Abstract
Seed aging is a problem during long-term seed storage, affecting the commercial and germplasm value of seeds. In this study, a widely cultivated rice (Oryza sativa L.) cultivar, Huanghuazhan, was used to investigate the effects of exogenous spermidine (Spd) on accelerated aging (AA). The results showed that the speed of germination and the activities of catalase (CAT), ascorbate peroxidase (APX) and β-amylase were reduced by AA, and more H2O2 accumulated in aged seeds than in normal seeds. As compared with aged seeds pretreated with water, seed vigor and the gibberellic acid (GA) content in aged seeds pretreated with Spd were increased by 47% and 17%, respectively. Furthermore, antioxidant enzyme activity and related gene expression were also higher in aged seeds pretreated with Spd. It is speculated that CAT and APX are the two primary enzymes involved in the effects of Spd to AA. These results suggest that the adverse effect of AA stress on seeds may be partially alleviated by the application of exogenous Spd, which may affect the scavenging of reactive oxygen species.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
As one of the earliest domesticated food crops, rice (Oryza sativa L.) is one of the most important staple foods for over half of the world’s population (Song et al. 2019). and sustains approximately 65% of the population in China (Cao et al. 2016; Feng et al. 2017).
Seeds with high viability are superior in both growth and production potential, which are closely correlated with grain yield and affect the success of agricultural production (Agacka-Mołdoch et al. 2015; Li et al. 2018; He et al. 2019). However, seed viability decreases as they undergo aging, leading to severe economic losses during seed marketing and a loss of germplasm resources (Agacka-Mołdoch et al. 2015). Therefore, studies on the regulation of seed deterioration and the maintenance of seed vitality not only contribute to the production and storage success of rice seed, but also contribute to effective management and conservation strategies for seed under long-term storage, which has great biological value and economic significance.
Numerous studies have investigated exploring the biochemical processes and internal mechanisms of seed deterioration and changes in vigor during storage (Sung and Chiu 1995; Nakabayashi et al. 2005; El-Maarouf-Bouteau et al. 2011; Kocsy 2015; Li et al. 2018). Seed aging has been suggested to be primarily associated with the expression of DNA and protein repair genes, telomere length, epigenetic regulation of DNA methylation, and changes in organelles and nuclear genomes (Fu et al. 2015). Aging is principally manifested as the accumulation of reactive oxygen species (ROS), mitochondrial damage, changes in the antioxidant system and lipid peroxidation (Waterworth et al. 2010; Michalak et al. 2015; Yin et al. 2016; Li et al. 2017). According to the “free radical theory”, the damage caused by excessive accumulation of ROS is part of the underlying mechanism in organism aging (Harman 2006). Higher temperatures, humidities and oxygen concentrations promote the formation of ROS in seeds, leading to the damage to crucial macromolecules, including proteins, lipids, and nucleic acids, which results in the destruction of mitochondria and affects the normal repair of their morphological structure (Benamar et al. 2007; Kocsy 2015). ROS are produced during aerobic cell metabolic processes and by mitochondria (respiratory electron transmission chain), peroxisomes and chloroplasts, and plant life activities are closely related to both important redox signaling pathways and participate in programmed cell death (PCD) (Penfield and King 2009; Waypa et al. 2016). To alleviate the oxidative damage caused by high concentrations of ROS, plants have evolved a complex ROS scavenging mechanism to maintain balance, which includes nonenzymatic (dependent on reductive substances, such as ascorbic acid, glutathione and flavonoids) and enzymatic scavenging mechanisms, including superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), peroxidase (POD), metallothionein (MT) and glutathione reductase (GR) (Apel and Hirt 2004; Qi et al. 2017).
Improving cellular antioxidant capabilities can partially reduce the damage from aging stress. For example, Zhou et al. (2012) expressed lotus-derived MT genes in Arabidopsis thaliana, increasing its SOD activity and thereby enhancing its anti-aging ability and seed viability. In addition, Xu et al. (2015) reported that decreased LOX3 expression could preserve rice grain quality during storage with no impact on grain yield.
Polyamines (PAs), including putrescine (Put), spermidine (Spd) and spermine (Spm), are present in almost all living organisms and function as important modulators (Huang et al. 2017). In many animal studies, PAs have been reported to be closely associated with anti-aging functions (de Cabo and Navas 2016; Bhukel et al. 2017). In plants, PAs are implicated in many physiological processes, including cell division, plant development, differentiation, response to abiotic and biotic stresses and germination (Galston 1990; Bajaj and Rajam 1995; Edreva et al. 2007; Fu et al. 2019). It has been demonstrated that PAs can reduce the injury from salt stress and drought stress during seed germination (Xin et al. 2010; Zheng et al. 2016). Under drought stress, exogenous Spd plays an important function as a stress-protective compound through scavenging of ROS in white clover (Li et al. 2014). Similarly, Spd acts as a free radical scavenger and protects the thylakoid membranes from oxidative damage in osmotically-stressed oat leaves (Besford et al. 1993). In addition, exogenous Spd can promote the accumulation of osmotic regulatory substances, improve the activity of antioxidant enzymes and reduce the accumulation of H2O2 and O2−·, thereby reducing damage resulting from saline and alkaline stress in tomato seedlings (Yi et al. 2014). In a transgenic European pear overexpressing spermidine synthase, the endogenous Spd content was shown to be associated with strong resistance to various stresses (Wen et al. 2009). During seed germination of sweet corn, Spd was demonstrated to promote fast seed germination and high seed vigor, as well as potentially playing an important role in maintaining cell membrane integrity (Huang et al. 2017).
PAs, including Spd, have been suggested to initiate plant defense systems and improve plant adaptability to various stress. However, there is little information regarding the role of exogenous Spd in the aging resistance of mature rice seeds. Therefore, in the present study, we applied exogenous Spd to mature seeds of the common rice cultivar, Huanghuazhan (HHZ), before accelerated aging (AA), a widely adopted method for testing seed vigor and longevity (Bailly et al. 1996; Li et al. 2017). Subsequently, the seed germination, phytohormone content, seedling growth, antioxidant activity and expression level of several genes (CATa, CATb, CATc, SOD1, SOD3, APX1, APX2, APX3 and APX4) involved in antioxidant systems were investigated to determine whether Spd has an anti-aging effect in stored rice seeds.
Materials and methods
Pretreatment and accelerated aging (AA)
The experiment was performed under laboratory conditions using the Indica rice (O. sativa L.) cultivar, Huanghuazhan (HHZ), as the study material. Seeds and Spd were obtained from the JinSeNongHua Seed Industry Co., Ltd., Hunan, People's Republic of China, and the Sinopharm Chemical Reagent Co., Ltd., Shanghai, People's Republic of China, respectively. Seeds without pretreatment and AA were used as controls (CK). Seeds pretreated with water or 0.5 mM Spd before AA were referred to as WBA and SBA, respectively. Mature seeds harvested in 2016 were weighed and then soaked in water or 0.5 mM Spd for 12 h (Huang et al. 2017). Subsequently, the pretreated seeds were air-dried at 28 °C for at least 3 days until recovering to their original weight. In the AA treatment, seeds were placed in an aging box (Thermo Fisher Scientific, incubator Model 3111, USA) at 43 °C for 4 days with a high relative humidity of 98 ± 1%, which was controlled using a saturated K2SO4 solution (Li et al. 2017). After AA treatment, seeds were dried at 28 °C for another 2 days before germination.
Seed germination and seedling growth
Before germination, 50 seeds with 3 replicates of each treatment were initially surface sterilized with 0.5% NaClO for 15 min, after which they were washed with tap water (Hu et al. 2017), and then incubated in 120 × 120 × 60 mm transparent boxes with 3 layers of moistened filter paper. The germination boxes were then placed in a germination chamber with a diurnal cycle of 8 h of light (30 °C) and 16 h of darkness (20 °C) for 14 days (Zhu et al. 2016; Hu et al. 2017). Water was supplied to each germination box daily to maintain the moisture level of the filter paper. Germinated seeds were counted daily, and seeds were considered to have germinated if the radicle reached half of the length of the seed. After 14 days, the germination energy (GE), mean germination time (MGT), germination index (GI) and germination percentage (GP) were calculated. GE and GP were calculated on days 4 and 14, respectively. MGT and GI were calculated as follows: GI = Σ(Gt/Dt) and MGT = Σ(Gt × Dt)/ΣGt, where Gt is the number of germinated seeds at Dt and Dt is the time corresponding to the record date since the beginning of imbibition (He et al. 2019). Ten seedlings in each replicate were manually evaluated on day 14, and the dry weight of the 10 seedlings was measured after drying at 80 °C for 24 h.
Determination of plant hormone contents
The extraction, purification and determination of endogenous levels of abscisic acid (ABA) and gibberellic acid (GA) were carried out according to Wang et al. (2012), Wu et al. (2019) and Song et al. (2019). At the early stage of germination, approximately 0.5 g of seeds were sampled and quickly ground into fine powder in liquid nitrogen. Subsequently, 10 mL of 80% (v/v) methanol with 1 mM butylated hydroxytoluene (an antioxidant) was added to each sample, and the samples were incubated for 4 h in a refrigerator at 4 °C. After that, the mixtures were centrifuged at 5000 × g for 15 min at 4 °C, and the supernatants were separated and purified using a ChromoSep C18 column (C18 Sep-Pak Cartridge, Waters, USA). Then, the extracts were dried using blowing nitrogen and then dissolved in 2 mL of phosphate buffer saline (PBS, pH 7.5) containing 0.1% (v/v) Tween 20 and gelatin. ELISA was performed to determine the contents of the hormones in the samples. Mouse monoclonal antigen and antibodies against free ABA and GA were produced at the Center of Crop Chemical Control of China Agricultural University, as previously described (Weiler et al. 1981). The specificity of the monoclonal antibody and other possible nonspecific immunoreactive interferences were previously investigated and shown to be reliable in the present study, and the quantifications of the two hormones were performed as described by Yang et al. (2001) and Wang et al. (2012). In the present study, the recovery rates during extraction were calculated according to internal standards and analyses and were above 90%.
Measurements of MDA content, CAT, SOD and APX activities
On the 3rd day of imbibition, approximately 0.3 g (fresh weight, FW) of seed samples in each replication were ground in 3 mL of phosphate buffered saline (PBS, 0.05 M, pH 7.8) in an ice bath. Then, the mixtures were centrifuged at 4000 × g for 15 min. The supernatants were stored at 4 °C for subsequent assays. The catalase (CAT) activity was determined according to the methods described by Zhu et al. (1990) and superoxide dismutase (SOD) activity was determined according Kraus and Fletcher (1994). In addition, ascorbate peroxidase (APX) activity was measured according to Yoshiyuki and Kozi (1981) and the malondialdehyde (MDA) contents were assayed via the thiobarbituric acid reaction, according to the method of Draper and Hadley (1990).
Measurements of H2O2
The determination of H2O2 in seeds was performed according to Sergiev et al. (1997). First, each 0.2 g of sample was ground with 1.5 mL of 0.1% trichloroacetic acid in an ice bath, after which the mixture was centrifuged at 8000 × g for 15 min at 4 °C. Then, 0.5 mL of the supernatant of each sample was transferred and added to 0.5 mL of PBS and 1 mL of KI, followed by incubation at 28 °C for 1 h to allow for the chromogenic reaction. Finally, the absorption of the samples was measured at 390 nm.
Measurements of α-amylase and β-amylase activity
To measure (α- and β-) amylase activities, 0.1 g of seeds from each replicate were added to 0.8 mL of distilled water and finely ground. The homogenates were then extracted for 15 min at room temperature, with blending every 5 min. Subsequently, the samples were centrifuged at 6000 × g for 10 min at room temperature, after which the supernatants were separated and diluted to 10 mL for amylase extraction. Then, 1 mL of these samples were diluted in 4 mL of distilled water as a diluent to determine the (α + β) amylase activities, which was measured using the 3,5-dinitrosalicylic acid colorimetric method at 540 nm, as described by Li (2000).
Determination of gene expression via quantitative real-time PCR
The expression levels of genes were measured by quantitative real-time polymerase chain reaction (qRT-PCR). The specific primers used for qRT-PCR were designed using NCBI Primer-Blast (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi) as shown in Table 1, and OsActin was used as an internal control. Total RNA was extracted using RNAiso Plus (Takara, Japan) and synthesized to cDNA using the PrimeScript RT Reagent Kit with gDNA Eraser (Takara, Japan) according to the manufacturer’s instructions. Seed samples were pretreated with Fruit-mate™ for RNA purification (Takara, Japan) to remove impurities such as polysaccharides. The transcript levels of genes were measured on the CFX 96 Real-time PCR system (Bio-Rad, USA) using SYBR green. The cycling conditions were as follows: denaturation at 95 °C for 120 s followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s, with a final cycle of 95 °C for 5 s, from 65 to 95 °C for melting curve with increment of 0.5 °C, and 95 °C for 5 s. Expression levels were determined according to the 2−∆∆t method with a cycle threshold (Ct) value determined for each sample (Livak and Schmittgen 2001); all experiments were repeated at least three times. Finally, data were calculated as the ratio of each treatment to control for the gene expression levels at each imbibition time and were then log-transformed (base 10). Lastly, the data were transformed to a heat map with Excel software (Microsoft, USA).
Data analysis
Statistical analyses were performed using analysis of variance (ANOVA) with the SAS Studio (https://odamid.oda.sas.com/SASStudio/index, SAS Institute, Inc., USA). The percentage data were transformed before analysis using the following equation: y = arcsin [sqr (x/100)]. Values from different parameters were used to calculate the means and separated using the LSD test (α = 0.05).
Results
Spd promotes the germination and seedling growth of AA seeds
As shown in Table 2, AA significantly decreased the seed germination rate. Significantly higher MGTs and lower GIs were observed in aged seeds, while no relevant inhibition of the GPs was observed. Seeds pretreated with Spd (SBA) partially alleviated the damage from AA compared with that of seeds pretreated with water (WBA). Seeds of SBA had significantly higher values for the GI and GE and a lower MGT compared with those of WBA. Moreover, SBA had higher seed vigor and better seedling growth than those of WBA.
Effects of Spd on endogenous hormone contents
The contents of two hormones in the three treatments are shown in Fig. 1. The AA and exogenous Spd application led to various changes in the different types of hormones at an early stage of imbibition. For the GA3 content, there was no significant differences at time 0 among the three treatments. At 12 h after imbibition (HAI), the GA3 contents in seeds of CK increased, while no significant change was observed in those of WBA, seeds of SBA increased after 12 h but less than those of the controls (CK). Regarding ABA, the ABA contents were lower in seeds of WBA and SBA than those of CK at time 0. At 12 HAI, there was no change in seeds of WBA and SBA, whereas reduced ABA contents were observed in seeds of CK, which decreased to the to same levels as those observed in the other treatments.
Changes in the content of GA3 and ABA in response to accelerated aging (AA) and spermidine (Spd). Seed samples were collected at 0 and 12 HAI, and three replications at each time point were used. CK seeds without AA, WBA seeds pretreated with water before AA, SBA seeds pretreated with 0.5 mM Spd before AA, GA3 gibberellin 3, ABA abscisic acid, HAI hours after imbibition. Different lowercase letters on the top of the bars indicate significant differences (LSD, α = 0.05) among treatments. Error bars indicate the standard error of the means (n = 3)
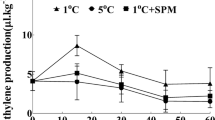
Effects of AA treatment and Spd on the activities of CAT, APX, SOD and amylase, H 2 O 2 production and MDA content
As shown in Table 3 and Fig. 2, on the third day of imbibition, the CAT and APX activities were lower in seeds of WBA than those in the other two treatments, whereas no significant differences in SOD activity were observed in seeds from among the three treatments, and AA reduced the MDA contents below that of CK. In addition, H2O2 levels was higher in imbibed seeds of WBA than those in the other two treatments. Furthermore, the activities of α-amylase activities among the three treatments remained at a similar levels without statistically and were not significantly different. However, significant differences were observed in activities of β-amylase, with WBA exhibiting the lowest value than the other two treatments. After 14 days, no significant differences in activities of CAT and SOD were observed in the seedlings that developed from the seeds among the three treatments. In addition, the APX activities were increased in the aging treatments, with that observed in the SBA being higher than WBA. Furthermore, the aging treatments also increased MDA levels in the seedlings with WBA being higher than SBA.
Effects of spermidine (Spd) application on the content of H2O2 and activities of α + β amylases in seeds subjected to accelerated aging (AA). Seed samples were collected on the third day after imbibition, and three replications at each time point were used. CK seeds without AA, WBA seeds pretreated with water before AA, SBA seeds pretreated with 0.5 mM Spd before AA. Different lowercase letters on the top of the bars indicate significant differences (LSD, α = 0.05) among treatments. Error bars indicate the standard error of the means (n = 3)
Effects of Spd on the expression of genes involved in the antioxidant system
The expression patterns of CAT-, SOD- and APX-related genes varied among the different treatments (Fig. 3). At 12 HAI, the AA treatment improved the expression levels of CATb but decreased those of CATa compared with those in CK, whereas little difference in the expression of CATc was observed between the three treatments, and there appeared to be little effect of Spd at this time point. On the third day after imbibition, expression levels of the three CAT genes in aged seeds were significantly lower than those in CK, with exogenous Spd partially reducing the decrease in CATb and CATc expression. On the 14th day, the expression levels of CATb in seedlings developing from AA seeds were significantly lower than those observed in seedlings from CK, and the expression levels of CATc were increased by 2.3-fold in WBA and by 3.5-fold in SBA. The expression levels of APX1, APX2 and APX3 in SBA and WBA increased to 1-fold higher than those observed in the CK on day 14. Furthermore, the expression of APX3 was significantly improved in SBA at 12 HAI. The expression of APX4 decreased by approximately 30–60% in SBA and WBA at 12 HAI and on the 3rd day, respectively, but increased at the 14th day compared with that observed in CK. For the SOD genes, there were no significant differences in CuZnsod1 (SOD 1) and Fesod1 (SOD 3) among the three treatments on both the 3rd and 14th days, however, Spd improved the expression levels of CuZnsod1 and Fesod1 by 2-fold at 12 HAI.
Expression patterns of CAT-, SOD- and APX-related genes in response to accelerated aging (AA) and spermidine (Spd) application. Seed samples were collected at 12 h, 3 days and 14 days after imbibition, and three replications at each time point were used. CK seeds without AA, WBA seeds pretreated with water before AA, SBA seeds pretreated with 0.5 mM Spd before AA. Data were calculated as the ratio of each treatment to the CK at each imbibition time and then log-transformed (base 10); the blue color means decrease, and the red color stands for a increase. (Color figure online)
Discussion
The viability of seeds during long-term storage will inevitably and continually decrease, and the maintenance of seed vigor during long-term storage is important for both agricultural production and the conservation of germplasm. Many studies have revealed that excessive accumulation of ROS is the key process of seed deterioration, resulting in a decrease in seed vigor (Bailly 2004; Kranner and Colville 2011).
PAs have been reported to play a key role in coping with diverse types of stresses in plants, which may be associated with their involvement in ROS scavenging and in maintaining the stability of proteins, nucleic acids and cell membranes (Galston 1990; Huang et al. 2017). In tomato seedlings, Spd triggers effective protection under salinity–alkalinity stress, probably by maintaining the structural integrity of chloroplasts and alleviating oxidative damage (Li et al. 2015). PAs have also been speculated to be one of the primary response factors for resistance to aging in wheat seeds (Anguillesi et al. 1990). The above results suggested that Spd participates in diverse stress defenses and in ROS scavenging. However, there is little information regarding the role of exogenous Spd in the aging of rice seeds. In the present study, germination tests were performed to determine the degree of seed deterioration under AA conditions. The results showed that seeds subjected to AA had a significantly slower germination rate and poorer seedling development than normal seeds (CK), including lower GE, GI, VI and SL values and higher MGT, which illustrated a decrease in seed vitality. This reduction in seed vigor could be partially restored by exogenous Spd.
GAs and ABA are the most important plant hormones involved in seed germination, where ABA inhibits seeds from germination, while GAs have a stimulatory effect on seed germination (Miransari and Smith 2014). In several plant species, ABA levels have been reported to decrease during seed imbibition (Grappin et al. 2000; Jacobsen et al. 2002). In Arabidopsis, the level of ABA decreased immediately after seed imbibition, reaching the basal level after 12 h (Kushiro et al. 2004). A similar decrease in ABA content was observed from 0 to 12 HAI in CK, but not in the aged seeds. It is speculated that the slight changes in contents of ABA in aged seeds may be responsible for the slower germination in aged seeds. Spd has been reported to decrease ABA levels and improve seed germination and vigor to some extent during the germination of maize seeds (Huang et al. 2017). In this study, Spd treatment resulted in little change in the content of ABA content, and its role in the germination and aging of rice seeds needs further investigation. In the study of Liu et al. (2014) GA3 content increased from 0 to 12 HAI in a non-dormant rice cultivar, G46B, with a faster germination rate, but only slightly fluctuated in rice cultivars, ZH11 and N22, with lower germination rates. Similarly, our results on GA3 content seemed to be in line with those of germination phenotypes, where AA seeds had lower content of GA3 and germination rates than those observed in CK, while Spd could mitigate these reductions in the AA seeds.
During rice seed germination, β-amylase activity has been suggested to be a reliable indicator of the germination ability and vigor, as this activity is absent in extensively deteriorated seeds (Nandi et al. 1995). The results showed that β-amylase activity was decreased in AA seeds on the third day of imbibition, and Spd partially alleviated this decrease suggesting that Spd aids in delaying the aging process. The enzymatic antioxidant system plays a prominent role in the preservation of seed vigor and the regulation of the stress response, including the protection of cellular components against oxidative injury (Miller et al. 2009; Kibinza et al. 2006; Sheteiwy et al. 2016). In previous studies, a low loss of seed viability during storage was shown to be associated with the efficient activities of antioxidant systems, such as SOD and CAT activities (Revilla et al. 2009; Yao et al. 2012). Generally, superoxide radicals are catalyzed to H2O2 by SOD (Raychaudhuri and Deng 2000). H2O2 can be catalyzed to H2O and O2 by CAT (Willekens et al. 1995). In addition, H2O2 scavenging may also take place in the ascorbate-glutathione cycle, which involves APX and GR. APX uses ascorbate to reduce H2O2 to water, along with the generation of monodehydroascorbate and/or dehydroascorbate (Noctor and Foyer 1998; Yao et al. 2012). In addition, the end-product of lipid peroxidation, MDA, is believed to be associated with the loss of seed viability during accelerated aging (Kibinza et al. 2006). Improving the antioxidant system helps to alleviate oxidative stress. It has been demonstrated that exogenous Spd has been shown to induce the antioxidation defense system and reduce the generation of O2.− to protect white clover from water stress and promote seed germination (Zhou et al. 2014). Here, we examined the activities of three primary ROS scavenging enzymes (CAT, SOD, and APX) and the contents of MDA and H2O2 in both imbibed seeds and seedlings. It seems that the activity of SOD was not significantly affected by WBA and SBA. On the third day of imbibition, activities of CAT and APX were reduced in WBA, these results were consistent with the research performed by Yin et al. (2016) who observed significantly decreased CAT and APX activities in aged rice seeds. As shown in our results, Spd increased the activities of CAT and APX in the AA treated seeds. In 14-day old shoots developed from the seeds, activities of APX were much lower than those observed in seeds, with WBA and SBA exhibiting higher activity of APX than CK, whereas no significant differences in CAT activity among the three treatments.
MDA and H2O2 content were used as an index of the degree of oxidative damage (Huang et al. 2017; Li et al. 2018). Our results showed that the MDA content in seeds of CK is significantly higher than those observed in the aged seeds. MDA contents were previously observed to increase during the early stage of seed germination of peas (Yang et al. 2012). Therefore, we speculated that the higher MDA accumulation may be associated with the faster germination rate in CK. For seedlings, WBA had the highest MDA content among the three treatments, which may suggest more oxidative injury in these seedlings. Similarly, the generation of H2O2 was increased by AA on the 3rd day after imbibition, which was consistent with the results of a previous study using tobacco seeds (Li et al. 2018), but this effect could be mitigated by Spd.
The above results revealed that the seed quality and activities of some antioxidant enzymes were significantly increased in AA seeds by Spd. We further investigated the transcriptional regulation of antioxidative enzymes in response to seed aging and Spd in different stages of development, including 12 HAI and 3 and 14 days after imbibition. According to our results, AA treatment increased the expression levels of CATb but decreased those of CATa as compared with those observed in CK. The transcriptional levels observed on the third day was in accordance with the activities of the enzymes. A significant decrease in the expression levels of the three CAT genes was observed in aged seeds, and the reduction in CATb and CATc expression was rescued by exogenous Spd. Similar expression patterns were observed in three APX genes on the third day, namely, APX1, APX2 and APX4. Furthermore, the expression of APX3 was significantly increased in SBA at 12 HAI. In contrast, SOD genes participated more at 12 HAI but not on the 3rd and 14th days. These results were consistent with the observed changes in SOD activity.
In summary, the results of this study suggest that the adverse effect of AA-induced stress on rice seeds can be partially alleviated by the application of Spd. However, further analysis of the resistance mechanisms underlying these anti-aging effects is necessary to elucidate the regulatory and signaling roles of Spd. The utilization of innovative technologies such as omics-based analysis (Zhu et al. 2017; Chen et al. 2020), phylogenetic comparison (Yang et al. 2019) and high throughput phenotyping system may narrow down the target of interests in this research direction. Nevertheless, the present study may also provide guidance for long-term storage during rice production, which may contribute to the production and storage of rice seed. However, progress in understanding seed aging and Spd antioxidation were restricted due to the complexity of metabolic and signaling pathways, and may vary in different genotypes. Therefore, the potential application of Spd in reducing aging-induced damage still requires further study.
Abbreviations
- AA:
-
Accelerated aging
- PAs:
-
Polyamines
- Spd:
-
Spermidine
- CAT:
-
Catalase
- APX:
-
Ascorbate peroxidases
- GA:
-
Gibberellin acid
- ABA:
-
Abscisic acid
- ROS:
-
Reactive oxygen species
- PCD:
-
Programmed cell death
- MDA:
-
Malondialdehyde
- POD:
-
Peroxidase
- MT:
-
Metallothionein
- GR:
-
Glutathione reductase
- H2O2 :
-
Hydrogen peroxide
- GE:
-
Germination energy
- GI:
-
Germination index
- GP:
-
Germination percentage
- HAI:
-
Hours after imbibition
- MGT:
-
Mean germination time
- SL:
-
Seedling length
- DW:
-
Dry weight of 10 seedlings
- qRT-PCR:
-
Quantitative real-time PCR
- CK:
-
Control
- WBA:
-
Seeds treated with water before AA
- SBA:
-
Seeds treated with Spd before AA
References
Agacka-Mołdoch M, Nagel M, Lewis R, Börner A (2015) Mapping quantitative trait loci determining seed longevity in tobacco (Nicotiana tabacum L.). Euphytica 202:1–8
Anguillesi MC, Grilli I, Tazziolo R, Floris C (1990) Polyamine accumulation in aged wheat seeds. Biol Plant 32:189–197
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Bailly C, Benamar A, Corbineau F, Come D (1996) Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiol Plant 97:104–110
Bailly C (2004) Active oxygen species and antioxidants in seed biology. Seed Sci Res 14:93–107
Bajaj S, Rajam MV (1995) Efficient plant regeneration from long-term callus cultures of rice by spermidine. Plant Cell Rep 14:717–720
Benamar A, Tallon C, Macherel D (2007) Membrane integrity and oxidative properties of mitochondria isolated from imbibing pea seeds after priming or accelerated ageing. Seed Sci Res 13:35–45
Besford R, Richardson C, Campos L, Tiburcio A (1993) Effect of polyamines on stabilization of molecular complexes in thylakoid membranes of osmotically stressed oat leaves. Planta 189:201–206
Bhukel A, Madeo F, Sigrist SJ (2017) Spermidine boosts autophagy to protect from synapse aging. Autophagy 13:444–445
Cao YY, Chen YH, Chen MX, Wang ZQ, Wu CF, Bian XC, Yang JC, Zhang JH (2016) Growth characteristics and endosperm structure of superior and inferior spikelets of indica rice under high-temperature stress. Biol Plant 60:1–11
Chen MX, Zhu FY, Gao B, Ma KL, Ye NH, Zhang YJ, Fernie AR, Chen X, Hu QJ, Tian Y, Zhang D, Liu TY, Zhang JH, Liu YG (2020) Full-length transcript-based proteogenomics of rice improves its genome and proteome annotation. Plant Physiol 182:1–17
de Cabo R, Navas P (2016) Spermidine to the rescue for an aging heart. Nat Med 22:1389
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Edreva AM, Velikova VB, Tsonev TD (2007) Phenylamides in plants. Russ J Plant Physl 54:287–301
El-Maarouf-Bouteau H, Mazuy C, Corbineau F, Bailly C (2011) DNA alteration and programmed cell death during ageing of sunflower seed. J Exp Bot 62:5003–5011
Feng F, Li Y, Qin X, Liao Y, Siddique K (2017) Changes in rice grain quality of indica and japonica type varieties released in China from 2000 to 2014. Front Plant Sci 8:1066–1072
Fu Y, Ahmed Z, Diederichsen A (2015) Towards a better monitoring of seed ageing under ex situ seed conservation. Conserv Physiol 3:v26
Fu YY, Gu QQ, Dong Q, Zhang ZH, Lin C, Hu WM, Pan RH, Guan YJ, Hu J (2019) Spermidine enhances heat tolerance of rice seeds by modulating endogenous starch and polyamine metabolism. Molecules 24:1395
Galston AW (1990) Polyamines in Plant Physiology. Plant Physiol 94:406–410
Grappin P, Bouinot D, Sotta B, Miginiac E, Jullien M (2000) Control of seed dormancy in Nicotiana plumbaginifolia: post-imbibition abscisic acid synthesis imposes dormancy maintenance. Planta 210:279–285
Harman D (2006) Free-Radical Theory of Aging. Ann N Y Acad Sci 717:1–15
He Y, Cheng J, He Y, Yang B, Cheng Y, Yang C, Zhang H, Wang Z (2019) Influence of isopropylmalate synthase OsIPMS1 on seed vigor associated with amino acid and energy metabolism in rice. Plant Biotechnol J 17:322–337
Hu Q, Lin C, Guan Y, Sheteiwy MS, Hu W, Hu J (2017) Inhibitory effect of eugenol on seed germination and pre-harvest sprouting of hybrid rice (Oryza sativa L.). Sci Rep 7(1):5295
Huang Y, Lin C, He F, Li Z, Guan Y, Hu Q, Hu J (2017) Exogenous spermidine improves seed germination of sweet corn via involvement in phytohormone interactions, H2O2 and relevant gene expression. BMC Plant Biol 17(1):1
Jacobsen JV, Pearce DW, Poole AT, Pharis RP, Mander LN (2002) Abscisic acid, phaseic acid and gibberellin contents associated with dormancy and germination in barley. Physiol Plant 115:428–441
Kibinza S, Vinel D, Côme D, Bailly C, Corbineau F (2006) Sunflower seed deterioration as related to moisture content during aging, energy metabolism and active oxygen species scavenging. Physiol Plant 128:496–506
Kocsy G (2015) Die or survive? Redox changes as seed viability markers. Plant Cell Environ 38:1008–1010
Kranner I, Colville L (2011) Metals and seeds: Biochemical and molecular implications and their significance for seed germination. Environ Exp Bot 72(1):93–105
Kraus T, Fletcher R (1994) Paclobutrazol Protects Wheat Seedlings from Heat and Paraquat Injury. Is detoxification of active oxygen involved? Plant Cell Physiol 35:45–52
Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, Hirai N, Koshiba T, Kamiya Y, Nambara E (2004) The Arabidopsis cytochrome P450 CYP707A encodes ABA 8 ‘-hydroxylases: key enzymes in ABA catabolism. Embo J 23:1647–1656
Li H (2000) Principle and technology of plant physiological and biochemical experiments. Higher Education Press, Beijing
Li Z, Peng Y, Zhang X, Ma X, Huang L, Yan Y (2014) Exogenous spermidine improves seed germination of white clover under water stress via involvement in starch metabolism, antioxidant defenses and relevant gene expression. Molecules 19:18003–18024
Li J, Hu L, Zhang L, Pan X, Hu X (2015) Exogenous spermidine is enhancing tomato tolerance to salinity-alkalinity stress by regulating chloroplast antioxidant system and chlorophyll metabolism. BMC Plant Biol 15:303
Li T, Zhang Y, Wang D, Liu Y, Dirk LMA, Goodman J, Downie AB, Wang J, Wang G, Zhao T (2017) Regulation of seed vigor by manipulation of raffinose family oligosaccharides in maize and Arabidopsis thaliana. Mol Plant 10:1540–1555
Li Z, Gao Y, Lin C, Pan R, Ma W, Zheng Y, Guan Y, Hu J (2018) Suppression of LOX activity enhanced seed vigour and longevity of tobacco (Nicotiana tabacum L.) seeds during storage. Conserv Physiol 6:y47
Liu Y, Fang J, Xu F, Chu J, Yan C, Schläppi MR, Wang Y, Chu C (2014) Expression patterns of aba and ga metabolism genes and hormone levels during rice seed development and imbibition: a comparison of dormant and non-dormant rice cultivars. J Genet Genomics 41:327–338
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 –∆∆CT Method. Methods 25:402–408
Michalak M, Plitta-Michalak BP, Naskręt-Barciszewska M, Barciszewski J, Bujarska-Borkowska B, Chmielarz P (2015) Global 5-methylcytosine alterations in DNA during ageing of Quercus robur seeds. Ann Bot-London 116:369–376
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2009) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Miransari M, Smith DL (2014) Plant hormones and seed germination. Environ Exp Bot 99:110–121
Nakabayashi K, Okamoto M, Koshiba T, Kamiya Y, Nambara E (2005) Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: Epigenetic and genetic regulation of transcription in seed. Plant J 41:697–709
Nandi S, Das G, Sen-Mandi S (1995) β-Amylase activity as an index for germination potential in rice. Ann Bot 75:463–467
Noctor G, Foyer C (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Phys 49:249–279
Penfield S, King J (2009) Towards a systems biology approach to understanding seed dormancy and germination. Proc R Soc B 276:3561–3569
Qi J, Wang J, Gong Z, Zhou JM (2017) Apoplastic ROS signaling in plant immunity. Curr Opin Plant Biol 38:92–100
Raychaudhuri SS, Deng XW (2000) The role of superoxide dismutase in combating oxidative stress in higher plants. Bot Rev 66:89–98
Revilla P, Butron A, Rodriguez VM, Malvar RA, Ordas A (2009) Identification of genes related to germination in aged maize seed by screening natural variability. J Exp Bot 60:4151–4157
Sergiev I, Alexieva V, Karanov E (1997) Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Comptes Rendus de I’ Académie Bulgare Sciences 51:121–124
Sheteiwy M, Fu Y, Hu Q, Nawaz A, Guan Y, Li Z, Huang Y, Hu J (2016) Seed priming with polyethylene glycol induces antioxidative defense and metabolic regulation of rice under nano-ZnO stress. Environ Sci Pollut R 23:19989–20002
Sheteiwy M, Shen H, Xu J, Guan Y, Song W, Hu J (2017) Seed polyamines metabolism induced by seed priming with spermidine and 5-aminolevulinic acid for chilling tolerance improvement in rice (Oryza sativa L.) seedlings. Environ Exp Bot 137:58–72
Song T, Xu F, Yuan W, Chen M, Hu Q, Tian Y, Zhang J, Xu W (2019) Combining alternate wetting and drying irrigation with reduced phosphorus fertilizer application reduces water use and promotes phosphorus use efficiency without yield loss in rice plants. Agric Water Manage 223:105686
Sung JM, Chiu CC (1995) Lipid peroxidation and peroxide-scavenging enzymes of naturally aged soybean seed. Plant Sci 110:45–52
Wang Y, Li B, Du M, Eneji AE, Wang B, Duan L, Li Z, Tian X (2012) Mechanism of phytohormone involvement in feedback regulation of cotton leaf senescence induced by potassium deficiency. J Exp Bot 63:5887–5901
Waterworth WM, Masnavi G, Bhardwaj RM, Jiang Q, Bray CM, West CE (2010) A plant DNA ligase is an important determinant of seed longevity. Plant J 63:848–860
Waypa GB, Smith KA, Schumacker PT (2016) O2 sensing, mitochondria and ROS signaling: the fog is lifting. Mol Aspects Med 47–48:76–89
Weiler EW, Jourdan PS, Conrad W (1981) Levels of indole-3-acetic acid in intact and decapitated coleoptiles as determined by a specific and highly sensitive solid-phase enzyme immunoassay. Planta 153:561–571
Wen XP, Ban Y, Inoue H, Matsuda N, Moriguchi T (2009) Aluminum tolerance in a spermidine synthase-overexpressing transgenic European pear is correlated with the enhanced level of spermidine via alleviating oxidative status. Environ Exp Bot 66:471–478
Willekens H, Inzé D, Montagu MV, Camp WV (1995) Catalases in plants. Mol Breeding 1:207–228
Wu Q, Du M, Wu J, Wang N, Wang B, Li F, Tian X, Li Z (2019) Mepiquat chloride promotes cotton lateral root formation by modulating plant hormone homeostasis. BMC Plant Biol 19:573
Xin SQ, Gao Y, Zhao JM, Liu XM (2010) Effect of seed soaking in spermidine (Spd) under salt stress on rice seed germination. North Rice 40(6):23–30
Xu H, Wei Y, Zhu Y, Lian L, Xie H, Cai Q, Chen Q, Lin Z, Wang Z, Xie H, Zhang J (2015) Antisense suppression of LOX3 gene expression in rice endosperm enhances seed longevity. Plant Biotechnol J 13:526–539
Yang S, Jianchun X, Quanfa L (2012) Oxidative response and antioxidative mechanism in germinating soybean seeds exposed to cadmium. Int J Environ Res Public Health 9:2827–2838
Yang JF, Chen MX, Zhang JH, Hao GF, Yang GF (2020) Genome-wide phylogenetic and structural analysis reveals the molecular evolutionary mechanisms of ABA receptor gene family. J Exp Bot 71:1322–1336
Yang Y, Xu CN, Wang BM, Jia JZ (2001) Effects of plant growth regulators on secondary wall thickening of cotton fibres. Plant Growth Regul 35:233–237
Yao Z, Liu L, Gao F, Rampitsch C, Reinecke DM, Ozga JA, Ayele BT (2012) Developmental and seed aging mediated regulation of antioxidative genes and differential expression of proteins during pre- and post-germinative phases in pea. J Plant Physiol 169:1477–1488
Yi Z, Li Z, Hu XH (2014) Exogenous spermidine-induced changes at physiological and biochemical parameters levels in tomato seedling grown in saline-alkaline condition. Bot Stud 55:1–8
Yin G, Whelan J, Wu S, Zhou J, Chen B, Chen X, Zhang J, He J, Xin X, Lu X (2016) Comprehensive mitochondrial metabolic shift during the critical node of seed ageing in rice. PLoS ONE 11:e148013
Yoshiyuki N, Kozi A (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Zheng M, Tao Y, Hussain S, Jiang Q, Peng S, Huang J, Cui K, Nie L (2016) Seed priming in dry direct-seeded rice: consequences for emergence, seedling growth and associated metabolic events under drought stress. Plant Growth Regul 78:167–178
Zhou Y, Chu P, Chen H, Li Y, Liu J, Ding Y, Tsang EWT, Jiang L, Wu K, Huang S (2012) Overexpression of Nelumbo nucifera metallothioneins 2a and 3 enhances seed germination vigor in Arabidopsis. Planta 235:523–537
Zhou L, Yan P, Li Z, Peng Y, Zhang XQ, Pan MH, Ma X, Huang LK, Yan YH (2014) Exogenous spermidine improves water stress tolerance of white clover (Trifolium repens L.) involved in antioxidant defence, gene expression and proline metabolism. Plant Omics 7:517–526
Zhu GL, Zhong HW, Zhang AQ (1990) The experiments of plant physiology. The Peking University Press, Beijing
Zhu LW, Cao DD, Hu QJ, Guan YJ, Hu WM, Nawaz A, Hu J (2016) Physiological changes and sHSPs genes relative transcription in relation to the acquisition of seed germination during maturation of hybrid rice seed. J Sci Food Agric 96:1764–1771
Zhu FY, Chen MX, Ye NH, Shi L, Ma KL, Yang JF, Cao YY, Zhang YJ, Yoshida T, Fernie AR, Fan GY, Wen B, Zhou R, Liu TY, Fan T, Gao B, Zhang D, Hao GF, Xiao S, Liu YG, Zhang JH (2017) Proteogenomic analysis reveals alternative splicing and translation as part of the abscisic acid response in Arabidopsis seedlings. Plant J 91:518–533
Acknowledgements
This work was supported by Grants from Shenzhen Science Technology and Innovation Commission (International Collaborative Funding), the China Postdoctoral Science Foundation (2018M633162 and 2019M663122), the National Natural Science Foundation of China (Nos. 31201279, 31371708 and 31671774), and the Jiangsu Collaborative Innovation Center for Modern Crop Production, People's Republic of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, QJ., Chen, MX., Song, T. et al. Spermidine enhanced the antioxidant capacity of rice seeds during seed aging. Plant Growth Regul 91, 397–406 (2020). https://doi.org/10.1007/s10725-020-00613-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-020-00613-4