Abstract
Woody cutting is customarily utilized as a material in research on grape adventitious root formation (ARF). However, phenotypic heterogeneity caused by the complex background influenced its use for molecular mechanism research of ARF of grape. The present study tested various types of explants from grape tissue culture plantlets and found that the whole leaf: blade with the petiole (LP) was the simplest unit that can easily form adventitious roots (ARs). LP explants which can be easily obtained, directly generate ARs via de novo organogenesis from the base of the petiole. Plantlet age, node position, blade size, the health condition of leaves, and light intensity have been demonstrated to affect the homogeneity of the ARF phenotype in LP. By controlling these parameters, selected LPs cultured on a medium with 6 g·L-1 agar and 10 g·L-1 sucrose under dark conditions started rooting at 6–7 days after culture (DAC) and reached 100% rooting rate within 13–14 DAC. Using this system, the core role of auxin on ARF was verified by exogenous application of indole butyric acid (IBA) and N-1-naphthylphthalamic acid (NPA). Strikingly, we found that light promoted ARF in the absence of sucrose, but inhibited ARF in the presence of sucrose (10 g·L-1), while a low concentration of 0.34 µM NPA partially relieved the inhibition. Finally, this study confirmed that exogenous plant growth regulators (PGRs), including 6-benzyl aminopurine (6-BA), gibberellic acid 3 (GA3), and 2,4-epibrassinolide (EBR), significantly inhibited ARF. This simple, rapid, quantifiable ARF research system provides a new approach to studying the factors influencing the formation and development of grape adventitious roots and establishes a framework for investigating the mechanism of grape adventitious root induction and initiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Grape (Vitis sp.) is one of the most economically important crops and occupies a pivotal position in the global fruit market. As perennial vines, the propagation of grapes is conventionally used by dormant hardwood cuttings that are taken from the current season’s canes. Cutting propagation guarantees characteristics of species or new varieties. In asexual propagation, adventitious root formation (ARF) is the premise of successful propagation (Steffens and Rasmussen 2016). However, adventitious roots are difficult to be induced in many grape species or varieties with important breeding utilization, such as V. davidii, V. quinquangularis (syn. V. kiusiana), and V. amurensis which are wild grapes native to East Asia (Shiozaki et al. 2013). The V. rotundifolia (Muscadine) (Castro et al. 1994), V. champinii, V. aestivalis (Keeley et al. 2004), V. berlandieri (Kracke et al. 1981; Smart et al. 2003), and some cultivars of V. vinifera are also known to be difficult to root from their woody cuttings.
In model plants, the formation of AR can be divided into three stages: induction, initiation, and expression (da Costa et al. 2013; Joshi and Ginzberg 2021). It is essentially a regenerative process that involves reprogramming cells and changes in cell fate, and the primary induction signal is wounding (Xu 2018). Environmental conditions, exogenous application, and endogenous biochemical substances all have an important influence on AR formation. Auxin plays a central role, and numerous inducible factors that respond to ARF will eventually be reflected in the concentration, transport, or homeostasis changes of auxin (Gonin et al. 2019). ARF also depends to a large extent on the nutritional status of the mother plant, including minerals and carbohydrates, and sink establishment at the rooting zone (Chen et al. 2014; Ahkami et al. 2013). The presence of some inhibitors may also make ARF difficult to achieve in some species. In addition, ethylene, cytokinin, jasmonic acid, gibberellin, abscisic acid, brassinolide, strigolactone, and other biochemical substances may regulate the production of ARs through molecular processes and crosstalk. These substances may both promote and inhibit ARF, which appears to depend on hormone type, dose, or species (Bannoud and Bellini 2021; Di Battista 2019; Omoarelojie 2021; Sharma et al. 2021).
In studies on grape adventitious roots, hardwood cuttings with dormant buds and greenwood leafy cuttings are usually utilized Jaleta 2019; Kaur et al. 2002; Thomas et al. 2003; Thomas and Schiefelbein 2004 ). Nevertheless, cuttings are a highly complex system in which endogenous hormone levels, transport, dormancy, storage, and inhibitory compounds affect adventitious root formation and growth, all of which depend on pretreatment (Smart et al. 2003). Moreover, the sprouting of buds on cuttings may affect rooting (Zhou et al. 2020; Smart et al. 2003), and the acquisition of cuttings has seasonality. These factors make it difficult to investigate the function and molecular regulatory mechanism of the factors affecting ARF in grapes by using cuttings.
Blade or whole leaves of Arabidopsis were utilized for adventitious root regeneration studies (Chen et al. 2014; Bustillo-Avendano et al. 2018), which is probably the smallest and simplest unit that can form ARs, and without the influence of sprouting. Nevertheless, the system has not been applied to grapes. Referring to this method, we found that ARs were regenerated when whole grape leaves (leaf with petiole, LP) were used without any added nutrients and hormones. We described some internal factors affecting the ARF of LP in grapes. Using this system, the role of auxin, carbohydrate, light, and exogenous plant growth regulators has been tested on grape ARF.
Materials and methods
Plant materials and culture conditions
Nine-year-old Vitis labruscana × Vitis vinifera ‘Shine Muscat’ (SM) and ‘Summer Black’ (SB), which were planted in a research field at Hunan Agricultural University (Changsha, China) were used in this experiment. Woody current canes with 3–5 nodes (N3, N4 and N5) were obtained from these vines in October 2020. Canes were then cut into single bud stem segments, placed into glass bottles with clear water, and cultured in a plant tissue culture chamber at 25℃ with a 16-h light (4000 lx, cool white fluorescent lamp) and 8-h dark photoperiod. Rooting data were recorded daily. The cutting with root (> 1 mm) was classified as a rooted cutting.
The explants of single bud stem section with leaf (SBS-L), single bud stem section (SBS), whole leaf (called a leaf with petiole, LP), blade (B), petiole (P), and stem section (SS) were obtained from field or tissue culture plants. The AR induction medium and culture conditions were changed according to the experiment. Without special instructions, the basic medium was 6 g·L-1 plant agar without (A) or with 10 g·L-1 sucrose (A + S), the pH value was adjusted to 5.8, and the culture conditions were 25℃ with 16 h photoperiod irradiance of 4000 lx (expressed by Light) or 24 h-dark (expressed by Dark).
In the field, leaves on a 9-year-old grapevine or 2-year-old cutting propagation plant of ‘Summer Black’ were also used in this experiment. From the top to the base, leaves at different nodes were cultured on medium A under the culture conditions mentioned above. Grape tissue culture explants from varieties ‘Vidal blanc’ were preserved in our laboratory. The medium for propagation was the base of Woody Plant Medium (WPM) with 10 g·L-1 sucrose and 3 g·L-1 activated carbon (WPM + S + AC). With no special description, the LP was taken from the 45-60-day-old tissue culture plantlets.
Hormonal and inhibition treatments
Exogenous plant growth regulators (PGRs) were used to investigate the effects of different hormones on grape ARF. Exogenous auxin (indole butyric acid, IBA) and N-1-naphthylphthalamic acid (NPA), a polar auxin transport inhibitor, were utilized to investigate the key functions of auxin in ARF. Cytokinin (6-benzaminopurine, 6-BA), ethephon (ET), abscisic acid (ABA), gibberellin acid 3 (GA3), jasmonic acid methyl ester (MeJA), and 2,4-epibrassinolide (EBR) were purchased from Sigma-Aldrich or Solarbio and used to investigate the effect on ARF in grapes.
ARF phenotype observation and analysis
The phenotype of ARF was determined by the appearance of AR protrusions (at the expression stage) observed under a stereomicroscope (SZX7, Olympus). The proportion of explants with the ARF phenotype was defined as the rooting rate, and the AR data curve was made by the dynamic change in rooting rate daily (day after culture, DAC) (Fig. S1).
Statistical analysis
Data values were statistically analyzed by ANOVA with Duncan’s multiple range test, using SPSS Statistics 20 software (IBM). Significant differences were collected with a 5% level of significance (P-value,0.05).
Results
Phenotypic heterogeneity of ARF in hardwood cuttings
The rooting of hardwood cuttings taken from nodes 3 to 5 (N3, N4, and N5) of Shine Muscat (SM) and Summer Black (SB) was investigated (Fig. 1(a) and (b)). Cuttings of the two cultivars were rooted after 22 DAC (F = 22 DAC). The ARF phenotype of SB was more consistent than SM, USB = 90 ~ 98%, and USM = 53 ~ 75% at 38 DAC. The rooting rate of SM was different in nodes. The N5 was higher than N3 and N4. From the first root induced to the stable rooting rate, SSB and SSM were taken for 16 days (S = 16 days). In addition, the standard deviation (E) of the rooting rate among the samples was large. Some of them were more than 22%. At the same time, there was also inconsistent rooting and sprouting in cuttings from the same node (Fig. S2).
Field and tissue culture materials were used to obtain different types of explants to study the phenotypic heterogeneity of ARF. Young and mature leaves (Blade or LP) were taken from top to base of current canes of SB and cultured on medium A or A with 0.48 µM IBA (A + IBA0.48) in light. After 60 days, till the leaves were scorched, no ARs appeared (Fig. S3). Then, the SBS explants from different nodes of the tissue culture plant were utilized for calculating the heterogeneity of ARF. Results showed that the rooting rate of the first and second nodes (N1 ~ N2) was low (U = 34.44 ~ 46.67%), while the rooting rate of N3 ~ N4 and the terminal shoot (NT) was 80.00%~88.89% at 45 DAC (Fig. 1(c)). The heterogeneity of the N1 ~ N2 samples was larger than that of N3 ~ N4, with a partial error (E) of more than 15% (Fig. 1(c)). These results indicated that explants from different nodes exhibit different rooting abilities.
ARF phenotype in light and exogenous carbohydrates
Six types of explants were selected from N3 ~ N6 of tissue culture plants, and these ARF phenotypes were compared (Fig. 2(a)). The explants were cultured on medium A in light and the results showed that the petiole (P) and stem segment (SS) did not regenerate roots at 60 DAC. The SBS-L, LP, and SBS began to root at 7–10 DAC, faster than the blade (F = 11 DAC). At 20 DAC, the highest rooting rate of explants was observed for LP (67.78%±14.99%); the rooting rate of SBS-L was 44.4% (44.44%±10.30%), and that of SBS was 10.0% (10.00%±2.70%) (Fig. 2(c)). However, only 1–2 explants of the blade (B) are rooted occasionally (N > 90) (Fig. 2(a)). Different types of explants were further cultured on medium A under dark conditions. After 20 DAC, only LP rooting was observed (17.80%±15.00%), and no rooting was observed until 30 DAC in other explants (Fig. 2(d)).
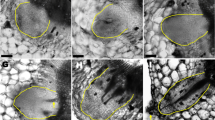
Characteristics of AR formed on explants
Rooting of different types of explants was displayed in (a). The arrow shows the growth of explants 7 days after root regeneration. The ARF process of LP was shown in (b). (c-f) showed rooting rates of different types of explants, which were cultured on medium A under light (c) or dark (d) conditions, and on medium A + S under light (e) or dark (f) conditions. The presence of ARs at the wounded site of the petiole was shown in (g). (h) shows the damage to leaves under high intensity for 24 h. Scale bars: 1 cm
ARF phenotype in dark and exogenous carbohydrates
Explants were then cultured on medium A + S under dark conditions. The results showed that P, B, and SS did not root at 30 DAC. However, after culturing for 45 days, 37.78% (37.78%±22.19%) of SS developed roots ((Fig. 2(a)), data not shown). These results indicate that neither leaves nor buds were necessary for ARF in this condition, while sugar and darkness were important factors promoting rooting. The rooting efficiency of SBS-L and LP on medium A + S in dark was significantly greater than that of medium A in light, and almost all the explants of SBS-L and LP could root at 13 DAC (U = 100%). The rooting rate of SBS also increased significantly and reached 73.33%±5.40% at 20 DAC (Fig. 2(f)).
Interestingly, when the explants were cultured on medium A + S in light, the rooting of SBS-L, SBS and LP were significantly slower than that in dark (Fig. 2(e) and 2(f)). The rooting rate of these explants was significantly reduced at 20 DAC, only 43.3%±8.20% for SBS-L and 10.00%±5.40% for SBS, and only 4.40%±4.20% for LP. Meanwhile, P and SS did not generate roots after continuous culture at 60 DAC, while B occasionally took root (Fig. 2(a)). These results suggest that light significantly inhibited the ARF of explants with a leaf in the presence of additional sucrose (10 g·L-1), which was confirmed by subsequent experiments.
ARF phenotypic characteristics of grape LP
Wounding has been found as the primary factor inducing AR formation in LP explants. When the petiole was picked with tweezers, the middle part of the petiole was occasionally clipped, and ARs were formed on these wounded sits (Fig. 2(g)). With attention to the clamping force, LP-induced AR appears more frequently approximately 2 mm above the cut site, rather than at the base of the cut. Observing under the stereomicroscope, AR formation did not undergo callus formation but was direct de novo organogenesis from the petiole (Fig. 2(b)).
High light intensity inhibited ARF in grape LP
In an accidental culture process, we noticed that when the light intensity was ≥ 6000 lx, the leaves cultured on medium A were damaged to varying degrees within 12 h under 16 h-photoperiod conditions. Part of the leaf margin was burnt, which seriously affected rooting (from 67.78% reduced to 12.22% at 20 DAC), although injured LP could occasionally take root (Fig. 2(h)). Therefore, the light intensity was determined to be 4000 lx in the subsequent experiments.
Leaf size influences the consistency of ARF formation in the LP explants
In tissue culture, the growth potential of the SBS propagated plantlets were not completely consistent at different ages, and the leaf size and growth state of the same plant at different nodes were also different. Usually, leaves at the basal nodes are small, and some of them may become brown and senile, while the middle and upper nodes are large and healthy, and the apical leaves are young and tender (Fig. 3(a)). These are different from the leaves of 12-day-old seedlings that are most suitable for ARF study in Arabidopsis thaliana by precisely controlling seedling age (Chen et al. 2014). Therefore, the blade size, expressed by length × width (L×W cm2), was analyzed for correlation with ARF capacity. In this study, the size (L×W) of healthy leaves of tissue culture plantlets ranged from 1 to 6. After culturing for 20 days in dark (D) on medium A, 17.80% LP could form ARs. Forty rooted (D-R) and unrooted (D-NR) LPs were randomly selected for statistical analysis, and it was shown that when the average L×W was less than 1.8 (median), most of the leaves had no spontaneous rooting ability. In LP, which could spontaneously root, the L×W was above 3.8. The same trend was observed in light culture (Fig. 3(b)). In addition, leaves with senescence at the base of the plant or withered patches at the leaf margin were found difficult to root under various conditions. These results suggest that leaf size and health status are important factors affecting the homogeneity of ARF.
Control of LP explants and their effect on phenotypic heterogeneity of ARF
The growth status of tissue culture plantlets was presented in (a). Arrows indicate nodes from the base up. The blue arrows indicate small leaves (2 < L×W), and the yellow arrows indicate aging or unhealthy leaves. The ARF characteristics of LP with different leaf sizes on medium A in dark or light were shown in (b). Different lowercase letters in each graph indicate significant differences (p ≤ 0.05). After controlling the leaf size (2 < L×W < 6) and node position (N3-N6), the rooting rates of LP were recorded under different cultural conditions (c-f)
Carbohydrate provision is a prerequisite in the formation of AR from LP
To further prove the importance of carbohydrates in ARF, N3-N6 nodes were selected according to the previous results, and the leaf size was controlled at (2 < L×W < 6). After controlling for blade size and node position (Controlled), the rooting rate of LP on medium A under dark conditions (16.7%±7.80% at 20 DAC) was not significantly different from that without control (Uncontrolled) (Fig. 3(c)). The rooting rate of LP with strict selection was increased (82.8%±6.80% at 20 DAC) under light culture on medium A compared with that without control, and the errors were significantly smaller during the rooting process (Fig. 3(d)). When sucrose (10 g·L-1) was supplemented in medium A, all healthy LP could root under dark conditions, and the rooting rate finally reached 100% after 13 DAC (Fig. 3(e)). These results suggest that the amount of nutrients and hormones stored in healthy leaves (under dark conditions) and the ability to synthesize photosynthates and hormones as determined by leaf photosynthetic area (under light conditions) determines the ARF ability. Therefore, leaf size and health status are the factors that need to be considered to affect the uniformity of ARF.
The importance of auxin supply in ARF of grape LP
In this study, grape LP could generate roots without adding exogenous auxin, suggesting that LP explants could provide endogenous auxin to meet the need for AR formation. On medium A + S, different concentrations of NPA (0.34, 3.36, and 10.09 µM) were added and cultured under dark conditions. The results showed that the inhibition of ARF by NPA was related to concentration. With increasing concentration, the inhibitory effect of ARF became more obvious, and 10.09 µM NPA completely inhibited rooting (Fig. 4(a)). On medium A, the light culture showed that 0.34 µM NPA had no noticeable inhibition of ARF, while 3.36 µM and 10.09 µM NPA could completely inhibit rooting (Fig. 4(b)). IBA was added to the medium A at 0.48 and 4.82 µM, respectively, and cultured under darkness. The results showed that 0.48 µM IBA significantly promoted ARF, and the rooting rate stabilized to 78.90%±10.30% after 11 DAC, whereas 4.82 µM IBA (36.70%±19.60%) had a less promoting effect on ARF than 0.48 µM IBA (Fig. 4(c)). In addition, the adventitious roots produced after IBA treatment continuously were different from the control, showing an increase in the number of roots, rooting sites were not limited to the base of the petiole, and AR elongation slowed down (Fig. 4(d) and 4(e)).
The effects of auxin, sucrose, and light on ARF of grape LP
The rooting rates of LP cultured on A + S with 0.34, 3.36, or 10.09 µM NPA in dark or light were shown in (a) and (b), respectively. The rooting rate of LP cultured in A with 0.48 or 4.82 µM IBA under dark conditions was shown in (c), and the state of LP after rooting was presented in (d). The arrow shows where AR occurs at 30 DAC. Scale bars: 1 cm. The number and length distribution of ARs of LP on medium A and A with IBA cultured in the dark at 20 DAC were shown in (e). Different lowercase letters in each graph indicate significant differences (p ≤ 0.05). The rooting rate of LP cultured on A + S with 0.34, 3.36, or 10.09 µM NPA in light was shown in (f)
Light inhibited rooting in the presence of exogenous sucrose
Higher plants can integrate multiple signal transduction pathways, such as light, auxin, and reactive oxygen, to finely regulate adventitious root formation to adapt to changing environmental conditions (Bai et al. 2020). Light exerts a strong influence on multiple aspects of the auxin system, controlling auxin level, transport, and responsiveness (Halliday et al. 2009). Sugar and light signal transduction is associated with auxin biosynthesis and root distribution changes (Garcia-Gonzalez et al. 2021). On medium A and under light culture, leaves could produce photosynthetic products through photosynthesis, partially satisfying ARF (Fig. 3(d)). However, as mentioned above, damage to leaves caused by high light intensity will affect ARF (Fig. 2(h)). In the medium supplemented with sucrose (A + S), the light was controlled at 4000 lx to prevent damage from strong light. After 20 DAC, only a few LP explants (4.40–5.60%) rooted, and the initiation of rooting was significantly delayed (F = 11 DAC) (Fig. 4(f)). Since the addition of sucrose may promote auxin synthesis, it is not clear whether the difficulty of rooting is due to the influence of auxin homeostasis (possibly including high concentration or polar transport). Under light conditions, on the medium A + S with 3.36 or 10.09 µM NPA (A + S + NPA3.36 and A + S + NPA10.09), LP did not root. However, LP in A + S + NPA0.34 can root (57.78%±8.75% at 20 DAC), which is significantly higher than the control A + S (Fig. 4(f)). Since NPA inhibits polar auxin transport, it is speculated that the difficulty of rooting A + S under light culture may be related to excessive polar auxin transport. In conclusion, light-induced inhibition of ARF of LP explants in grapes after sugar addition, while a low concentration of NPA partially relieved this inhibition, suggests that light and sugar signals may interact, but molecular biological evidence is needed.
Investigation of PGRs affecting ARF phenotype of LP
Exogenous hormones may promote or inhibit rooting and may be dose- or species-dependent (Bannoud and Bellini 2021). Based on the established LP study system for rapid and stable ARF phenotype identification, this study preliminarily investigated the effect of exogenous PGRs on grape ARF. Under dark conditions, LP explants could not root on medium A + S with 2.60 µM GA3 or 4.40 µM 6-BA. Meanwhile, 24-epibrassinolide (EBR) (1.87 µM) also showed significant inhibition of ARF, and ABA had no significant effect on ARF (Fig. 5(a)). The base of the LP petiole treated with 6-BA expanded significantly (Fig. S4). Lower concentrations of GA3 (0.03 and 0.26 µM) have also been found to inhibit rooting completely (Fig. 5(f)). SBS, SBS-L, and woody cuttings were treated with GA3 (0.26 µM), and the rooting inhibition effect was also obvious. The results showed that GA3 had a significant inhibitory effect on grape ARF. Studies have shown that the inhibition of ARF by GA may be due to the inhibition of auxin polar transport (Mauriat et al. 2014). Based on this, LP was cultured on A + S with GA3 (0.26 µM) and IBA (4.82 µM) under dark conditions, and partial rooting of LP was found (Fig. 5(b)). It indicates that IBA (4.82 µM) could partially reduce the inhibitory effect of GA3 (0.26 µM).
Investigation of PGRs affecting ARF phenotype of LP
The rooting rate of LP under dark conditions on A + S with 4.40 µM 6-BA, 2.60 µM GA3, 1.87 µM EBR or 3.75 µM ABA medium was shown in (a). The rooting rate of LP under dark conditions on medium A + S with GA3 and/or IBA was shown in (b). The white arrow indicates ARs. The rooting rate of LP under dark conditions on A + S with ET 0.06, 0.59 or 5.88 µM medium was shown in (c). Rooting rates of LP on A + S with MeJA 0.42 or 4.24 µM medium under dark and light conditions were shown in (d) and (e), respectively. The growth state of LP explants cultured on A + S with GA3 0.03, 0.26 or 2.60 µM medium in dark was presented in (f)
Endogenous ethylene and jasmonic acid (JA) have been tested. After culturing on A + S with 0.06, 0.59, or 5.88 µM ethephon (ET) in dark, there were no significant effects on the ARF of grapes (Fig. 5(c)). The rooting rate was reduced after culture with MeJA (0.42 and 4.24 µM) on medium A + S under darkness (Fig. 5(d)). After being cultured on medium A with MeJA (0.42 and 4.24 µM) in light, the rooting rate increased, but there was no significant difference. In addition, leaves treated with MeJA were uniformly yellow (Fig. S4), which may be related to the initiation of systemic defense by MeJA.
Discussion
When culture on medium A + S in dark, grape LP could form AR within 6–7 days, and the rooting rate reached 100% at 13–14 DAC (Fig. 3(e)). LP is the smallest and simplest ARF unit in grapes. Owing to the absence of exogenous mineral nutrients and hormones, and the absence of sprouting, the system eliminated other factors affecting ARF to the maximum extent and could screen key genes for AR induction and initiation more conveniently. We determined the conditions under which LP uniformly produces the ARF phenotype and verified the influence of related factors on ARF. This system can be used to study the factors affecting adventitious root formation, and it offers a better approach to studying adventitious root induction and initiation.
In plants, endogenous auxin and sugars are recognized as key influencing factors of ARF. However, the relationship between the two factors is far from being explained. Under the premise of satisfying the carbohydrate supply, the utilization of NPA confirmed the key role of auxin in grape ARF. NPA is an inhibitor of auxin polar transport, and its concentration on grapes was determined to be greater than or equal to 10.09 µM, which can guarantee complete inhibition of LP rooting (Fig. 4(a) and 4(b)). The rooting rate of LP was increased when IBA was added to medium A for continuous dark culture (Fig. 4(c)). Moreover, the relationship between the effect of auxin on ARF and its concentration was confirmed by quantitative data.
The crucial role of sucrose and its relationship with the light on ARF were revealed in this paper. LP was difficult to root on medium without sucrose in dark, while it was easy to root on medium with 10 g·L-1 sucrose under dark conditions or without sucrose but under light conditions (Figs. 2 and 3). Sucrose can affect auxin homeostasis in the form of signaling molecules (Rolland et al. 2006). However, it has yet to be studied whether sugar only serves as a carbon supply or whether it acts as a signaling molecule on ARF (Ahkami et al. 2013; Bellini et al. 2014) summarized the effects of light on ARF, but based on existing studies, it remains difficult to distinguish the roles of light, carbohydrates, and auxin in ARF. The relationship between sugar, light, and auxin and their function in ARF were embodied in the experiment of this system. Sugar may affect the homeostasis of endogenous auxin together with light by affecting PIF (Sairanen et al. 2012), which may be the justification why light inhibits rooting in the presence of exogenous sugar. These hypotheses will be tested in future studies.
In application, AR can be induced from asexually propagated cuttings. However, when exogenous auxin is added and/or other culture conditions are combined, some species or varieties may fail to achieve the goal of rooting, suggesting that there may be other physiological factors or compounds that inhibit rooting in these plants (Wei et al. 2019). Many studies support the hypothesis that endogenous cytokinin, gibberellin, brassinolide, and jasmonic acid are suppressors of ARF, but this varies depending on species or hormone type and concentration (Druege et al. 2019; Ford et al. 2002; Li 2021; Niu et al. 2013; Mauriat et al. 2014; Mensuali-Sodi et al. 1995). In this study, the application of 6-BA, GA3, and EBR severely inhibited the rooting of grapes, while the function of MeJA on ARF is complex (Fig. 5).
Studies in model plants show that the various hormones and their crosstalk are extremely intricate, but this is not illustrative of the real situation in grapes. This study developed a simple and rapid LP system suitable for the study of AR in grapes, which can assist in explaining the particularity of ARF in grapes. LP materials from tissue culture are readily available. The ARF phenotype of the constructed system was easy to observe, and the rooting rate data were easy to quantify. The design of various processing can facilitate the data analysis, which is conducive to the detailed investigation of ARF influencing factors. Based on these characteristics, we revealed some interesting effects of sucrose and hormone treatment on grape ARF. Much remains to be done, and the system will show its advantages.
References
Ahkami AH, Melzer M, Ghaffari MR, Pollmann S, Ghorbani Javid M, Shahinnia F, Hajirezaei MR, Druege U (2013) Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 238(3):499–517. doi:https://doi.org/10.1007/s00425-013-1907-z
Bai Z, Zhang J, Ning X, Guo H, Xu X, Huang X, Wang Y, Hu Z, Lu C, Zhang L, Chi W (2020) A kinase-phosphatase-transcription factor module regulates adventitious root emergence in Arabidopsis root-hypocotyl junctions. Mol Plant 13(8):1162–1177. doi:https://doi.org/10.1016/j.molp.2020.06.002
Bannoud F, Bellini C (2021) Adventitious rooting in populus species: Update and perspectives. Front Plant Sci 12:668837. doi:https://doi.org/10.3389/fpls.2021.668837
Bellini C, Pacurar DI, Perrone I (2014) Adventitious roots and lateral roots: similarities and differences. Annu Rev Plant Biol 65:639–666. doi:https://doi.org/10.1146/annurev-arplant-050213-035645
Bustillo-Avendano E, Ibanez S, Sanz O, Sousa Barros JA, Gude I, Perianez-Rodriguez J, Micol JL, Del Pozo JC, Moreno-Risueno MA, Perez-Perez JM (2018) Regulation of hormonal control, cell reprogramming, and patterning during de novo root organogenesis. Plant Physiol 176(2):1709–1727. doi:https://doi.org/10.1104/pp.17.00980
Castro PRC, Melotto E, Soares FC, Passos IRS, Pommer CV (1994) Rooting stimulation in muscadine grape cuttings. Sci Agr 51(3):436–445. doi:https://doi.org/10.1590/s0103-90161994000300009
Chen X, Qu Y, Sheng L, Liu J, Huang H, Xu L (2014) A simple method suitable to study de novo root organogenesis. Front Plant Sci 5:208. doi:https://doi.org/10.3389/fpls.2014.00208
da Costa CT, de Almeida MR, Ruedell CM, Schwambach J, Maraschin FS, Fett-Neto AG (2013) When stress and development go hand in hand: main hormonal controls of adventitious rooting in cuttings. Front Plant Sci 4:133. doi:https://doi.org/10.3389/fpls.2013.00133
Di Battista F, Maccario D, Beruto M, Grauso L, Lanzotti V, Paolo Curir P, Monroy F (2019) Metabolic changes associated to the unblocking of adventitious root formation in aged, rooting-recalcitrant cuttings of Eucalyptus gunnii Hook. Plant Growth Regul 89:73–82. doi:https://doi.org/10.1007/s10725-019-00515-0. Myrtaceae
Druege U, Hilo A, Perez-Perez JM, Klopotek Y, Acosta M, Shahinnia F, Zerche S, Franken P, Hajirezaei MR (2019) Molecular and physiological control of adventitious rooting in cuttings: phytohormone action meets resource allocation. Ann Bot 123(6):929–949. doi:https://doi.org/10.1093/aob/mcy234
Ford YY, Taylor JM, Blake PS, Marks TR (2002) Gibberellin A3 stimulates adventitious rooting of cuttings from cherry (Prunus avium). Plant Growth Regul 37:127–133. doi:https://doi.org/10.1023/A:1020584627919
Garcia-Gonzalez J, Lacek J, Retzer K (2021) Dissecting hierarchies between light, sugar and auxin action underpinning root and root hair growth. Plants (Basel) 10(1):111. doi:https://doi.org/10.3390/plants10010111
Gonin M, Bergougnoux V, Nguyen TD, Gantet P, Champion A (2019) What makes adventitious roots? Plants (Basel) 8(7):240. doi:https://doi.org/10.3390/plants8070240
Halliday KJ, Martinez-Garcia JF, Josse EM (2009) Integration of light and auxin signaling. Cold Spring Harb Perspect Biol 1(6):a001586. doi:https://doi.org/10.1101/cshperspect.a001586
Jaleta A (2019) A Review on the effect of rooting media on rooting and growth of cutting propagated grape (Vitis vinifera L). World J Agric Soil Sci 3(4):1–8. doi:https://doi.org/10.33552/wjass.2019.03.000567
Joshi M, Ginzberg I (2021) Adventitious root formation in crops-potato as an example. Physiol Plant 172(1):124–133. doi:https://doi.org/10.1111/ppl.13305
Kaur S, Cheema SS, Chhabra BR, Talwar KK (2002) Chemical induction of physiological changes during adventitious root formation and bud break in grapevine cuttings. Plant Growth Regul 37:63–68. doi:https://doi.org/10.1023/A:1020355505105
Keeley K, Preece JE, Taylor BH, Dami IE (2004) Effects of high auxin concentrations, cold storage, and cane position on improved rooting of Vitis aestivalis Michx. Norton cuttings. Am J Enol Viticult 55(3):265–268. doi:https://doi.org/10.1021/bk-2004-0887.ch012
Kracke H, Cristoferi G, Marangoni B (1981) Hormonal changes during the rooting of hardwood cuttings of grapevine rootstocks. Am J Enol Viticult 32(2):135–137. doi:https://doi.org/10.1016/S0065-2628(08)60296-7
Li SW (2021) Molecular bases for the regulation of adventitious root generation in plants. Front Plant Sci 12:614072. doi:https://doi.org/10.3389/fpls.2021.614072
Mauriat M, Petterle A, Bellini C, Moritz T (2014) Gibberellins inhibit adventitious rooting in hybrid aspen and Arabidopsis by affecting auxin transport. Plant J 78(3):372–384. doi:https://doi.org/10.1111/tpj.12478
Mensuali-Sodi A, Panizza M, Tognoni F (1995) Endogenous ethylene requirement for adventitious root induction and growth in tomato cotyledons and lavandin microcuttings in vitro. Plant Growth Regul 17:205–212. doi:https://doi.org/10.1007/BF00024727
Niu SH, Li ZX, Yuan HW, Fang P, Chen XY, Li W (2013) Proper gibberellin localization in vascular tissue is required to regulate adventitious root development in tobacco. J Exp Bot 64(11):3411–3424. doi:https://doi.org/10.1093/jxb/ert186
Omoarelojie LO, Kulkarni MG, Finnie JF, van Staden J (2021) Strigolactone inhibits hydrogen peroxide and plasma membrane H+-ATPase activities to downregulate adventitious root formation in mung bean hypocotyls. Plant Growth Regul 94:11–21. doi:https://doi.org/10.1007/s10725-021-00691-y
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57(1):675–709
Sairanen I, Novak O, Pencik A, Ikeda Y, Jones B, Sandberg G, Ljung K (2012) Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. Plant Cell 24(12):4907–4916. doi:https://doi.org/10.1105/tpc.112.104794
Sharma M, Singh D, Saksena HB, Sharma M, Tiwari A, Awasthi P, Botta HK, Shukla BN, Laxmi A (2021) Understanding the intricate web of phytohormone signalling in modulating root system architecture. Int J Mol Sci 22(11):5508. doi:https://doi.org/10.3390/ijms22115508
Shiozaki S, Makibuchi M, Ogata T (2013) Indole-3-acetic acid, polyamines, and phenols in hardwood cuttings of recalcitrant-to-root wild grapes native to east asia: Vitis davidii and Vitis kiusiana. J Bot 2013:1–9. doi:https://doi.org/10.1155/2013/819531
Smart DR, Kocsis L, Andrew Walker M, Stockert C (2003) Dormant buds and adventitious root formation by vitis and other woody plants. J Plant Growth Regul 21(4):296–314. doi:https://doi.org/10.1007/s00344-003-0001-3
Steffens B, Rasmussen A (2016) The physiology of adventitious roots. Plant Physiol 170(2):603–617. doi:https://doi.org/10.1104/pp.15.01360
Thomas P, Lee MM, Schiefelbein J (2003) Molecular identification of proline-rich protein genes induced during root formation in grape (Vitis vinifera L.) stem cuttings. Plant Cell Environ 26:1497–1504. doi:https://doi.org/10.1046/j.1365-3040.2003.01071.x
Thomas P, Schiefelbein JW (2004) Roles of leaf in regulation of root and shoot growth from single node softwood cuttings of grape (Vitis vinifera). Ann Appl Biol 144(1):27–37. doi:https://doi.org/10.1111/j.1744-7348.2004.tb00313.x
Wei K, Ruan L, Wang L, Cheng H (2019) Auxin-induced adventitious root formation in nodal cuttings of Camellia sinensis. Int J Mol Sci 20(19):4817
Xu L (2018) De novo root regeneration from leaf explants: wounding, auxin, and cell fate transition. Curr Opin Plant Biol 41:39–45. doi:https://doi.org/10.1016/j.pbi.2017.08.004
Zhou Q, Gao B, Li WF, Mao J, Yang SJ, Li W, Ma ZH, Zhao X, Chen BH (2020) Effects of exogenous growth regulators and bud picking on grafting of grapevine hard branches. Sci Hort 264:109186. doi:https://doi.org/10.1016/j.scienta.2020.109186
Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2021YFD1200200), Natural Science Foundation of Hunan Province, China (No. 2021JJ30310), and Research Foundation of Education Department of Hunan Province, China (No. 19B278).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation and data collection were performed by XYC, KZ, YZY, PYN, JM, HL, and SYG. Data analysis was performed by XYC and MB. The first draft of the manuscript was written by GSY and MB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Communicated by Vaclav Motyka.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
XinYu Chang, Kai Zhang and Yunzhang Yuan have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, X.Y., Zhang, K., Yuan, Y. et al. A simple, rapid, and quantifiable system for studying adventitious root formation in grapevine. Plant Growth Regul 98, 117–126 (2022). https://doi.org/10.1007/s10725-022-00838-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-022-00838-5