Abstract
Ca2+-dependent protein kinases (CDPKs or CPKs) are essential primary sensors of Ca2+ in plants and are known to play important roles in plant abiotic and biotic stress responses. Vitis amurensis is a wild grapevine species with a high level of cold and disease resistance. It has previously been shown that transcription of 10 CDPK genes of V. amurensis was elevated under salt, desiccation, high mannitol, cold, and heat stress conditions. Expression of VaCPK29 was induced under high and low temperatures, water deficit, and high mannitol stress in plant cuttings of V. amurensis. The present study revealed that the callus cell cultures of V. amurensis and soil-grown plants of Arabidopsis thaliana overexpressing VaCPK29 exhibited higher tolerance to heat and high mannitol stress in comparison with the control transformed with the empty vector. Cold, salt, and drought stress tolerance of the transgenic V. amurensis calli and A. thaliana plants was comparable to that of the controls. The stress-responsive genes AtDREB1A, AtDREB2A, AtRD29A, AtRD29B, and AtABF3 were up-regulated in the VaCPK29-overexpressing A. thaliana plants under heat stress. Taken together, the data indicate that the VaCPK29 gene may act as a positive regulator in the grapevine response to heat and osmotic stresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple environmental and developmental factors such as hormones, light, or environmental stresses initiate stimulus-specific elevations in cytoplasmic Ca2+ concentration ([Ca2+]). The spatially- and temporally-distinct changes in [Ca2+], designated as “Ca2+ signatures”, are also characterized by a specific amplitude, frequency, sub-cellular location, duration, and shape and are considered to induce proper responses to environmental or developmental signals (Reddy et al. 2011; Batistič and Kudla 2012). Perturbations in [Ca2+] are recognized, decoded, and transmitted by a number of Ca2+ sensor proteins including calmodulin (CaM), calmodulin-like proteins (CMLs), Ca2+-dependent protein kinases (CDPKs), calcineurin B-like proteins (CBLs), and their interacting kinases (CIPKs) (Hashimoto and Kudla 2011; Batistič and Kudla 2012). CDPKs (EC 2.7.1.37) are Ca2+-regulated Ser/Thr protein kinases encoded by a large multigene family that can be found in plants, green algae, protists, and oomycetes (Valmonte et al. 2014). CDPKs are composed of a N-terminal variable domain, a kinase or catalytic domain, and a CDPK activation domain (CAD) that includes a pseudosubstrate segment and a CaM-like Ca2+-binding domain (Liese and Romeis 2013). The pseudosubstrate segment is known to inhibit phosphorylation and keeps CDPKs inactive at low Ca2+ concentrations (Harper et al. 1994; Wernimont et al. 2010; Liese and Romeis 2013). Upon Ca2+ entry, CDPKs perceive the information encoded in the Ca2+ signatures and translate it into the phosporylation of specific target proteins (Hashimoto and Kudla 2011).
An increasing body of evidence has shown that CDPKs are important players in plant abiotic and biotic stress responses, immune signaling, and development (Asano et al. 2012a; Schulz et al. 2013; Boudsocq and Sheen 2013). The transcription levels and kinase activities of CDPKs are affected by various environmental stress conditions (Jaworski et al. 2011; Das and Pandey 2010; Franz et al. 2011; Dubrovina et al. 2013; Zhang et al. 2015). Various plant species have been transformed with certain CDPK genes from Arabidopsis thaliana, Oryza sativa, Populus euphratica, or Zea mays. Overexpression of these CDPK genes improved plant tolerance to various abiotic stresses (Zou et al. 2010; Asano et al. 2012b; Chen et al. 2013b; Wei et al. 2014). On the contrary, CDPK overexpression has also been shown to confer greater stress sensitivity (Ma and Wu 2007; Franz et al. 2011; Weckwerth et al. 2015). Thus, CDPKs are implicated in both positive and negative regulation of plant abiotic stress adaptation. Activated CDPKs have been shown to phosphorylate proteins (e.g., membrane channels, NADPH oxidase, or transcription factors) involved in stomatal movements, oxidative burst, and gene expression regulation (Choi et al. 2005; Kobayashi et al. 2007; Geiger et al. 2010). Nevertheless, the precise physiological functions of most plant CDPKs are still not clear.
Cultivated grapevine Vitis vinifera L. is an important fruit crop worldwide and represents a valuable source for wine production. Whole-genome sequencing of V. vinifera cv. PN40024 demonstrated the presence of 17 or 19 CDPK genes in its genome, depending on the study (Velasco et al. 2007; Jaillon et al. 2007; Chen et al. 2013a; Zhang et al. 2015). Several initial studies of Vitaceae CDPKs focused on VvCPK7 (ACPK1) of V. vinifera and indicated that the gene is involved in fruit development and seed germination but not in abiotic stress responses (Shen et al. 2004; Yu et al. 2006, 2007). Later, Chen et al. (2013a) identified 17 CDPK genes in the 12x genome sequence of V. vinifera and analyzed the expression profiles of these CDPKs in various grapevine organs at different developmental stages and under certain abiotic stress conditions using the publicly available Affymetrix microarray data. Some V. vinifera CDPKs (VvCPK3, 7, 11, 12, 13, 14, 17) were found to be up-regulated in response to drought and salt stresses (Chen et al. 2013a). In a recent study, Zhang et al. (2015) identified 19 CDPK genes of V. vinifera during a genome-wide analysis of the grapevine 12x genome. The authors also analyzed the expression of the 19 homologous CDPK genes in the wild grape V. pseudoreticulata under various abiotic, biotic, and phytohormone treatments using quantitative real-time RT-PCR (qRT-PCR). The analysis revealed that a large number of VpCDPK genes were markedly up-regulated under the tested stress conditions, indicating the important roles VpCPKs play in abiotic and biotic stress resistance to V. pseudoreticulata. We recently identified 13 CDPK genes in the wild grapevine V. amurensis Rupr. and characterized their organ-specific and stress-induced expression patterns (Dubrovina et al. 2013). The wild grapevine V. amurensis possesses a remarkable abiotic and biotic stress tolerance, especially to freezing, drought, and microbial pathogens, and is currently used for wine production and as a breeding parent (Ma et al. 2010; Liu and Li 2013).
According to our data, transcription of 10 CDPK genes of V. amurensis (VaCPK1, 2, 3, 9, 13, 16, 20, 21, 26, and 29) was induced under different abiotic stress treatment conditions, including water deficit, high salinity, as well as high mannitol and temperature stresses. Expression of the remaining three CDPK genes (VaCPK3a, 25, and 30) was not responsive to these stresses (Dubrovina et al. 2013). Overexpression of the VaCPK20 gene in callus cell cultures of V. amurensis and in transgenic plants of A. thaliana improved resistance to cold and drought stresses, while overexpression of the VaCPK21 gene improved resistance to salt stress, demonstrating that the VaCPK20 and VaCPK21 genes may play a role in the positive regulation of signaling pathways involved in cold, drought, and salt stress adaptation in V. amurensis (Dubrovina et al. 2015, 2016a). In similar overexpression experiments, the VaCPK3a and VaCPK9 genes were shown to function in growth regulation of V. amurensis but not in its abiotic stress adaptation (Kiselev et al. 2013; Dubrovina et al. 2016b). Nevertheless, the functions of most grape CDPKs in wild grape abiotic stress adaptation are largely unknown at the present time.
This study aimed to investigate the involvement of the CPK29 gene of V. amurensis in the plant’s adaptation to abiotic stress. Transcription of the VaCPK29 gene was found to be elevated under desiccation, high mannitol, as well as cold and heat stresses in the plant cuttings of V. amurensis (Dubrovina et al. 2013), therefore VaCPK29 has been suggested to function in stress resistance of V. amurensis. Transgenic callus cultures of V. amurensis and plants of A. thaliana overexpressing the VaCPK29 gene exhibited increased resistance under heat stress and mannitol-induced osmotic stress. Thus, VaCPK29 may play a role in the positive regulation of the signaling pathways involved in heat and osmotic stress resistance in V. amurensis.
Materials and methods
Plant materials and growth conditions
The V2 callus culture of wild grapevine V. amurensis Rupr. (Vitaceae) was established in 2002 as described previously (Kiselev et al. 2007). The KA-0 empty vector-transformed callus cell culture was obtained in 2012 by co-cultivation of the V2 cell suspension with Agrobacterium tumefaciens GV3101::pMP90 strain containing pZP-RCS2-nptII (Tzfira et al. 2005), which contained only the kanamycin (Km) resistance gene, nptII, under the control of the double cauliflower mosaic virus (CaMV 35S) promoter as described previously (Kiselev et al. 2007, 2013). The VaCPK29-transgenic callus cell cultures of V. amurensis (designated KA10-I, KA10-II, KA10-III, and KA10-IV) were obtained in 2013 by transformation of the V2 cell suspension with A. tumefaciens strain GV3101::pMP90 containing pZP-RCS2-VaCPK29-nptII as described previously (Aleynova et al. 2015). The grape callus cell cultures were cultivated at 30-day subculture intervals in the dark as described (Kiselev et al. 2009; Aleynova et al. 2015). Plants (Arabidopsis thaliana ecotype Columbia L., stored by our lab) were grown in pots filled with commercially available rich soil in a controlled environmental chamber at 22 °C (Sanyo MLR-352, Panasonic, Japan) kept on a 16/8 h day/night cycle at a light intensity of ~120 μmol m−2 s−1.
Isolation and sequencing of VaCPK29
Full-length cDNA coding sequence (1638 bp) of VaCPK29 (VaCPK1a) was amplified using primers based on the predicted sequence of VvCPK29-like gene of V. vinifera (GB acc. no KC488317), subcloned, and sequenced previously (Dubrovina et al. 2013; Aleynova et al. 2015). Multiple sequence alignments were done with the BioEdit 7.0.8 program (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). The amino acid sequence homology analysis of VaCPK29 and other plant CDPKs was performed using NCBI BLAST (http://blast.ncbi.nlm.nih.gov) by blastp algorithm (protein–protein BLAST). PROSITE (http://prosite.expasy.org/) was used to analyze domain structure of the CDPK proteins.
Overexpression of VaCPK29 in cell cultures of V. amurensis
The plasmid constructions for plant cell transformations were obtained previously as described (Aleynova et al. 2015). Briefly, to generate the construction, the full-length cDNA of VaCPK29 (GB acc. no KC488317) was amplified by PCR as described previously (Dubrovina et al. 2013; Aleynova et al. 2015). The full-length cDNA of VaCPK29 was cloned into the pSAT1 vector under the control of the double CaMV 35S promoter (Tzfira et al. 2005). Then, the expression cassette with VaCPK29 was cloned from pSAT1 into the pZP-RCS2-nptII (Tzfira et al. 2005). Plasmid DNA samples (pSAT1 and pZP-RCS2-nptII) were kindly provided by Professor Alexander Krichevsky (State University of New York, Stony Brook, USA). The overexpression construct of VaCPK29 (pZP-RCS2-VaCPK29-nptII) or empty vector (pZP-RCS2-nptII) was introduced into the A. tumefaciens strain GV3101::pMP90 and transformed into the V. amurensis suspension culture V2 by co-cultivation with the bacterial cells as described (Aleynova-Shumakova et al. 2014; Aleynova et al. 2015). Transgenic callus cell cultures were selected as described (Aleynova-Shumakova et al. 2014; Aleynova et al. 2015).
Salt, cold, and heat treatments of transgenic callus cell cultures
Growth analysis and abiotic stress treatments of transgenic callus cell cultures were conducted as described (Dubrovina et al. 2015, 2016a). Briefly, salt treatment was applied by adding 50 and 100 mM of NaCl to the WB/A culture media. Mannitol treatment was applied by adding 200 and 300 mM of d-mannitol to the medium. Cold and heat treatments were performed by culturing the transgenic cells at 16 and 33 °C in a growth chamber (TSO-1/80 SPU, SKTB, Smolensk, Russia). The average growth rates were assessed after 30 days of cultivation under the control and stress conditions.
Overexpression of VaCPK29 gene in A. thaliana
To create A. thaliana lines overexpressing the VaCPK29 gene, we used the same plasmid construction as for overexpression of VaCPK29 in cell cultures of V. amurensis (described above). The overexpression construct of VaCPK29 (pZP-RCS2-VaCPK29-nptII) or empty vector (pZP-RCS2-nptII) was introduced into the A. tumefaciens strain GV3101::pMP90 and transformed by floral dip method into wild-type A. thaliana (Zhang et al. 2006). Transgenic plants were selected and confirmed by PCR as described (Dubrovina et al. 2015, 2016a) using primers S1 5′GAA TGG GGG ATG AAG CGA CT designed to the 3′ end of the VaCPK29 protein coding region and A1 5′GAG AGA CTG GTG ATT TTT GCG designed to the CaMV 35 S terminator in the pSAT1 vector. The PCR products were verified by DNA sequencing as described (Kiselev et al. 2015). The transgenic lines used in this study were homozygous plants with single copy insertion. Four representative independent T3 homozygous lines (L1, L2, L3, and L4) with high mRNA levels of VaCPK29 were chosen for detailed analyses.
Drought, salt, cold, and heat tolerance analysis of transgenic A. thaliana
The plants were subjected to heat, freezing, drought, and salt stress treatments as described (Dubrovina et al. 2015, 2016a). Briefly, the sterilized transgenic seeds of Arabidopsis were germinated on plates and the 7-day-old seedlings were transferred to commercially available rich well-watered soil in a controlled environmental chamber at standard conditions. Then, the plants were subjected to drought by culturing without additional irrigation for 5 weeks, and then re-watered. For salt stress treatments, the transferred seedlings were cultivated without additional irrigation for 2 weeks, and then the plants were well-irrigated with 350 mM NaCl solution. No signs of drought were observed for plants before irrigation with the NaCl solution. One week after irrigation with NaCl, the pots were placed in 3 cm deep fresh water for 4 h to leach the salt from the soil. For cold tolerance assays, normally cultured A. thaliana plants (3-week-old) were stressed in a −10 °C freezer for 1.5 h and then cultured at 8 °C for 2 h. For heat tolerance assays, normally cultured plants (3-week-old) were stressed at 45 °C in a controlled incubator for 3 h. The survival rates were determined as the number of visibly green plants 3 days after re-watering (drought), 1 week after heat and cold stress treatments, and 1 week after salt leaching (salt stress). Two pots of plants (10 seedlings per pot) were grown for each transgenic line and each treatment in one experiment. The experiments were repeated eight times for each stress treatment type. We also assessed responses to mannitol of 1-week-old A. thaliana seedlings grown on the 1/2 Murashige and Skoog (MS) plates. Six-day-old VC and 35S-VaCPK29 seedlings were transferred on plates with the 1/2 MS medium supplemented with 500 mM mannitol. Survival rates were determined as the number of visibly green seedlings 6 days after the transfer.
Nucleic acid isolation and qRT-PCR
Total DNA isolation from all plants was performed as described previously (Kiselev et al. 2015). Total RNA isolation was performed using the cetyltrimethylammonium bromide-based extraction (Kiselev et al. 2012). Total RNA was isolated from the 3-week-old normally cultured and heat-stressed A. thaliana plants (cultivated at +22 or +45 °C for 1 h 20 min). The leaves of the control and heat-stressed plants were collected for RNA extraction after the stress treatments. Complementary DNAs were synthesized using 1.5 µg of total RNA by the MMLV RT Kit (Silex M, Moscow, Russia) as described (Kiselev et al. 2007). cDNAs of VaCPK29, AtGAPDH, AtActin, and the stress-responsive genes of A. thaliana (ABF3, DREB1A, DREB2A, RD29A, RD29B, CSD1, CSD2, CAT1) were amplified as described (Dubrovina et al. 2015). To evaluate the VaCPK29 transcript levels in the transgenic A. thaliana plants, we used the S1 and A1 primers (mentioned above). The oligonucleotide primers, used for evaluating expression of the stress-responsive genes were designed and applied as described (Dubrovina et al. 2015). qRT-PCR data were obtained and analyzed as described previously (Dubrovina and et al. 2015). Expression was calculated by the 2−ΔΔCT method (Livak and Schmittgen 2001). After the calculations, the highest expressing sample was assigned the value 1 in the relative mRNA calculation in each qPCR reaction.
Statistical analysis
All analyses of Arabidopsis survival rates in this work were independently repeated at least eight times with 20 replicates. The data for fresh biomass accumulation in the callus cultures were obtained from two independent experiments with ten replicates each. The data are presented as mean ± standard error (SE) and were tested by Student’s t test. The data from qRT-PCRs (expression analysis of selected stress-responsive genes) were obtained from two independent experiments with eight replicates each. The data are presented as mean ± standard error of the mean (SE) and were subjected to a one-way analysis of variance (ANOVA) with Tukey’s Honestly Significant Difference (HSD) test at P ≤ 0.05 with Tukey’s Honestly Significant Difference (HSD) test at P ≤ 0.05 using the Microsoft Office Excel 2003 program.
Results
Stress tolerance of VaCPK29-overexpressing callus cell cultures of V. amurensis
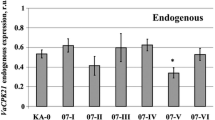
To investigate the effects of VaCPK29 overexpression on the growth of V. amurensis cells cultivated in vitro, we used independent clonal callus cell cultures of V. amurensis transformed with the VaCPK29 gene under the control of the double CaMV 35S promoter. The cell cultures were previously obtained from the V2 cell suspension culture of V. amurensis (Aleynova et al. 2015). Briefly, A. tumefaciens strains bearing pZP-RCS2-nptII or pZP-RCS2-VaCPK29-nptII constructs were inoculated in multiple separate flasks with cell suspensions of V. amurensis to establish the independently-transformed KA-0 (empty vector), KA10-I, KA10-II, KA10-III, and KA10-IV callus cell cultures. The VaCPK29 and nptII genes were cloned under the control of the double CaMV 35S promoter in the constructs. We selected transgenic cell aggregates in the presence of Km, as described (Aleynova et al. 2015). The KA-0 cell culture was used as a control in all further experiments. The KA10-I, -II, -III, and -IV cell cultures represented friable, vigorously-growing, homogenous tissues that did not appear to have undergone differentiation (Fig. S1). qRT-PCR revealed that the KA10-I,-III, and -IV calli expressed the exogenous VaCPK29 gene at a high level, while the KA10-II calli did not actively express it (Aleynova et al. 2015). Expression of endogenous VaCPK29 in the KA10 callus cell cultures did not significantly differ from that in the control KA-0 culture and was at approximately the same level in all four KA10 calli.
Under standard cultivation conditions, the KA10 cell cultures accumulated biomass at approximately the same rate as the control KA-0 during the 30 d of cultivation (Table 1). To assess the effect of heat, cold, salt and osmotic stress conditions, we cultivated the transgenic calli at 16 °C (i.e., cold stress conditions) and 33 °C (i.e., heat stress conditions) for 30 days and in the presence of NaCl or mannitol for 30 days (Table 1). Under cold and salt stress conditions, the growth of the KA10 cell cultures was reduced to approximately the same level as the growth of the control KA-0 calli. Three KA10 calli showed a higher tolerance to heat stress in comparison with the tolerance of the KA-0 control calli. Under heat stress conditions, growth of the KA10-I, -III, and -IV cell cultures (active transgene expression) was reduced 1.5–2.4-fold, while the growth of KA-0 (control) and KA10-II (weak VaCPK29 expression) was reduced 3.9- and 3.1-fold, respectively (Table 1). In response to osmotic stress generated by mannitol, the KA10-I, -II, -III, and -IV cell cultures showed an increased growth rate in comparison with that of the KA-0 control. Growth of the KA10 calli in the presence of mannitol was not inhibited or was reduced to a less degree than that of the KA-0 (Table 1). Thus, overexpression of the VaCPK29 gene did not have a consistent and considerable effect on cold and salt stress resistance levels of the transgenic cell cultures of V. amurensis, while it improved growth of the KA10 calli under heat stress and high mannitol conditions. Taken together, these data indicate that a high level of VaCPK29 expression improved the resistance of V. amurensis calli to heat stress and mannitol-induced osmotic stress.
Stress tolerance of VaCPK29-overexpressing A. thaliana plants
To investigate the physiological role of VaCPK29 in abiotic stress responses, the overexpression constructs pZP-RCS2-VaCPK29-nptII and pZP-RCS2-nptII were transformed in Arabidopsis. Four independent fertile T3 homozygous lines of A. thaliana transformed with VaCPK29 (L1, L2, L3, and L4) and a T3 homozygous KA-0 line of A. thaliana transformed with the empty vector (VC) were chosen for further experiments. Since the VC and wild type controls exhibited similar survival rates under the stress conditions tested earlier (Dubrovina et al. 2015, 2016a), the wild type control was not used in the present investigation. No consistent differences in growth and morphologies were observed between the VC, L1, L2, L3, and L4 A. thaliana lines. qRT-PCR demonstrated that all four transgenic 35S-VaCPK29 expressed the VaCPK29 gene at a high level and there were no considerable differences in VaCPK29 expression between all four lines (Fig. 1a).
Characterization of VaCPK29-transformed A. thaliana lines. a qRT-PCR analysis of the VaCPK29 gene in transgenic lines. b Response of transgenic lines to mannitol-induced osmotic stress in vitro. Six-day-old seedlings, pre-cultured on half-strength MS medium for 1 week, were grown on semisolid half-strength MS medium supplemented with 500 mM mannitol. Survival rates were recorded as the number of visibly green seedlings present at day 6. *, **Significantly different from the vector control at P ≤ 0.05 and 0.01, respectively, according to the Student’s t test
The stress tolerance assays showed that the survival rates of the VC and 35S-VaCPK29 lines were affected by freezing, drought, and salinity stress treatments to approximately the same degree (Fig. 2a–c). For the heat stress treatment, the data obtained indicated that the survival rates of 35S-VaCPK29 A.thaliana lines were higher than that of the VC control (Fig. 2d). In all, 30–44% of the 35S-VaCPK29 L1, L2, L3, and L4 transgenic lines survived, whereas only 27% of the VC plants survived. However, the increase in heat stress tolerance was slight and was statistically significant for only two KA10 transgenic lines.
Response of VaCPK29-transformed A. thaliana lines to abiotic stresses. a Survival rates of A. thaliana under cold stress. Three-week-old plants were cold stressed at −10 °C for 1.5 h and then transferred to normal conditions for recovery. b Survival rates of A. thaliana under drought stress. One-week-old plants were transplanted to soil, watered, and then cultivated for an additional 5 weeks without watering to induce drought stress, and finally were re-watered. c Survival rates of A. thaliana under salt stress. Three-week-old plants were irrigated with 350 mM NaCl solution. Free NaCl solution was removed and the plants were cultured for 1 week. Then, the pots were placed in fresh water to leach the salt from the soil. d Survival rates of A. thaliana under heat stress. Three-week-old plants were heat stressed at +45 °C for 3 h and then transferred to normal conditions for recovery. Survival rates were recorded as the number of visibly green plants after 7 days (cold, salt, and heat) or 3 days (drought). *, **Significantly different from the vector control at P ≤ 0.05 and 0.01, respectively, according to the Student’s t test
We also analyzed the survival rates of the VC and KA10 lines when the A. thaliana seedlings were exposed to hyperosmotic stress by transferring the seedlings to plates containing 1/2 MS medium supplemented with 500 mM mannitol (Fig. 1b; Fig. S2). The analysis showed that the 35S-VaCPK29 L1, L2, L3, and L4 plants exhibit a higher tolerance for mannitol-induced osmotic stress than the VC control plants. The results indicated that the VaCPK29 gene conferred heat and osmotic stress tolerance to the transgenic Arabidopsis at a low level and did not affect its drought, freezing, and salt stress resistance.
Expression of the stress-associated genes in VaCPK29-overexpressing A. thaliana plants
In order to investigate the role of the VaCPK29 gene in the heat stress response pathway, the expression levels of eight stress-related genes were analyzed by qRT-PCR in the VC control plants and in the 35S-VaCPK29 transgenic lines. We analyzed transcription levels of the stress-inducible genes (AtABF3, AtDREB1A, AtDREB2A, AtRD29A, AtRD29B) and antioxidant genes (AtCSD1, AtCSD2, AtCAT1) in the 3-week-old VC and VaCPK29-overexpressing L1–L4 Arabidopsis plants exposed to control and heat stress conditions (Fig. 3). Under standard cultivation conditions, all lines showed the same level of expression of the stress-associated genes, with the exception of the transcription levels of RD29A in L4 and CSD2 in L3 compared to that in VC and some other lines. However, under heat stress, expression levels of AtABF3, AtDREB1A, AtDREB2A, AtRD29A, and AtRD29B were up-regulated in the VaCPK29-overexpressing L1, L2, L3, and L4 lines but not in the VC control line. The expression of the heat-inducible gene AtDREB2A (Sakuma et al. 2006b; Schramm et al. 2008) displayed the most considerable up-regulation in the VaCPK29-overexpressing plants. Expression of AtABF3, AtDREB1A, AtDREB2A, AtRD29A, and AtRD29B in the control VC plant line was induced to a lesser extent or was not affected at all. In response to heat stress, expression of the AtCSD1 gene significantly increased only in the L3 and L4 KA10 lines (CSD1 in L3 and L4 under heat vs. that under control conditions). Expression of the AtCSD2 gene was down-regulated in both the VC and all four KA10 transgenic lines in response to heat stress. Thus, overexpression of VaCPK29 enables Arabidopsis to cope with heat stress by regulating expression of the stress-inducible genes AtDREB1A, AtDREB2A, AtRD29A, AtRD29B, and AtABF3.
Expression analysis of selected stress-responsive genes a AtDREB1A, b AtDREB2A, c AtRD29A, d AtRD29B, e AtABF3, f AtCAT1, g AtCSD1, and h AtCSD2 in the 3-week-old normally cultured (+22 °C for 1 h 20 min, white bars) and heat-stressed (+45 °C for 1 h 20 min, dark grey bars) VaCPK29-transformed A. thaliana lines. Means with common letters are not significantly different at P ≤ 0.05, according to Tukey’s HSD test
Discussion
As a result of the recent sequencing of the V. vinifera genome (Jaillon et al. 2007; Velasco et al. 2007) and the high stress resistance of wild-growing V. amurensis (Ma et al. 2010; Liu and Li 2013), the wild grape species is considered a promising experimental system to investigate plant stress adaptation mechanisms. Unraveling the mechanisms of wild grape resistance to abiotic and biotic stresses could potentially provide the knowledge to develop new efficient strategies to improve crop yield. The VaCPK20 and VaCPK21 genes have recently been shown to contribute to the stress resistance of V. amurensis by regulating its cold, drought, and salt stress responses (Dubrovina et al. 2015, 2016a). A previous study also revealed that VaCPK29 is likely involved in abiotic stress responses in V. amurensis (Dubrovina et al. 2013). In this study, the effects of VaCPK29 overexpression on the growth of the callus cell cultures of V. amurensis and the survival rates of A. thaliana plants were studied under high salinity, cold, heat, drought, and osmotic stress conditions. The stress tolerance assays showed that overexpression of VaCPK29 improved growth of V. amurensis calli and survival rates of A. thaliana plants under heat stress and mannitol-induced osmotic stress. These results suggest that the VaCPK29 gene is involved in the grapevine response to heat and osmotic stress conditions as a weak positive regulator, and that it likely contributes, along with other CDPKs, to the enhanced stress tolerance of V. amurensis.
To further understand the function of VaCPK29 in abiotic stress responses, we analyzed whether the transcription levels of select stress-associated and antioxidant genes were altered in VaCPK29-overexpressing A. thaliana plants experiencing heat stress. The transcription levels of ABF3, DREB1A, DREB2A, RD29A, RD29B, CSD1, CSD2, and CAT1 were compared in VaCPK29-overexpressing plants and control Arabidopsis plants transformed with empty vector. Under heat stress, expression levels of AtABF3, AtDREB1A, AtDREB2A, AtRD29A, and AtRD29B were up-regulated in the VaCPK29-overexpressing A. thaliana lines but not in the VC. The higher transcription levels of ABF3, DREB1A, DREB2A, RD29A, and RD29B suggest that the VaCPK29 gene may act upstream to these genes in the heat stress response. The Arabidopsis abscisic acid responsive element-binding factor 3 (ABF3) is known to function in drought, cold, heat, and oxidative stress responses via regulation of stress-responsive gene transcription levels (Kim et al. 2004; Abdeen et al. 2010; Choi et al. 2013). Recent studies suggest that ABF3 could promote stomatal closure and reduce water loses under water deficit and high temperature conditions (Choi et al. 2013). The transcription factors DRE-BINDING PROTEIN 1A and 2A (DREB2A and DREB1A) were reported to regulate dehydration-responsive element (DRE)-mediated transcription of target genes under dehydration, high salinity, and cold stress conditions (Liu et al. 1998; Kasuga et al. 1999; Sakuma et al. 2006a). DREB2A had also been reported to be transcriptionally-regulated by heat shock and to play important roles in heat stress tolerance, e.g., by controlling HsfA3 transcription (Sakuma et al. 2006b; Schramm et al. 2008). Notably, in the present investigation, the most pronounced stress-responsive activation of transcription in the VaCPK29-overexpressing plants was observed for the DREB2A gene. Gene expression levels of the RESPONSIVE TO DEHYDRATION29 (RD29A and RD29B) proteins are known to be induced not only by dehydration conditions but also by salinity, cold, and/or osmotic stress (Msanne et al. 2011). CSD1, CSD2, and CAT1 encode antioxidant enzymes that can detoxify reactive oxygen species (ROS) generated in plants under abiotic stress conditions (Locato et al. 2008; You and Chan 2015). It is known that ROS production increases during abiotic stress, including heat stress (Locato et al. 2008). In the present study, we found that heat stress induced the level of CSD1 transcription in some VaCPK29-overexpressing A. thaliana lines but not in the VC line, while there were no considerable differences in CSD2 and CAT1 transcriptional responses in all transgenic lines. Thus, it is possible that CSD1 is responsible for ROS scavenging under heat stress in the heat-resistant VaCPK29-overexpressing Arabidopsis. Taken together, the results suggest that VaCPK29 is implicated in the induction of transcription of some stress-responsive genes, thereby improving plant tolerance to heat and osmotic stress conditions.
Amino acid sequence homology analyses revealed a high degree of similarity between the deduced amino acid sequence of VaCPK29 and other CDPKs present in different plant species as shown in Table 2 and Fig. S3. VaCDPK29 shares the highest degree of similarity to VvCPK15 of V. vinifera and VpCPK15 of V. pseudoreticulata identified by Chen et al. (2013a) and Zhang et al. (2015). qRT-PCR data presented by Zhang et al. (2015) revealed that the CPK15 gene of wild grape V. pseudoreticulata was up-regulated in response to heat, salt, and cold stresses. The elevation in VpCPK15 expression was moderate under cold and heat stress conditions and was slight under high salinity conditions. Zhang et al. (2015) did not analyze VpCPK transcription in response to osmotic stress. The data by Zhang et al. (2015) on the expression of VpCPK15 in response to abiotic stresses do not contradict our hypothesis that VaCPK29 is involved in heat stress response in grapevine. As for homologous CDPKs from Arabidopsis, it has been shown that AtCPK21 activates SLAC1 and SLAH3 channels in response to abscisic acid (ABA) and functions as a positive regulator in ABA-induced stomatal closure (Geiger et al. 2010). A study by Franz et al. (2011) reported that AtCPK21 was biochemically activated in vivo in response to hyperosmotic stress. However, overexpression of AtCPK21 in Arabidopsis did not have a positive effect on salt and osmotic stress resistance and suggested that AtCPK21 acted as a negative regulator in abiotic stress signaling (Franz et al. 2011). According to Zuo et al. (2013), the PtCDPK19 gene was up-regulated in response to drought stress as shown by microarray analysis. The potential biological function of AtCPK9, AtCPK29, OsCPK1, OsCPK15, and PtCDPK24 in plant stress responses remain largely unknown.
In conclusion, the characterization of the VaCPK29 functioning suggests that the gene plays a role in regulating the signaling pathways involved in heat and osmotic stress resistance in V. amurensis. This finding helps to clarify details of the CDPK-mediated abiotic stress response of V. amurensis.
References
Abdeen A, Schnell J, Miki B (2010) Transcriptome analysis reveals absence of unintended effects in drought-tolerant transgenic plants overexpressing the transcription factor ABF3. BMC Genom 11:69
Aleynova OA, Dubrovina AS, Manyakhin AY, Karetin YA, Kiselev KV (2015) Regulation of resveratrol production in Vitis amurensis cell cultures by calcium-dependent protein kinases. Appl Biochem Biotechnol 175:1460–1476
Aleynova-Shumakova OA, Dubrovina AS, Manyakhin AY, Karetin YA, Kiselev KV (2014) VaCPK20 gene overexpression significantly increased resveratrol content and expression of stilbene synthase genes in cell cultures of Vitis amurensis Rupr. Appl Microbiol Biotechnol 98:5541–5549
Asano T, Hayashi N, Kikuchi S, Ohsugi R (2012a) CDPK-mediated abiotic stress signaling. Plant Signal Behav 7:817–821
Asano T, Hayashi N, Kobayashi M, Aoki N, Miyao A, Mitsuhara I, Ichikawa H, Komatsu S, Hirochika H, Kikuchi S, Ohsugi R (2012b) A rice calcium-dependent protein kinase OsCPK12 oppositely modulates salt stress tolerance and blast disease resistance. Plant J 69:26–36
Batistič O, Kudla J (2012) Analysis of calcium signaling pathways in plants. Biochim Biophys Acta 1820:1283–1293
Boudsocq M, Sheen J (2013) CDPKs in immune and stress signaling. Trends Plant Sci 18:30–40
Chen F, Fasoli M, Tornielli GB, Dal Santo S, Pezzotti M, Zhang L, Cai B, Cheng ZM (2013a) The evolutionary history and diverse physiological roles of the grapevine calcium-dependent protein kinase gene family. PLoS ONE 8:e80818
Chen J, Xue B, Xia X, Yin W (2013b) A novel calcium-dependent protein kinase gene from Populus euphratica, confers both drought and cold stress tolerance. Biochem Biophys Res Commun 441:630–636
Choi HI, Park HJ, Park JH, Kim S, Im MY, Seo HH, Kim YW, Hwang I, Kim SY (2005) Arabidopsis calcium-dependent protein kinase AtCPK32 interacts with ABF4, a transcriptional regulator of abscisic acid responsive gene expression, and modulates its activity. Plant Physiol 139:1750–1761
Choi YS, Kim YM, Hwang OJ, Han YJ, Kim SY, Kim JI (2013) Overexpression of Arabidopsis ABF3 gene confers enhanced tolerance to drought and heat stress in creeping bentgrass. Plant Biotechnol Rep 7:165–173
Das R, Pandey GK (2010) Expressional analysis and role of calcium regulated kinases in abiotic stress signaling. Curr Genom 11:2–13
Dubrovina AS, Kiselev KV, Khristenko VS (2013) Expression of calcium-dependent protein kinase (CDPK) genes under abiotic stress conditions in wild-growing grapevine Vitis amurensis. J Plant Physiol 170:1491–1500
Dubrovina AS, Kiselev KV, Khristenko VS, Aleynova OA (2015) VaCPK20, a calcium-dependent protein kinase gene of wild grapevine Vitis amurensis Rupr., mediates cold and drought stress tolerance. J Plant Physiol 185:1–12
Dubrovina AS, Kiselev KV, Khristenko VS, Aleynova OA (2016a) VaCPK21, a calcium-dependent protein kinase gene of wild grapevine Vitis amurensis Rupr., is involved in grape response to salt stress. Plant Cell Tissue Organ Cult 124:137–150
Dubrovina AS, Kiselev KV, Aleynova OA (2016b) Influence of overexpression of the true and false alternative transcripts of calcium-dependent protein kinase CPK9 and CPK3a genes on the growth, stress tolerance, and resveratrol content in Vitis amurensis cell cultures. Acta Physiol Plant 38:78
Franz S, Ehlert B, Liese A, Kurth J, Cazalé AC, Romeis T (2011) Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana. Mol Plant 4:83–96
Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S, Liese A, Wellmann C, Al-Rasheid KA, Grill E, Romeis T, Hedrich R (2010) Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci USA 107:8023–8028
Harper JF, Huang JF, Lloyd SJ (1994) Genetic identification of an autoinhibitor in CDPK, a protein kinase with a calmodulin-like domain. BioChemistry 33:7267–7277
Hashimoto K, Kudla J (2011) Calcium decoding mechanisms in plants. Biochimie 93:2054–2059
Jaillon O, Aury JM, Noel B, Policriti A, Clepet C, Casagrande A, Choisne N et al (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449:463–467
Jaworski K, Szmidt-Jaworska A, Kopcewicz J (2011) Two calcium dependent protein kinases are differently regulated by light and have different activity patterns during seedling growth in Pharbitis nil. Plant Growth Regul 65:369–379
Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1999) Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 17:287–291
Kim JB, Kang JY, Kim SY (2004) Over-expression of a transcription factor regulating ABA-responsive gene expression confers multiple stress tolerance. Plant Biotechnol J 2:459–466
Kiselev KV, Dubrovina AS, Veselova MV, Bulgakov VP, Fedoreyev SA, Zhuravlev YN (2007) The rolB gene-induced overproduction of resveratrol in Vitis amurensis transformed cells. J Biotechnol 128:681–692
Kiselev KV, Dubrovina AS, Bulgakov VP (2009) Phenylalanine ammonia-lyase and stilbene synthase gene expression in rolB transgenic cell cultures of Vitis amurensis. Appl Microbiol Biotechnol 82:647–655
Kiselev KV, Shumakova OA, Manyakhin AY, Mazeika AN (2012) Influence of calcium influx induced by the calcium ionophore, A23187, on resveratrol content and the expression of CDPK and STS genes in the cell cultures of Vitis amurensis. Plant Growth Regul 68:371–381
Kiselev KV, Dubrovina AS, Shumakova OA, Karetin YA, Manyakhin AY (2013) Structure and expression profiling of a novel calcium-dependent protein kinase gene, CDPK3a, in leaves, stems, grapes, and cell cultures of wild-growing grapevine Vitis amurensis Rupr. Plant Cell Rep 32:431–442
Kiselev KV, Tyunin AP, Ogneva ZV, Dubrovina AS (2015) Age-associated alterations in the somatic mutation level in Arabidopsis thaliana. Plant Growth Regul 75:493–501
Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H (2007) Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 19:1065–1080
Liese A, Romeis T (2013) Biochemical regulation of in vivo function of plant calcium-dependent protein kinases (CDPK). Biochim Biophys Acta-Mol. Cell Res 7:1582–1589
Liu L, Li H (2013) Review: Research progress in amur grape, Vitis amurensis Rupr. Can J Plant Sci 93:565–575
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10:1391–1406
Locato V, Gadaleta C, De Gara L, De Pinto MC (2008) Production of reactive species and modulation of antioxidant network in response to heat shock: a critical balance for cell fate. Plant Cell Environ 31:1606–1619
Ma SY, Wu WH (2007) AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Mol Biol 65:511–518
Ma YY, Zhang YL, Shao H, Lu J (2010) Differential physio-biochemical responses to cold stress of cold-tolerant and non-tolerant grapes (Vitis L.) from China. J Agron Crop Sci 196:212–219
Msanne J, Lin J, Stone JM, Awada T (2011) Characterization of abiotic stress-responsive Arabidopsis thaliana RD29A and RD29B genes and evaluation of transgenes. Planta 234:97–107
Reddy AS, Ali GS, Celesnik H, Day IS (2011) Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell 23:2010–2032
Sakuma Y, Maruyama K, Osakabe Y, Qin F, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2006a) Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 18:1292–1309
Sakuma Y, Maruyama K, Qin F, Osakabe Y, Shinozaki K, Yamaguchi-Shinozaki K (2006b) Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc Natl Acad Sci USA 103:18822–18827
Schramm F, Larkindale J, Kiehlmann E, Ganguli A, Englich G, Vierling E, von Koskull-Döring P (2008) A cascade of transcription factor DREB2A and heat stress transcription factor HsfA3 regulates the heat stress response of Arabidopsis. Plant J 53:264–274
Schulz P, Herde M, Romeis T (2013) Calcium-dependent protein kinases: hubs in plant stress signaling and development. Plant Physiol 163:523–530
Shen YY, Duan CQ, Liang XE, Zhang DP (2004) Membrane-associated protein kinase activities in the developing mesocarp of grape berry. J Plant Physiol 161:15–23
Tzfira T, Tian GW, Lacroix B, Vyas S, Li J, Leitner-Dagan Y, Krichevsky A, Taylor T, Vainstein A, Citovsky V (2005) pSAT vectors: a modular series of plasmids for autofluorescent protein tagging and expression of multiple genes in plants. Plant Mol Biol 57:503–516
Valmonte GR, Arthur K, Higgins CM, MacDiarmid RM (2014) Calcium-Dependent Protein Kinases in plants: evolution, expression and function. Plant Cell Physiol 55:551–569
Velasco R, Zharkikh A, Troggio M, Cartwright DA, Cestaro A, Pruss D, Pindo M et al (2007) A high quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS ONE 2:e1326
Weckwerth P, Ehlert B, Romeis T (2015) ZmCPK1, a calcium-independent kinase member of the Zea mays CDPK gene family, functions as a negative regulator in cold stress signalling. Plant Cell Environ 38:544–558
Wei S, Hu W, Deng X, Zhang Y, Liu X, Zhao X, Luo Q, Jin Z, Li Y, Zhou S, Sun T, Wang L, Yang G, He G (2014) A rice calcium-dependent protein kinase OsCPK9 positively regulates drought stress tolerance and spikelet fertility. BMC Plant Biol 14:133
Wernimont AK, Artz JD, Finerty, Lin YH, Amani M, Allali-Hassani A, Senisterra G, Vedadi M, Tempel W, Mackenzie F et al (2010) Structures of apicomplexan calcium-dependent protein kinases reveal mechanism of activation by calcium. Nat Struct Mol Biol 17:596–601
You J, Chan Z (2015) ROS regulation during abiotic stress responses in crop plants. Front Plant Sci 6:1092
Yu XC, Li MJ, Gao GF, Feng HZ, Geng XQ, Peng CC, Zhu SY, Wang XJ, Shen YY, Zhang DP (2006) Abscisic acid stimulates a calcium-dependent protein kinase in grape berry. Plant Physiol 140:58–579
Yu XC, Zhu SY, Gao GF, Wang XJ, Zhao R, Zou KQ, Wang XF, Zhang XY, Wu FQ, Peng CC, Zhang DP (2007) Expression of a grape calcium-dependent protein kinase ACPK1 in Arabidopsis thaliana promotes plant growth and confers abscisic acid-hypersensitivity in germination, postgermination growth, and stomatal movement. Plant Mol Biol 64:31–538
Zhang XR, Henriques R, Lin SS, Niu QW, Chua NH (2006) Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc 1:641–646
Zhang K, Han YT, Zhao FL, Hu Y, Gao YR, Ma YF, Zheng Y, Wang YJ, Wen YQ (2015) Genome-wide identification and expression analysis of the CDPK gene family in grape, Vitis spp. BMC Plant Biol 15:164
Zou JJ, Wei FJ, Wang C, Wu JJ, Ratnasekera D, Liu WX, Wu WH (2010) Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol 154:1232–1243
Zuo R, Hu R, Chai G, Xu M, Qi G, Kong Y, Zhou G (2013) Genome-wide identification, classification, and expression analysis of CDPK and its closely related gene families in poplar (Populus trichocarpa). Mol Biol Rep 40:2645–2662
Acknowledgements
This work was supported by a Grant from the Russian Science Foundation (14-14-00366).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dubrovina, A.S., Kiselev, K.V., Khristenko, V.S. et al. The calcium-dependent protein kinase gene VaCPK29 is involved in grapevine responses to heat and osmotic stresses. Plant Growth Regul 82, 79–89 (2017). https://doi.org/10.1007/s10725-016-0240-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-016-0240-5