe expression significantly existed in gradients across the predentin and was degraded near the mineralization front (Fig. 2e, f, g, h). The precise functions of Mepe in predentin during tooth development have not been clearly defined. Mepe expression was found only in predentin but not in mineralized dentin, indicating that it might involve in the formative processes of dentinal tubules. It raises the possibility that Mepe is responsible for inhibiting conversion of predentin to dentin at the mineralization front. However, Mepe specifically acted on functions of promotion or inhibition, still or combined blend the both, which remain unclear.
For Mepe biological roles, previous study reported that knockout of Mepe gene in mice increased bone formation and bone mass (Gowen et al. 2003). Liu et al. (2005) reported that Mepe-null mice showed improved mineralization and increased osteoblastic markers, including DMP1, osteocalcin and Osterix. Despite this large body of information on the role of Mepe as a negative regulator of mineralization, however, its exact role as a regulator of mineralization is still controversial. Recent studies have found that given domains of Mepe have different biological functions and Mepe may be acted either as a positive or a negative regulator in the process of mineralization. The evidences for this possibility include that Rowe et al. (2005) reported that a small peptide released from COOH-terminus of Mepe was able to inhibit mineralization processes in vitro whereas Hayashibara et al. (2004) reported that another fragment from N-terminal Mepe with RGD motif accelerated mineralization. Analysis of these data from prior work raises the question that which fragment existed in predentin in our study is derived from which portion of Mepe protein, answering this would need further experimentations.
A significant finding of our data is Mimecan equally accumulation and homogenously distribution in the predentin and dental pulp excluding differentiated odontoblasts (Fig. 3), which has not been described previously. The co-localization of Mimecan and Mepe at the same time in the unmineralized predentin suggested they perhaps had significant and similar function in mice tooth germ. One explanation for the results is that some proteoglycans may inhibit the mineralization of the dentin matrix (Fukae et al. 1994). On the other hand, large proteoglycans mainly synthesized within the predentin matrix, play important roles in matrix formation and in prevention of premature mineralization (Waddington et al. 2003). However, at present, there is no published information about Mimecan exact role as a regulator of dentin mineralization. Hence, identifying the inhibitory or excitatory roles of Mimecan in the mineralization of dentin and understanding the interactions between Mimecan and other matrix molecules requires abundant further studies.
However, our studies are different from previous studies (Ruggeri et al. 2009), because we failed to observe the expression of Versican within the intertubular dentine and predentin matrix. The reasons for these discrepancies are not clear, although notable differences exist with regard to experiment staging, age and techniques for assessing tooth development as well as species–species variations and genetic background.
Notably, strong reactions for Versican appeared in the subodontoblastic layer of the coronal pulp until completion of crown formation, which was similar to Mimecan and Dspp expression at PN 5. Yamauchi et al. (1997) made a conclusion that Versican in dental pulp could form large hydrated proteoglycan aggregates that fill the extracellular space, support odontoblasts, and/or facilitate the transport function of metabolites and nutrients within the tissue. The co-localization of Mimecan, Dspp and Versican in the subodontoblastic layer suggested they could offer stronger support to the odontoblasts and maintain more extensive nervous and/or vascular systems. These molecules may cooperate with each other in remodeling the extracellular environment and in developing these tissues.
Although Mepe is an extracellular matrix protein, it is interesting to note that its expression was detectable in both the cytoplasm and nucleus (Fig. 2c, d) which was in agreement with previous report (Chen et al. 2008b). However, its role in the nucleus remains obscure.
Additionally, a novel finding of our data is the transient presence of strong Mepe expression in secretory ameloblasts of tooth germ (Fig. 2c, d). The secretion of ameloblast-derived Mepe is short-lived, correlates to the establishment of the dentino-enamel junction (DEJ). Such a localized spatial–temporal pattern of Mepe protein expression may contribute to remarkable crack-resistant properties of the DEJ, which resembles that of Dspp (White et al. 2007). Before predentin mineralization, signaling molecules can migrate from one cell type to the other, whereas, predentin mineralization acts as a physical barrier, which interrupts the oral epithelium and underlying mesenchyme interaction (Bleicher et al. 1999). It raises the question that the expression of Mepe in polarized ameloblasts could be directly or indirectly regulated by an unknown signaling molecule secreted by odontoblasts.
Although the roles of Dspp, Mepe, Mimecan and Versican in dentin formation are not fully understood, it is clear that the expression seems to be highly regulated with a spatial distribution that is not restricted to odontoblasts. They may play a role in two important processes of dental morphogenesis, i.e. epithelial-mesenchymal interactions and mineralization of matrices.
In conclusion, the observed similarities of temporal and spatial expression patterns among Dspp, Mepe, Mimecan and Versican have suggested that those proteins were involved in the early and later stages of odontogenesis, not independently but complementarily and/or collaboratively. Such expression analyses should prove useful for future studies that will assess the individual functions of these extracellular matrices proteins in vivo.
References
Baba O, Qin C, Brunn JC, Jones JE, Wygant JN, McIntyre BW et al (2004) Detection of dentin sialoprotein in rat periodontium. Eur J Oral Sci 112:163–170
Bleicher F, Couble ML, Farges JC, Couble P, Magloire H (1999) Sequential expression of matrix protein genes in developing rat teeth. Matrix Biol 18:133–143
Boukpessi T, Septier D, Bagga S, Garabedian M, Goldberg M, Chaussain-Miller C (2006) Dentin alteration of deciduous teeth in human hypophosphatemic rickets. Calcif Tissue Int 79:294–300
Butler WT, Ritchie HH (1995) The nature and functional significance of dentin extracellular matrix proteins. Int J Dev Biol 39:169–179
Chen S, Chen L, Jahangiri A, Chen B, Wu Y, Chuang HH, Qin C, MacDougall M (2008a) Expression and processing of small integrin-binding ligand N-linked glycoproteins in mouse odontoblastic cells. Arch Oral Biol 53:879–889
Chen S, Gluhak-Heinrich J, Martinez M, Li T, Wu Y, Chuang H-H et al (2008b) Bone morphogenetic protein 2 mediates dentin sialophosphoprotein expression and odontoblast differentiation via NF-Y signaling. J Biol Chem 283:19359–19370
Cheng H, Caterson B, Yamauchi M (1999) Identification and immunolocalization of chondroitin sulfate proteoglycans in tooth cementum. Connect Tissue Res 40:37–47
Dash JR, Pace DR, Avis PD, Bentz H, Chu S (1993) Characterization of monoclonal antibodies recognizing bovine bone osteoglycin. Connect Tissue Res 30:11–21
Dong J, Amor D, Aldred MJ, Gu T, Escamilla M, MacDougall M (2005) DLX3 mutation associated with autosomal dominant amelogenesis imperfecta with taurodontism. Am J Med Genet A 133A:138–141
D’Souza RN, Cavender A, Sunavala G, Alvarez J, Ohshima T, Kulkarni AB, MacDougall M (1997) Gene expression patterns of murine dentin matrix protein 1(Dmp1) and dentin sialophosphoprotein (Dspp) suggest distinct developmental functions in vivo. J Bone Miner Res 12:2040–2049
Fukae M, Tanabe T, Yamada M (1994) Action of metalloproteinases on porcine dentin mineralization. Calcif Tissue Int 55:426–435
Gowen LC, Petersen DN, Mansolf AL, Qi H, Stock JL, Tkalcevic GT, Simmons HA, Crawford DT, Chidsey-Frink KL, Ke HZ, McNeish JD, Brown TA (2003) Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J Biol Chem 278:1998–2007
Hao Jianjun, Ramachandran Amsaveni, George Anne (2009) Temporal and spatial localization of the dentin matrix proteins during dentin biomineralization. J Histochem Cytochem 57:227–237
Harlamb SC, Messer HH (1996) The identification of proteoglycanassociated mRNAs in human dental pulp cells. Arch Oral Biol 41:1097–1100
Hayashibara T, Hiraga T, Yi B, Nomizu M, Kumagai Y, Nishimura R (2004) A synthetic peptide fragment of human Mepe stimulates new bone formation in vitro and in vivo. J Bone Miner Res 19:455–462
Jiang BZ, Yokohama-Tamaki T, Wang ZL, Obara N, Shibata S (2010) Expression, localisation and synthesis of versican by the enamel organ of developing mouse molar tooth germ: an in vivo and in vitro study. Arch Oral Biol 55:995–1006
Kim JW, Simmer JP (2007) Hereditary dentin defects. J Dent Res 86:392–399
Kim JW, Nam SH, Jang KT, Lee SH, kim CC, Hahn SH et al (2004) A novel splice acceptor mutation in the Dspp gene causing dentinogenesis imperfecta type II. Hum Genet 115:248–254
Larjava H, Hakkinen L, Rahemtulla F (1992) A biochemical analysis of human periodontal tissue proteoglycans. Biochem J 284(Pt 1):267–274
Liu H, Li W, Shi S, Habelitz S, Gao C, Denbesten P (2005) Mepe is downregulated as dental pulp stem cells differentiate. Arch Oral Biol 50:923–928
MacDougall D, Gu SimmonsTT, Dong J (2002) Mepe/OF45, a new dentin/bone matrix protein and candidate gene for dentin diseases mapping to chromosome 4q21. Connect Tissue Res 43:320–330
McKnight DA, Suzanne Hart P, Hart TC, Hartsfield JK, Wilson A, Wright JT, Fisher LW (2008) A comprehensive analysis of normal variation and disease-causing mutations in the human Dspp gene. Hum Mutat 29:1392–1404
Park E-S, Cho H-S, Kwon T-G, Jang S-N, Lee S-H, An C-H, Shin H-I, Kim J-Y, Cho J-Y (2009) Proteomics analysis of human dentin reveals distinct protein expression profiles. J Proteome Res 8:1338–1346
Rajpar MH, Koch MJ, Davies RM, Mellody KT, Kielty CM, Dixon MJ (2002) Mutation of the signal peptide region of the bicistronic gene Dspp affects translocation to the endoplasmic reticulum and results in defective dentine biomineralization. Hum Mol Genet 11:2559–2565
Rowe PS, Garrett IR, Schwarz PM, Carnes DL, Lafer EM, Mundy GR (2005) Surface plasmon resonance (SPR) confirms that Mepe binds to PHEX via the Mepe-ASARM motif: a model for impaired mineralization in X-linked rickets (HYP). Bone 36:33–46
Ruggeri A, Orsini G, Mazzoni A, Nato F, Papa V, Piccirilli M, Putignano A, Mazzotti G, De Stefano Dorigo E, Breschi L (2009) Immunohistochemical and biochemical assay of versican in human sound predentine/dentine matrix. Eur J Histochem 53:125–133
Sato R, Yamamoto H, Kasai K, Yamauchi M (2002) Distribution pattern of versican, link protein and hyaluronic acid in the rat periodontal ligament during experimental tooth movement. J Periodontal Res 37:15–22
Shibata S, Baba O, Oda T, Yokohama-Tamaki T, Qin C, Butler WT, Sakakura Y, Takano Y (2007) An immunohistochemical and ultrastructural study of the pericellular matrix of uneroded hypertrophic chondrocytes in the mandibular condyle of aged c-src-deficient mice. Arch Oral Biol 53:220–230
Sone S, Nakamura M, Maruya Y, Takahashi I, Mizoguchi I, Mayanagi H, Sasano Y (2005) Expression of versican and ADAMTS during rat tooth eruption. J Mol Histol 36:281–288
Song YL, Wang CN, Fan MW, Su B, Bian Z (2008) Dentin phosphoprotein frameshift mutations in hereditary dentin disorders and their variation patterns in normal human population. J Med Genet 45:457–464
Sreenath T, Thyagarajan T, Hall B, Longenecker G, D’Souza R, Hong S, Wright JT, MacDougall M, Sauk J, Kulkarni AB (2003) Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem 278:24874–24880
Waddington RJ, Hall RC, Embery G, Lloyd DM (2003) Changing profiles of proteoglycans in the transition of predentin to dentin. Matrix Biol 22:153–161
Wang H, Kawashima N, Iwata T, Xu J, Takahashi S, Sugiyama T, Suda H (2010) Differentiation of odontoblasts is negatively regulated by Mepe via its C-terminal fragment. Biochem and Biophys Res Commun 398:406–412
Wei X, Wu LP, Ling JQ, Liu L (2009) Expression of matrix extracellular phosphoglycoprotein in human dental pulp cells undergoing odontoblastic differentiation. Zhonghua Kou Qiang Yi Xue Za Zhi 44:524–528
White SN, Paine ML, Ngan AY, Miklus VG, Luo W, Wang H, Snead ML (2007) Ectopic expression of dentin sialoprotein during amelogenesis hardens bulk enamel. J Biol Chem 282:5340–5345
Wight TN (2002) Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol 14:617–623
Yamauchi S, Cheng H, Neame P, Caterson B, Yamauchi M (1997) Identification, partial characterization, and distribution of versican and link protein in bovine dental pulp. J Dent Res 76:1730–1736
Acknowledgments
We are grateful to Dr. Larry Fisher (National Institute of Dental and Craniofacial Research, Maryland, USA) for providing the antiserums of Dspp, Mepe, Mimecan and Versican. This study was supported by Natural Science Foundation of Shandong Province of China (ZR2010HM050). The authors report no conflicts of interest related to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, C., Liu, Z.X., Tang, K.L. et al. Developmental changes and regional localization of Dspp, Mepe, Mimecan and Versican in postnatal developing mouse teeth. J Mol Hist 43, 9–16 (2012). https://doi.org/10.1007/s10735-011-9368-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-011-9368-9