Abstract
Mitogen-activated protein kinase pathways are involved in plant resistance to a variety of adverse environmental processes, and their downstream component MAPKs play an important role in this process. However, the function of MAPKs in abiotic stresses is still far from being clear in grape (Vitis vinifera L.). Here, we isolated a novel group B MAPK gene (VvMAPK9) from grape, which is induced by different abiotic stresses such as salt, drought and high temperature (42 °C). Overexpressing VvMAPK9 in Arabidopsis thaliana significantly enhanced the tolerance to salt stress. Compared with wild type plants, the transgenic lines exhibited higher germination rate and longer root length as well better growth status under salt stress. In addition, overexpression of VvMAPK9 in grape callus also increased the salt stress tolerance and enhanced the callus’s ability to scavenge reactive oxygen species (ROS), which correlated with higher activity of ROS-related antioxidant enzymes. These results indicate that VvMAPK9 may positively regulate salt stress by regulating the antioxidative system.
Key message
Grape VvMAPK9 positively regulates salt tolerance in Arabidopsis and grape callus through regulating the antioxidative system
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are constantly exposed to a variety of abiotic stress throughout their life cycle, including high salinity, drought and extreme temperatures (Qin et al. 2011; Tuteja 2007). In order to cope with these stresses, plants have changed their physiological structure, morphology and evolved different signaling pathways to sense and transmit various signals (Bohnert et al. 1995). Among these signaling pathways, mitogen-activated protein kinase (MAPK) cascade pathways are highly conserved and involved in environmental stress resistance such as high salinity and drought (Danquah et al. 2014; Sun et al. 2015). A typical MAPK cascade is composed of three kinases: MAPK kinase kinase (MAPKKK or MEKK), MAPK kinase (MAPKK or MEK) and MAPK, forming the MAPKKK-MAPKK-MAPK signaling pathway. Currently, many MAPKs have been identified from different plants, there are 20 MAPKs in Arabidopsis, 17 in rice, 21 in poplar, and 14 in grapevine. According to amino acid sequence similarity, MAPKs are divided into four subfamilies (A–D group). Group A, B and C all have a common phosphorylation motif TEY in their active loop, while group D MAPKs contains a TDY motif (Jonak et al. 1999; Hamel et al. 2006; MAPK Group 2002). In addition, groups A, B and C possess a conserved C-terminal docking domain, whereas it could not be found in the sequence of group D (MAPK Group 2002).
It is well documented that plant MAPK protein kinases are involved in different abiotic stresses, such as salt, drought, cold and high temperature. For example, OsMAPK5 played a positive regulatory role in high salinity, drought and low temperature stress in rice (Xiong and Yang 2003). In Arabidopsis, AtMAPK3 and AtMAPK6 enhanced the ability of plants to resist salt stress and oxidative stress (Zhou et al. 2017; Pérez-Salamó et al. 2014), and the MEKK18-MKK3-MPK1/2/7/14 cascade pathway was involved in osmotic stress and ABA signaling (Danquah et al. 2015; Li et al. 2017). In Zea mays, ZmMAPK1 have been reported to participate in both drought and high temperature stresses (Wu et al. 2015). Moreover, Durum wheat TMKP1 phosphatase enhanced salt tolerance of plants (Zaidi et al. 2016). All these results suggested that the MAPK genes have important application values in the improvement of stress tolerance in crops.
Abiotic stresses always resulted in the rapid production of reactive oxygen species (ROS), particularly H2O2 and O2·−. It is well known that ROS, at low concentration, are important signaling molecules, while high concentrations of ROS may result in oxidative stress and cause irreversible damage to biological organisms (Kovtun et al. 2000). Therefore, moderate the accumulation of ROS is critical to regulate many biological processes of plants. Previous studies reported that MAPK cascades are involved in the maintaining of ROS homeostasis. In Arabidopsis, the MEKK1-MKK2-MPK4/6 cascade is known to participate in the regulation of ROS production under abiotic stress (Teige et al. 2004; Xing et al. 2008), and AtMAPK8 can negatively regulate the ROS accumulation (Takahashi et al. 2011). In Nicotiana benthamiana, NPK1-MEK1-NTF6 cascade was reported to enhance the ROS accumulation by promoting the expression of NbRbohb, which increased the plant tolerance to environmental stresses (Asai et al. 2008). Recent study reported that under salt stress the PdMAPK3/6 were activated to negatively regulate the ROS production to reduce the oxidative damage in Populus (Lu et al. 2020). Taken together, the cross-talk between ROS and the MAPK cascade in the signal transduction network is very complex, and further studies are required to clarify these mechanisms.
Grape (Vitis vinifera L.) is one of the important economically fruit crops in the world. However, grape is constantly exposed to a variety of environment stresses during growth and development stages, which severely inhibit its growth, yield and economic value. Among these abiotic stresses, soil salinization is the main limiting factor that seriously restricts the development of grape industry. Developing salinity-tolerant grape varieties is considered as one of the most effective ways for increasing the yield of grape in high saline soil. However, it is difficult to breed highly halotolerant grapevine varieties by traditional breeding methods, whereas genetic engineering is an economic and more effective strategy on screening and introducing salinity–tolerant varieties. Therefore, it is necessary to reveal salt tolerance mechanisms and search for salt resistant genes. The important function of MAPK in abiotic stresses in plants has been revealed, but studies were mainly concentrated on the model plants, less on fruit trees, especially grapevine. In this study, a group B MAPK gene, VvMAPK9 from grape was isolated and its expression pattern under abiotic stress was analyzed. Then, the function of VvMAPK9 was investigated by overexpressing it in Arabidopsis and grape callus. This study would not only enrich our understanding of MAPK signaling in grape, but also provide the theoretical foundation for the application of VvMAPK9 in grapevine rootstock breeding.
Materials and methods
Plant materials and stress treatments
The issue culture seedlings of grape rootstock A35 were cultured in MS solid medium supplemented with 0.2 mM indolebutyric acid (IBA) at 25 °C with a 16 h light/8 h dark cycle. 2-month-old grape seedlings were treated with 100 µM abscisic acid (ABA), 200 mM mannitol, 200 mM NaCl, and high temperature (42 °C), respectively. For tissue-specific expression analyses, young leaves, mature leaves, petioles, stems and roots were harvested from the same plants, frozen in liquid nitrogen, and stored at − 80 °C.
‘Crimson seedless’ grape calluses were cultured as previous described (Xu et al. 2019), which were used for gene transformation and salt tolerance assay. The grape callus was cultured on MS medium supplemented with 0.59 g/L 2-(N-Morpholino) ethanesulfonic acid, 10 mg/L picloram, and 2.2 mg/L thidiazuron, at 25 °C under dark conditions.
Arabidopsis thaliana were used for gene transformation and salt tolerance assay. The leaves of Nicotiana benthamiana seedlings were used for transient gene expression. Arabidopsis thaliana and Nicotiana benthamiana were all planted in plastic pots filled with vermiculite under a greenhouse conditions at 22 °C with a 16 h light/8 h dark photoperiod.
RNA extraction, cDNA synthesis and quantitative real-time PCR
Total RNA of grape was isolated by an improved cetyltrimethyl ammonium bromide (CTAB) method, and total RNA of Arabidopsis thaliana was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized using the PrimeScript™ RT reagent kit with gDNA Eraser (Vazyme, Nanjing, China). According to the supplier’s instructions, the qRT-PCR was performed using the SYBR® PrimeScript™ RT-PCR Kit (TaKaRa, Dalian, China) in the CFX96TM Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The grape β-actin and the N. benthamiana β-actin gene were used as the internal reference. All primers used in this study are listed in Table S1.
Isolation of the VvMAPK9 open reading frame sequence
The open reading frame (ORF) of VvMAPK9 was isolated by PCR amplification with the specific primers VvMPK9-F and VvMPK9-R (Table S1) that was designed and synthesized by Biosune Biotechnological Company, Shanghai, China. The PCR products were purified and combined with pMD19-T vector (TaKaRa, Dalian, China) and then transformed into E. coli cells (DH5α) for sequencing (Biosune Biotechnological Company, Shanghai, China).
Subcellular localization of VvMAPK9
The ORF sequence of VvMAPK9 was fused to the N-terminus of the green fluorescent protein (GFP) gene controlled by the 35S promoter. Cells of Agrobacterium tumefaciens GV3101 containing the recombinant plasmid cultured overnight were collected and resuspended in osmotic solution (10 mM MES, 10 mM MgCl2, and 150 mM acetosyringone), and injected into leaves from 1-month-old N. benthamiana seedlings after placed in the dark for 3 h. After 2–3 days of transformation, the fluorescent signal was detected by a confocal microscope (LSM 510 META, Carl Zeiss). Leaves expressing the 35S-GFP construct were used as a control (Shi et al. 2011).
Transformation of VvMAPK9 into grape callus and Arabidopsis thaliana
The full-length cDNA of VvMAPK9 was inserted into the binary vector PBI121 controlled by the 35S promoter. Then, the recombinant plasmid was introduced into A. tumefaciens GV3101 strain and transformed into Arabidopsis thaliana using a floral dip method as previously described (Clough and Bent 1998). The transgenic seedlings were selected on 1/2 MS agar medium containing 50 mg/L kanamycin, and homozygous lines were screened and further confirmed by PCR and qRT-PCR. Subsequently, three homozygous transgenic lines (OE1, OE2, and OE3) were selected for further studies.
The grape callus with VvMAPK9 overexpression were obtained as described by Xu et al (2019). Firstly, the recombinant plasmid was introduced into Agrobacterium strain LBA4404. Secondly, grape calluses were put in the Agrobacterium suspension for 20 min, blotted dry using sterile filter paper and cultured on solid MS medium with 100 µM acetosyringone in darkness at 25 °C. After 2 days, the calluses were screened on the MS medium with 100 mg/L kanamycin and 300 mg/L cefalexin. 2 months later, most of the calluses had died, and the surviving callus were subcultured on screening medium at 4-week intervals until the callus no longer turns black and dies. Finally, the transgenic callus were confirmed by qRT-PCR.
Salt tolerance assays
In Arabidopsis, wild type (WT) and transgenic Arabidopsis seeds (T3 generation) were disinfected and sowed on 1/2 MS medium containing different concentrations of NaCl for seed germination and root length analysis. Seed germination was monitored every 12 h, and root length was measured after 7 days of vertical culture. In addition, 2-week-old WT and transgenic Arabidopsis seedlings were irrigated with 200 mM NaCl solution, and the control seedlings were irrigated with water. The plant growth status was observed every day. After 2 weeks of salt stress treatment, the plants were photographed. Each experiment was performed at least three independent biological replicates.
For the salt assay of grape callus, the same size callus were inoculated on MS medium containing 150 or 200 mM NaCl, and photographed after 10 days treatments. The relative electric conductivity was measured as described by Zhou and Leul (1998). Total protein concentrations were quantified with the BCA Protein Assay Kit (Suzhou Kerming Biotechnology co. LTD, China). The activities of anti-O2·−, superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and ascorbate peroxidase (APX) were determined by spectrophotometry according to the instruction of the corresponding assay kit (Suzhou Kerming Biotechnology co. LTD, China), respectively. The expression levels of antioxidant enzymes genes were determined by qRT-PCR. Each experiment was conducted at least three times.
Bioinformatic and statistics analysis
Amino acid sequences of other plants MAPK genes were retrieved from GenBank (http://www.ncbi.gov/Genbank). Amino acid sequence alignments were done using the DNAMAN5.2.2 (https://www.lynnon.com). The phylogenetic tree was constructed by the Neighbor-Joining (NJ) method using MEGA 4 software (https://www.megasoftware.net/mega4/mega.html). The promoter sequence of VvMAPK9 were performed using PlantCARE datebase (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). Statistical significance was analyzed using Duncan’s multiple range tests with analysis of variance (ANOVA), and calculations were performed with SPSS Statistics.
Results
Sequence analysis of VvMAPK9
The full-length ORF sequence of VvMAPK9 (XP_002278860.1) is 1128 bp, encoding a 375 amino acid peptide with a predicted molecular weight of 42.615 kD and an isoelectric point of 6.24. Multiple sequence alignments with other plant MAPKs demonstrated that VvMAPK9 contains a conserved phosphorylation motif TEY in the active loop and a CD domain in the C-terminal, which shares high homology (76.80–84.31%) with other group B MAPKs, such as AtMAPK4, BnMAPK4, ZmSIMK1 and OsMAPK4 (Fig. 1A). The Phylogenetic analysis revealed that VvMAPK9 displayed high similarity to the members of the MAPK group B (Fig. 1B), as previously reported (Çakır and Kılıçkaya 2015). These results indicated that VvMAPK9 is a member of MAPK group B.
Phylogenetic tree analysis and sequence alignment. A Alignment of the amino acid sequences of Vitis vinifera (VvMAPK9, XP_002278860.1) with Arabidopsis thaliana (AtMPK4, NP_192046), Brassica napus (BnMAPK4, ABB69023), Zea mays (ZmSIMK1, NP_001105239.2) and Oryza sativa (OsMAPK4, BAC99508.1). Identical amino acids are shaded in black. The phosphorylated TEY motif and the CD domain are marked by red frame. B Phylogenetic analysis of VvMAPK9 and Arabidopsis thaliana MAPK proteins. The neighbour-joining phylogenetic tree was constructed using MEGA 4.0. The numbers above or below the branches indicate the bootstrap values (> 50%) from 500 replicates
Subcellular localization
To investigate the localization of VvMAPK9, two constructs, 35S::GFP and 35S::VvMAPK9:GFP (Fig. 2A), were transferred individually into the epidermal cells of tobacco leaves by Agrobacterium GV3101 mediated transient transformation. As shown in Fig. 2B, the fluorescence signals of both VvMAPK9:GFP fusion protein and 35S::GFP were detected in the cytoplasm and nucleus by the laser confocal microscope, which indicated that VvMAPK9 protein may function in the nucleus and cytoplasm.
Subcellular localization of VvMAPK9 in N. benthamiana leaves. A Schematic diagram of the 35S::VvMAPK9:GFP fusion construct and 35S::GFP construct. B Transient expression of the 35S::VvMAPK9:GFP fusion construct and the 35S::GFP construct in N. benthamiana leaves. Green fluorescence was observed with an LSM 880 META confocal microscope (Carl Zeiss)
Expression pattern analysis in different tissues of grapevine
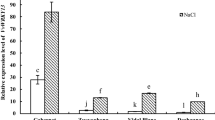
In order to identify the organ-specific expression pattern of VvMAPK9 in grape, qRT-PCR was used. The RNA of 2-month-old grape seedlings from tissue culture was extracted for qRT-PCR. The results showed that the expression level of VvMAPK9 was mainly expressed in young leaves, mature leaves and roots, but relatively low in petiole and stem (Fig. 3), which suggested that the expression of VvMAPK9 was tissue specific.
Expression patterns of VvMAPK9 in different tissues of grape. Tissue-specific expression of VvMAPK9 was detected in the roots, stems, petiole, young leaves and mature leaves of 2-month old grape seedlings by qRT-PCR. The β-actin gene was used as the reference gene. The data are the means ± standard error (SE) of three independent experiments (n = 3). Different letters above the bar indicate significant differences (P ≤ 0.01) based on Duncan’s multiple range tests
Expression of VvMAPK9 under different abiotic stresses
To investigate the potential functions of VvMAPK9 under different abiotic stresses, 2-month-old grape tissue culture seedlings were exposed to various abiotic stresses and the expression profile of VvMAPK9 was examined by qRT-PCR. As shown in Fig. 4A, the expression of VvMAPK9 increased significantly under salt treatment and peaked after 8 h. After drought simulated by mannitol treatment, the expression of VvMAPK9 increased at first and then decreased, and the peak appeared at 12 h (Fig. 4B). Under heat treatment, the transcription level of VvMAPK9 dramatically increased and reached a peak at 4 h (Fig. 4C). In addition, the VvMAPK9 transcription had a significant rise under ABA treatment, and expression peak appeared at 3 h (Fig. 4D). All these results indicated that VvMAPK9 seem to be involved in responses to a variety of abiotic stresses.
VvMAPK9 expression under various abiotic stresses. VvMAPK9 expression levels were analyzed on 2-month-old grape seedlings treated with 200 mM NaCl (A), 200 mM Mannitol treatment (B), 42 °C (C) and 100 µM ABA (D), respectively. The β-actin gene from Vitis vinifera was used as an internal control. In addition, the expression levels were normalized to grape without any stress treatment (0 h). The data are means ± SE of three independent experiments (n = 3). Different letters above the bar indicate significant differences (P ≤ 0.01) based on Duncan’s multiple range tests
Analyses of cis-elements in promoter sequence of VvMAPK9
To further investigate the mechanism that the VvMAPK9 responds to abiotic stresses, 2000 bp upstream sequence of the VvMAPK9 was analysed by the PlantCARE database. Many putative cis-acting elements were predicted in the promoter sequence of VvMAPK9, which are related to abiotic and biotic stress responses and light responsiveness (Table 1). Specifically, stress response element (STRE) is involved in responses to osmotic stress. Ethylene responsive element (ERE), MYB and MYC elements are participated in responses to drought stress. W-box (combined with WRKY transcription factor binding site) is related to inducer, injury and pathogen responses. In addition, some of these cis-elements have been shown to be involved in low temperature and salicylic acid responsiveness.
Overexpression of VvMAPK9 in Arabidopsis enhanced salt tolerance
To investigate the role of VvMAPK9 in abiotic stress resistance in plants, VvMAPK9 was overexpressed in Arabidopsis. Three transgenic lines (OE1, OE2, and OE3) with different expression levels of VvMAPK9 were selected for further experiments. Under non-stressful conditions, the seed germination and development of WT and OE plants were no significant different. However, following treatment with NaCl, the seeds of the OE lines germinated much earlier, and the germination rate of OE lines were significantly higher than that of WT. At 48 h, the germination rates of OE1, OE2, and OE3 were about 1.3-, 2- and 2.5-fold of WT, respectively (Fig. 5). At the same time, the seeds of WT and OE lines with the same germination status were spread in 1/2 MS medium containing 0, 100 or 200 mM NaCl. After 2 weeks, on MS medium without NaCl, the root length of WT and OE line seedlings was consistent. However, under NaCl treatment, the root length of OE seedlings was significantly longer than that of WT (Fig. 6A, B). To further examine salt tolerance in the transgenic Arabidopsis during the vegetative growth stages, 2-week-old WT and OE seedlings were treated with 200 mM NaCl for 14 days. It was found that the leaves of WT were significantly withered and even died, while the OE lines grew significantly better (Fig. 6C). These results showed that overexpression of the VvMAPK9 gene confers tolerance to salt stress in the early growth of Arabidopsis.
The germination phenotypes of WT and VvMAPK9-overexpressing (OE) lines under salt stress. A Seedling phenotype of WT and OE lines in 1/2 MS medium with or without NaCl. B The germination rates of WT and OE plants grown on 1/2 MS medium with or without NaCl. Three independent experiments were carried out using 64 seeds in each
The phenotype of WT and VvMAPK9-overexpressing plants under salt stress. A Root phenotypes of WT and OE lines in 1/2 MS medium containing NaCl (0, 100, 200 mM). B Root length of WT and OE lines in 1/2 MS medium containing NaCl (0, 100, 200 mM). C Phenotypes of WT and OE seedlings were treated with 200 mM NaCl for 14 days
Overexpression of VvMAPK9 improved the salt tolerance of grape callus
To further examine whether VvMAPK9 participates in salt stress tolerance in grape, VvMAPK9 was overexpressed in grape callus, three transgenetic lines (OE1, OE2, and OE3) exhibiting different expression levels of VvMAPK9 were obtained and used for further experiments. WT and transgenic grape callus with the same size and growth state were transferred to the medium containing different concentrations of NaCl for 10 days. Under the normal condition, the growth of WT and transgenic callus showed no significant differences. However, under salt stress treatment, the transgenic grape callus grew faster and were significantly larger than WT. Moreover, on the medium with 200 mM NaCl, most of WT callus stopped growing and their color changed to brown, while the transgenic callus grew better with a pale yellow color (Fig. 7A). Additionally, under normal conditions, the relative conductivity had no significant differences between WT and transgenic callus. However, after salt stress, the relative conductivity of transgenic lines was significantly lower than that of WT (Fig. 7B). These results suggested that the overexpression of VvMAPK9 improved the salt-tolerance ability of grape callus.
The phenotypes, relative conductivity and anti-superoxide anion activity of the WT and VvMAPK9-overexpressing grape callus after cultured on the medium containing NaCl (0, 150, 200 mM) for 10 days. A The phenotypes. B The relative conductivity. C The anti-superoxide anion activity. The data are means ± SE of three independent experiments (n = 3). Different letters above the bar indicate significant differences (P ≤ 0.01) based on Duncan’s multiple range tests
Salinity imposes osmotic stress on plants, which will lead to ROS overproduction and cause ROS-associated injury (Krasensky and Jonak 2012). Therefore, the anti-superoxide anion activity of callus was measured in this study. Under normal growth conditions, the anti-superoxide anion activity of transgenic callus was obviously lower than that of WT. However, after salt stress treatment, the anti-superoxide anion activity of transgenic callus was significantly higher than that of WT (Fig. 7C). The results indicated that the VvMAPK9-overexpressing callus had a strong ability to remove ROS under salt stress.
VvMAPK9 participates in the metabolism of ROS under salt stress
To explore the possible mechanisms underlying the increased activity of anti-O2·−, the antioxidant enzyme activity in grape callus were further examined. As shown in Fig. 8, under normal condition, the POD activity of transgenic callus was not significantly different from that of the WT callus, and SOD activity was significantly lower than that of WT callus. After 200 mM NaCl treatment, the POD and SOD activities of wild-type callus were all decreased. However, the POD and SOD activities of transgenic callus were greatly increased and significantly higher than that of WT callus decreased (Fig. 8A and B). The activities of CAT and APX in transgenic callus were all higher than that in WT under normal condition, but the upregulation degree was not significantly different from the WT after salt treatment (Fig. 8C and D). Synthesizing the above results, VvMAPK9 mainly improves salt resistance of grape callus by regulating VvPOD and VvSOD, and enhancing POD and SOD activity.
Antioxidant enzyme activity of WT and VvMAPK9-overexpressed grape callus. The grape callus was cultured on the medium containing NaCl (0, 200 mM) for 10 days. The activity of A POD, B SOD, C CAT and D APX in WT and transgenic callus were measured, respectively. The data are means ± SE of three independent experiments (n = 3). Different letters above the bar indicate significant differences (P ≤ 0.01) based on Duncan’s multiple range tests
Discussion
MAPK pathways play an important role in plant response to various adverse environmental stimuli. It is a signal transduction pathway ubiquitous in eukaryotic organisms, in plant cells it transmits various external signals from the cell surface to the nucleus in a cascade signaling pathway, thus regulating plant growth and development and stress response (Nakagami et al. 2005; Zhang and Klessig 2001). Therefore, it is of great significance to study the MAPK pathway in plants. However, most studies on MAPK genes are limited to model plants, and there are few studies on MAPK in other plants, especially in fruit trees. In this study, VvMAPK9, a group B MAPK gene, was isolated from grape, which possesses the typical features of group B MAPKs, such as the TEY activation motif and CD domain (Fig. 1) (MAPK group 2002). Overexpression of VvMAPK9 in Arabidopsis thaliana and grape callus enhanced their tolerance to salt stress.
As the most downstream kinase of the MAPK cascade pathway, MAPK, when phosphorylated, can not only continue to stay in the cytoplasm to activate other proteins, but also enter the nucleus to activate transcription factors and regulate the expression of genes (Xu and Zhang 2015). Through the subcellular localization of MAPK, a lot of information can be learned, including its upstream genes and the interaction mechanism between MAPK and protein substrates. In this present study, the analysis of subcellular localization revealed that VvMAPK9 protein was localized in both the cytoplasm and the nucleus (Fig. 4), suggesting that VvMAPK9 may function in both the cytoplasm and the nucleus.
MAPKs have been confirmed to participate in the regulation of abiotic stress (Ichimura et al. 2000). For example, AtMPK4 participated in salt stress through a cascade reaction of MEKK1-MKK2-MPK4 (Brader et al. 2007; Furuya et al. 2014). Overexpression of ZmSIMK improved the salt and drought tolerance of plants (Gu et al. 2010; Wang et al. 2014a, b). OsMAPK4 can activate OsWRKY30 to improve salt tolerance through the MKK1-MPK4 cascade pathway (Wang et al. 2014a, b). Moreover, proteomic analysis showed that BnMAPK4 activation affects multiple pathways, such as stress and defense responses (Zhang et al. 2019). The present study revealed that VvMAPK9 has high homology with these MAPK genes, therefore, we speculate that VvMAPK9 may has the same function with them. The analysis of VvMAPK9 promoter suggested that some cis-acting elements related to abiotic stress, including MYC, MYB, ERE, STRE etc., were identified from the promoter region. Further, the expression of VvMAPK9 was induced by drought, salt, high temperature and ABA, which suggested that VvMAPK9 might be involved in regulating responses to various abiotic stresses. In addition, overexpression of VvMAPK9 in Arabidopsis significantly enhanced salt stresses tolerance, with higher germination rates, longer root length, and better growth condition under salt stress. Moreover, the VvMAPK9 transgenic grape callus displayed better salt tolerance than WT as a result of larger volume and lower relative conductivity under salt stress.
Salt stress can result in the accumulation of excessive ROS, which have been proved to have a negative effect on abiotic stress resistance in plants. Therefore, an increased ROS-scavenging ability might be beneficial to plant tolerance to abiotic stresses (Gill and Tuteja 2010). Previous studies revealed that MAPK pathways play an important role in mediating the antioxidative system under abiotic stresses (Jalmi and Sinha 2015). In the present study, grape callus overexpressing VvMAPK9 had higher anti-superoxide anion activity than WT under salt stress treatment, indicating that transgenic callus could eliminate excessive ROS in time. Antioxidant enzymes POD and SOD play a key role in ROS clearance, which can reduce or eliminate the damage caused by salt stress (Liang et al. 2003). This study suggested that the activities of SOD and POD were significantly higher in the VvMAPK9-overexpressing grape callus than in the WT callus after salt stress, indicating that VvMAPK9 might improve the salt tolerance of grape callus by positively regulating the ROS pathway.
In conclusion, a grape group B MAPK gene, VvMAPK9, was isolated and characterized. The expression of VvMAPK9 was induced by various abiotic stresses, and it was proved that this gene was involved in the process of salt stress resistance in Arabidopsis and grape callus. VvMAPK9 can positively regulate the antioxidative system to reduce accumulation of ROS under salt stress. These findings not only extend our knowledge of the group B MAPKs but als provide new clues in the regulation of salt tolerance in grape.
Data availability
The amino acid sequences of Arabidopsis was downloaded from The Arabidopsis Information Resource (https://www.arabidopsis.org).
Code availability
Amino acid sequences of other plants MAPK genes were retrieved from GenBank (http://www.ncbi.gov/Genbank). Amino acid sequence alignments were done using the DNAMAN program (version 5.2.2). Analysis of the promoter sequence of VvMAPK9 were performed using PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). The phylogenetic tree was constructed by the NJ (Neighbor-Joining) method using MEGA 4. Statistical significance was analyzed using Duncan’s multiple range tests with analysis of variance (ANOVA), and calculations were performed with SPSS Statistics.
References
Asai S, Ohta K, Yoshioka H (2008) MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell 20(5):1390–1406. https://doi.org/10.1105/tpc.107.055855
Bohnert HJ, Nelson DE, Jensen RG (1995) Adaptations to environmental stresses. Plant Cell 7(7):1099–1111. https://doi.org/10.1105/tpc.7.7.1099
Brader G, Djamei A, Teige M, Palva ET, Hirt H (2007) The MAP kinase kinase MKK2 affects disease resistance in Arabidopsis. Mol Plant Microbe Interact 20(5):589–596. https://doi.org/10.1094/MPMI-20-5-0589
Çakır B, Kılıçkaya O (2015) Mitogen-activated protein kinase cascades in Vitis vinifera. Front Plant Sci 6:556. https://doi.org/10.3389/fpls.2015.00556
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16(6):735–743. https://doi.org/10.1046/j.1365-313x.1998.00343.x
Danquah A, de Zelicourt A, Colcombet J, Hirt H (2014) The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol Adv 32(1):40–52. https://doi.org/10.1016/j.biotechadv.2013.09.006
Danquah A, de Zélicourt A, Boudsocq M, Neubauer J, Frei Dit Frey N, Leonhardt N, Pateyron S, Gwinner F, Tamby JP, Ortiz-Masia D, Marcote MJ, Hirt H, Colcombet J (2015) Identification and characterization of an ABA-activated MAP kinase cascade in Arabidopsis thaliana. Plant J 82(2):232–244. https://doi.org/10.1111/tpj.12808
Furuya T, Matsuoka D, Nanmori T (2014) Membrane rigidification functions upstream of the MEKK1-MKK2-MPK4 cascade during cold acclimation in Arabidopsis thaliana. FEBS Lett 588(11):2025–2030. https://doi.org/10.1016/j.febslet.2014.04.032
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gu L, Liu Y, Zong X, Liu L, Li DP, Li DQ (2010) Overexpression of maize mitogen-activated protein kinase gene, ZmSIMK1 in Arabidopsis increases tolerance to salt stress. Mol Biol Rep 37(8):4067–4073. https://doi.org/10.1007/s11033-010-0066-6
Hamel LP, Nicole MC, Sritubtim S, Morency MJ, Ellis M, Ehlting J, Beaudoin N, Barbazuk B, Klessig D, Lee J, Martin G, Mundy J, Ohashi Y, Scheel D, Sheen J, Xing T, Zhang S, Seguin A, Ellis BE (2006) Ancient signals: comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci 11(4):192–198. https://doi.org/10.1016/j.tplants.2006.02.007
Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozaki K (2000) Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J 24(5):655–665. https://doi.org/10.1046/j.1365-313x.2000.00913.x
Jalmi SK, Sinha AK (2015) ROS mediated MAPK signaling in abiotic and biotic stress-striking similarities and differences. Front Plant Sci 6:769. https://doi.org/10.3389/fpls.2015.00769
Jonak C, Ligterink W, Hirt H (1999) MAP kinases in plant signal transduction. Cell Mol Life Sci 55(2):204–213. https://doi.org/10.1007/s000180050285
Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97(6):2940–2945. https://doi.org/10.1073/pnas.97.6.2940
Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63(4):1593–1608. https://doi.org/10.1093/jxb/err460
Li Y, Cai H, Liu P, Wang C, Gao H, Wu C, Yan K, Zhang S, Huang J, Zheng C (2017) Arabidopsis MAPKKK18 positively regulates drought stress resistance via downstream MAPKK3. Biochem Biophys Res Commun 484(2):292–297. https://doi.org/10.1016/j.bbrc.2017.01.104
Liang Y, Chen Q, Liu Q, Zhang W, Ding R (2003) Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J Plant Physiol. 160(10):1157–1164. https://doi.org/10.1078/0176-1617-01065
Lu Y, Su W, Bao Y, Wang S, He F, Wang D, Yu X, Yin W, Liu C, Xia X (2020) Poplar PdPTP1 gene negatively regulates salt tolerance by affecting ion and ROS homeostasis in populus. Int J Mol Sci 21(3):1065. https://doi.org/10.3390/ijms21031065
MAPK Group (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 7(7):301–308. https://doi.org/10.1016/s1360-1385(02)02302-6
Nakagami H, Pitzschke A, Hirt H (2005) Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci 10(7):339–346. https://doi.org/10.1016/j.tplants.2005.05.009
Pérez-Salamó I, Papdi C, Rigó G, Zsigmond L, Vilela B, Lumbreras V, Nagy I, Horváth B, Domoki M, Darula Z, Medzihradszky K, Bögre L, Koncz C, Szabados L (2014) The heat shock factor A4A confers salt tolerance and is regulated by oxidative stress and the mitogen-activated protein kinases MPK3 and MPK6. Plant Physiol 165(1):319–334. https://doi.org/10.1104/pp.114.237891
Qin F, Shinozaki K, Yamaguchi-Shinozaki K (2011) Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol 52(9):1569–1582. https://doi.org/10.1093/pcp/pcr106
Shi J, Zhang L, An H, Wu C, Guo X (2011) GhMPK16, a novel stress-responsive group D MAPK gene from cotton, is involved in disease resistance and drought sensitivity. BMC Mol Biol 12:22. https://doi.org/10.1186/1471-2199-12-22
Sun W, Chen H, Wang J, Sun HW, Yang SK, Sang YL, Lu XB, Xu XH (2015) Expression analysis of genes encoding mitogen-activated protein kinases in maize provides a key link between abiotic stress signaling and plant reproduction. Funct Integr Genomics 15(1):107–120. https://doi.org/10.1007/s10142-014-0410-3
Takahashi F, Mizoguchi T, Yoshida R, Ichimura K, Shinozaki K (2011) Calmodulin-dependent activation of MAP kinase for ROS homeostasis in Arabidopsis. Mol Cell 41(6):649–660. https://doi.org/10.1016/j.molcel.2011.02.029
Teige M, Scheikl E, Eulgem T, Dóczi R, Ichimura K, Shinozaki K, Dangl JL, Hirt H (2004) The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell 15(1):141–152. https://doi.org/10.1016/j.molcel.2004.06.023
Tuteja N (2007) Abscisic acid and abiotic stress signaling. Plant Signal Behav 2(3):135–138. https://doi.org/10.4161/psb.2.3.4156
Wang F, Jing W, Zhang W (2014a) The mitogen-activated protein kinase cascade MKK1-MPK4 mediates salt signaling in rice. Plant Sci 227:181–189. https://doi.org/10.1016/j.plantsci.2014.08.007
Wang L, Liu Y, Cai G, Jiang S, Pan J, Li D (2014b) Ectopic expression of ZmSIMK1 leads to improved drought tolerance and activation of systematic acquired resistance in transgenic tobacco. J Biotechnol 172:18–29. https://doi.org/10.1016/j.jbiotec.2013.11.006
Wu L, Zu X, Zhang H, Wu L, Xi Z, Chen Y (2015) Overexpression of ZmMAPK1 enhances drought and heat stress in transgenic Arabidopsis thaliana. Plant Mol Biol 88(4–5):429–443. https://doi.org/10.1007/s11103-015-0333-y
Xing Y, Jia W, Zhang J (2008) AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J 54(3):440–451. https://doi.org/10.1111/j.1365-313X.2008.03433.x
Xiong L, Yang Y (2003) Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid-inducible mitogen-activated protein kinase. Plant Cell 15(3):745–759. https://doi.org/10.1105/tpc.008714
Xu J, Zhang S (2015) Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci 20(1):56–64. https://doi.org/10.1016/j.tplants.2014.10.001
Xu L, Xiang G, Sun Q, Ni Y, Jin Z, Gao S, Yao Y (2019) Melatonin enhances salt tolerance by promoting MYB108A-mediated ethylene biosynthesis in grapevines. Hortic Res 6:114. https://doi.org/10.1038/s41438-019-0197-4
Zaidi I, Ebel C, Belgaroui N, Ghorbel M, Amara I, Hanin M (2016) The wheat MAP kinase phosphatase 1 alleviates salt stress and increases antioxidant activities in Arabidopsis. J Plant Physiol 193:12–21. https://doi.org/10.1016/j.jplph.2016.01.011
Zhang S, Klessig DF (2001) MAPK cascades in plant defense signaling. Trends Plant Sci 6(11):520–527. https://doi.org/10.1016/s1360-1385(01)02103-3
Zhang T, Chhajed S, Schneider JD, Feng G, Song WY, Chen S (2019) Proteomic characterization of MPK4 signaling network and putative substrates. Plant Mol Biol 101(3):325–339. https://doi.org/10.1007/s11103-019-00908-9
Zhou WJ, Leul M (1998) Uniconazole-induced alleviation of freezing injury in relation to changes in hormonal balance, enzyme activities and lipid peroxidation in winter rape. Plant Growth Regul 26(1):41–47. https://doi.org/10.1023/a:1006004921265
Zhou S, Chen Q, Sun Y, Li Y (2017) Histone H2B monoubiquitination regulates salt stress-induced microtubule depolymerization in Arabidopsis. Plant Cell Environ 40(8):1512–1530. https://doi.org/10.1111/pce.12950
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant No. 31972358), the Natural Foundation of Shandong Province (Grant No. ZR2018MC022) and Shandong Provincial Key Research and Development Project (Grant No. 2019JZZY010727).
Author information
Authors and Affiliations
Contributions
QS and BL conceived and designed the experiments. XJ, CS, and YY conducted the experiments. XJ and XL analyzed the data. XJ and QS wrote the manuscript. All authors read and approve the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Henryk Flachowsky.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11240_2021_2218_MOESM1_ESM.tif
Fig. S1 Identification of the transgenic plants of VvMAPK9. (A) Screening of overexpressing strains by antibiotic resistance. (B) Identification of transgenic seedlings by PCR. (C) The expression of VvMAPK9 in the leaves of WT and transgenic plants. Supplementary file1 (TIF 2444 KB)
11240_2021_2218_MOESM2_ESM.tif
Fig. S2 Screening and identification of VvMAPK9-overexpressing grape callus. (A) Wild-type grape callus. (B) Agrobacterium tumefaciens and grape callus were cultured together. (C) Screening transgenic grape callus by antibiotic resistance. (D) Transgenic grape callus was obtained (circled in red). (E) The transgenic grape callus was cultured by screening medium. (F) The expression level of VvMAPK9 in WT and transgenic grape callus. Supplementary file2 (TIF 2894 KB)
Rights and permissions
About this article
Cite this article
Ji, X., Sui, C., Yu, Y. et al. Grape VvMAPK9 positively regulates salt tolerance in Arabidopsis and grape callus through regulating the antioxidative system. Plant Cell Tiss Organ Cult 148, 609–622 (2022). https://doi.org/10.1007/s11240-021-02218-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02218-9