Abstract
Pre-harvest sprouting (PHS) is a primary cause of decreases in quality and economic value in rice seeds. This study examined the inhibitory effects of four combinations of maleic hydrazide, eugenol and uniconazole on rice PHS and seed quality. After the four treatments, delayed grain germination was observed compared with the control, mean germination time was prolonged by 17.5 % and germination index was decreased by an average of 18.2 %. Activity of α-amylase was significantly reduced, by 26.7–83.5 %, during grain germination and early seedling growth. We then used qRT-PCR to further analyze the mRNA expression levels of four genes involved in α-amylase biosynthesis. The expression of OsAMY3B and OsAMY3E was significantly down-regulated by the four combinations of chemicals. In field trials, each of the four combinations resulted in a significantly lower sprouting rate and sprouting index compared with the control. In addition, freshly harvested rice seeds showed no significant differences in the mean germination time or germination index between the four combination treatments and the control. After 1 month of storage, there was still no significant difference between the four combination treatments and the control with regard to the mean germination time and germination index. These results suggest that the selected combinations strongly inhibited the seed germination speed and PHS of rice in the field. However, these combinations had no inhibitory effects on the final germination percentage and showed no negative effect on seed quality after short-term storage. The combinations of inhibitors used in this study can be applied to decrease the PHS damage caused by high temperatures and moist weather during the production of hybrid rice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice is an important staple food that is planted worldwide (Maclean et al. 2002; Valipour et al. 2014; Valipour 2014, 2015). Pre-harvest sprouting (PHS) occurs when grains (e.g., in rice, wheat, barley and maize) germinate in ears before field harvesting (Groos et al. 2002). Prior to harvest, physiologically mature grains sprout easily within ears under conditions of high humidity and temperature (Gubler et al. 2005; Yang et al. 2007). PHS generally reduces the cereal yield and degrades seed quality for planting, resulting in severe economic losses of cultivars with high rates of PHS (Chono et al. 2006; Depauw et al. 2009; Ullrich et al. 2009). PHS also complicates seed separation and processing because the sprouting seeds must be sorted and discarded. Moreover, the lixivium of sprouting seeds may affect other, normal seeds during seed soaking before sowing (Wang et al. 2008). Rice hybrids showing a yield advantage of 20 % over conventional varieties have been cultivated in China since the 1970s, and these varieties occupy approximately 58 % of the rice cultivation area in China and more than 5.2 million ha in other countries. However, these hybrid varieties are more prone to ear sprouting than conventional rice. In China, the annual incidence of PHS in rice is approximately 2–5 %, though it increases to 7–50 % if rainfall occurs during harvest time. This rate can be as high as 90 % for hybrid rice cultivars (Zhou et al. 2011). Therefore, establishing ways to reduce or eliminate PHS is crucial for rice cultivation.
Many plant growth regulators have been tested for the inhibition of PHS, including ethephon, maleic hydrazide, uniconazole, paclobutrazol, abscisic acid and coumaric acid (Duan et al. 1996; Wang et al. 2000). Combinations of coumaric acid and paclobutrazol show a greater inhibitory effect on PHS than treatment with either agent individually, decreasing the grain sprouting rate to approximately 61 % of that in the control (Tan et al. 2006).
Based on previous results, we designed four combinations of three chemicals with different inhibitory effects: maleic hydrazide (MH), uniconazole and eugenol. MH, known for its strong inhibitory effect, has been widely used for the regulation of seed dormancy and germination as well as for axillary bud inhibition in plants. Duan et al. (1996) found that admixtures of MH with other chemicals might have a stronger inhibitory effect on rice PHS than MH by itself. In addition, fewer abnormal seedlings were observed when MH mixtures were used compared with the single MH treatment. However, the use of MH has decreased due to its toxic effect on cells and chromosomes (Marcano et al. 2004; Yurdakok et al. 2014). Recently, an increasing number of plant growth retardants have been studied. Uniconazole is an efficient and degradable plant growth retardant that is widely used in plant production (Fletcher and Hofstra 1990; Saito et al. 2006). Wang et al. (2000) found that both uniconazole and ABA effectively inhibit PHS in hybrid rice, and a concentration of uniconazole greater than 800 mg/L had an inhibitory effect similar to that of 75 mg/L of ABA. However, ABA was not used in the present study due to its high cost.
In contrast to MH and uniconazole, eugenol is a natural plant extract and is mainly used in medicine and the cosmetic and perfume industries. Eugenol protects plants with its bacteriostatic effect (Cervenka et al. 2008; Walsh et al. 2003). In addition, Darabi et al. (2011) found that wheat seeds soaked in a eugenol solution exhibited a significantly lower germination percentage than those that were soaked in water, indicating that eugenol might inhibit seed germination.
Previous work has suggested various chemicals to control PHS (Duan et al. 1996; Wang et al.2000). While the mechanisms of most of the inhibitors have not been studied in depth, some traditional chemicals are not environmentally friendly. The current investigation sought to develop new combinations of chemicals with good inhibitory effects and without negative effects on seedlings, as well as no adverse environmental effects. The objectives of this study were (1) to investigate the effects of combinations of three inhibitors on rice seed germination, α-amylase activity and genes related to α-amylase biosynthesis; and (2) to evaluate the influence of these combinations on the inhibition of pre-harvest sprouting and seed quality in hybrid rice after harvest and short-term storage.
Materials and methods
Experimental materials
The hybrid rice variety Qianyou 1, which is widely cultivated in eastern China, was supplied by the Zhejiang Nongke Seed Industry Co., Ltd., Hangzhou, People’s Republic of China. Uniconazole was purchased from the Jiangsu Sword Agrochemicals Co., Ltd., Changzhou, People’s Republic of China. Maleic hydrazide (MH) and eugenol were obtained from the Sinopharm Chemical Reagent Co., Ltd., Shanghai, People’s Republic of China. The three chemicals were formulated as follows: certain quantity of uniconazole and maleic hydrazide calculated according to corresponding concentration in Table 1 were resolved in distilled water, then eugenol was added to the mixture solution, finally, stirred the solution in a magnetic stirrer for 10 min. 10 mL solution of each combination was applied to pre-treat F1 seeds of Qianyou 1 in germination test and pre-harvest sprouting simulation in the laboratory, while 100 mL solution was sprayed onto ears of Qianyou 1 female plants in a field trial.
Seed germination and seedling growth
Before germination, surface-sterilized seeds were soaked in 10 mL distilled water (control) or the indicated combinations of chemicals for 1 h and dried at room temperature. For each combination, three replicates of 50 seeds were germinated in covered germination boxes (12 × 12 × 6 cm) containing 3 layers of moistened filter paper and placed in germination chambers under a diurnal cycle of 8 h of light, at 30 °C, and 16 h of darkness, at 20 °C, for 14 days (Wang et al. 2012). Next, 1 mL of water was supplied to each germination box every day. The seeds were noted as germinated when the radicle reached half the length of the seed. The numbers of geminated seeds were counted daily. The germination energy (GE) and germination percentage (GP) were calculated on Day 4 and Day 14, respectively. The germination index (GI) and mean germination time (MGT) were calculated as GI = Σ(Gt/Dt) and MGT = Σ(Gt × Dt)/ΣGt, where Gt is the number of germinated seeds on Day t, and Dt is the time corresponding to Gt in days (Zhang et al. 2007). Primary root length (RL) and shoot height (SH) were manually measured in ten randomly selected seedlings on Day 14; the dry weight of the ten seedlings was measured after drying at 80 °C for 24 h (Zhang et al. 2007).
Pre-harvest sprouting simulation (laboratory conditions)
A total of 50 seeds, with 3 replications were soaked in 10 mL distilled water (control) or each combination treatment for 1 h. Air-filled plastic bags were used for packaging the pre-treated seeds and placed in germination chambers with a diurnal cycle of 12 h of light, at 35 °C, and 12 h of darkness, at 25 °C. The sprouting rate and sprouting index were calculated after 3 days, at which point the seeds were classified into four levels. Level 0: ungerminated seeds; Level 1: the micropyle had broken, but the embryo had not yet penetrated; Level 2: the radicle had extended to less than half the length of the whole seed; and Level 3: the radicle length had extended to more than half the length of the whole seed. Sprouting rate = (number of level 2 + number of level 3)/total number of seeds; sprouting index = (1 × number of level 1 + 3 × number of level 2 + 5 × number of level 3)/total number of seeds (Hu et al. 2002).
Measurement of α-amylase activity
Seeds from the control (soaked in distilled water) and the four combination treatments were collected at 12, 24, 48, 72 and 96 h after imbibition (HAI), quickly frozen in liquid nitrogen and stored at −80 °C. After all of the samples were ready, each seed sample was hulled and ground into fine powder, which was finely homogenized with 10 mL of distilled water, and the mixtures were centrifuged at 5000 rpm for 10 min. The supernatants were collected for chromogenic reactions. α-Amylase activity was measured via the 3,5-dinitrosalicylic acid colorimetric (DNS) method, as described by Li (2000). Here, enzyme activity = M × T/[R × W × t], where M is the maltose content (mg); T is the total volume of the extract; R is the volume of the extract used for the reaction; W is the weight of the seed sample; and t is the reaction time.
Extraction of total RNA and qRT-PCR
Seeds collected at 12, 24, 48 and 72 HAI were quickly frozen in liquid nitrogen and stored at −80 °C. Total RNA was extracted from frozen seeds with an RNeasy Plant Mini Kit (Waryong, Beijing, China), and then 1 μg of RNA was reverse-transcribed into cDNA using a PrimeScript RT Reagent Kit (Takara, Otsu, Shiga, Japan). Transcripts of each gene were measured by real-time qRT-PCR using a LightCycler (Roche, Switzerland) and SYBR Green II (Takara, Otsu, Shiga, Japan) according to the manufacturers’ guidelines. The cycling conditions included annealing at 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s and 60 °C for 1 min, and a final cycle of 95 °C for 10 s, 65 °C for 60 s and 97 °C for 1 s. qRT-PCR primers are listed in Table S1. Gene expression was analyzed using the 2−ΔΔCT method (Livak and Schmittgen 2001), and the experiments were repeated three times. Actin was used as the internal standard gene to normalize the relative mRNA expression levels (Zhu et al. 2009; Zhong et al. 2015). Finally, data were log-transformed (base 10) for the value φ, which was calculated as the ratio of each combination treatment to the control for the α-amylase biosynthetic gene expression level at each imbibition time.
Field trial
The field trial was carried out on June 3, 2013, at the experimental farm of Zhejiang University, Hangzhou, People’s Republic of China. The Qianyou 1 rice hybrid was used to evaluate the inhibitory effect of the selected combinations of chemicals on PHS. For each rice variety, four rows of the female parent and one row of the male parent were grown. The control and combination treatments, with three replicates, were arranged randomly according to a random complete block design. A total of 100 mL of each selected chemical combination was sprayed to rice ears in each field plot (2.5 square meters) on September 4 (early yellow maturity stage), and the control was treated with an equal amount of water. Rain simulators sprayed water for 16 min every hour from 6:00 am to 6:00 pm from September 5 to September 17 (harvest time) to establish a moist environment with a relative humidity of approximately 85 % (weather data in Table S2). Twenty ears harvested from the female rice plants in each replicate were used for the analysis of the seed sprouting rate and sprouting index (as described in Hu et al. 2002). The ungerminated seeds from each combination treatment were used for germination tests before and after storage at room temperature.
Statistical analysis
Statistical analyses of all data were performed using analysis of variance (ANOVA) with SAS software, version 8.0 (SAS Institute, Cary, NC). The percentage data were transformed according to y = arcsin [sqr (x/100)] before analysis. When a significant difference occurred in the treatments, the least significant difference was calculated (α = 0.05, LSD).
Results
Effects of the combinations of inhibitors on seed germination and seedling growth
Compared with the control, the combinations of inhibitors significantly prolonged the mean germination time (MGT) and reduced the germination index (GI), which coincided with germination phenotypes (Figure S1). With the exception of combination 14, the other three combinations significantly reduced the germination energy (GE) compared with the control. Furthermore, no significant differences in the final germination percentage (GP) were observed between the control and the combination treatments (Table 2). Regarding seedling growth, the four combinations resulted in significantly lower values in the length and dry weight of the roots and shoots and significantly higher root length (RL)/shoot height (SH) and root dry weight (RDW)/shoot dry weight (SDW) ratios compared with the control (Table 3; Figure S2). In addition, during the PHS simulation under laboratory conditions, combinations 8, 10, 12 and 14 resulted in lower sprouting rates and sprouting indexes compared with the control, and combination 14 showed a stronger inhibitory effect than the other three combination treatments (Fig. 1).
Effects of the combinations of inhibitors on the sprouting rate (A) and sprouting index (B) of Qianyou 1 during the PHS simulation. Control: seeds soaked in water; 8, 10, 12 and 14: seeds soaked in the combinations shown in Table 1. The data are the means of three replications, and the standard deviation is indicated by bars. Small letter(s) on top of bars indicate significant differences (α = 0.05, LSD) between the control and the combinations
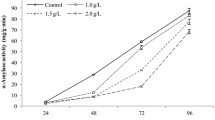
Effects of combinations of inhibitors on α-amylase enzyme activity
α-Amylase enzyme activity increased dramatically from 12 to 96 HAI in the control and the four combination treatments (Fig. 2). However, the α-amylase enzyme activities observed in the four combination treatments were all significantly lower than those in the control at 48, 72 and 96 HAI, and the lowest values were observed at 72 h. In addition, no significant difference was found among the four combination treatments at each evaluated time point.
Effects of the four combinations of inhibitors on α-amylase activities during seed imbibition of Qianyou 1. Control: seeds soaked in water; 8, 10, 12 and 14: seeds soaked in the combinations shown in Table 1. The data are the means of three replicates, and the standard deviation is indicated by bars. Small letter(s) on top of the bars indicate significant differences (α = 0.05, LSD) between the control and the combined treatments. \(\theta\): the ratio of means of each combined treatment to mean of control in α-amylase activity values at each imbibition time; \(\log\theta\): the logarithm to base 10 of the value \(\theta\)
Effects of combinations of inhibitors on expression of α-amylase biosynthetic genes
Four genes involved in the α-amylase biosynthesis showed different expression patterns during seed imbibition in the four combinations of inhibitors (Fig. 3). Compared with the control, OsAMY3B expression in the four combination treatments obviously increased from 12 to 24 h, decreased from 24 to 48 h, reached a significant lowest level at 48 h and then increased from 48 to 72 h (Fig. 3). For combinations 8, 10 and 14, OsAMY3A, OsAMY3E and OsAMY1A expression levels decreased sharply from 24 to 48 h, and OsAMY3A and OsAMY3E levels increased from 48 to 72 h (Fig. 3). For combination 12, gradual increases were observed in OsAMY3A and OsAMY1A expression levels from 12 to 72 h, while OsAMY3E obviously decreased from 12 to 48 h and then increased from 48 to 72 h.
Effects of combined treatments on α-amylase biosynthetic genes (OsAMY3A, OsAMY3B, OsAMY3E and OsAMY1A) expression during seed imbibition from 12 to 72 h in rice cultivar Qianyou 1. A–D: represented 8, 10, 12 and 14 treatments shown in Table 1; Control: seeds soaked in water. \(\phi\): the ratio of each combined treatment to control in α-amylase biosynthetic genes expression level at each imbibition time; \(\log\phi\): the logarithm to base 10 of the value \(\phi\)
Effects of combinations of inhibitors on PHS in the field trial
As shown in Table 4, the rice ears sprayed with the four combinations of chemicals showed a significantly lower sprouting rate and sprouting index than the control. No significant differences in these values were observed between the four combination treatments. However, combination 10 presented slight advantages over the other three.
Effects of combinations of inhibitors on the quality of freshly harvested seeds in the field trial
With the exception of combination 8, the combinations significantly prolonged the MGT and reduced the GE compared with the control (Table 5). There were no significant differences in the GP, shoot height or root/shoot length ratio between seeds that were freshly harvested from the control and combinations of inhibitors. The control had a significantly higher GI than the four combination treatments.
Effects of combinations of inhibitors on seed quality after 1 month of storage
After 1 month of storage, there were no significant differences in the GE, GP, root length or root/shoot length ratio between the control and the four combination treatments (Table 6). With the exception of combination 14, the combinations resulted in no significant differences in the MGT and GI compared with the control. In addition, each of the combination treatments resulted in a significantly lower shoot height.
Discussion
In addition to genetic improvement, the application of exogenous chemicals is an effective way to reduce PHS damage to seed production (Wang et al. 2008). Maleic hydrazide and uniconazole are well-known plant growth regulators (Paterson et al. R1952; Tso et al. 1965; Izumi et al. 1988). In our preliminary experiment, maleic hydrazide, uniconazole and eugenol postponed or inhibited seed germination. The combinations of the three chemicals used in the present study were designed based on previous results. Because of its possible toxicity, MH was limited to a lower dosage of 75–120 mg/L, which was approximately one-tenth the dosage of each of the other two chemicals.
Treatments with the combinations of chemicals significantly slowed seed germination speed compared with the control but resulted in no significant difference in the final germination percentage, indicating that these combination treatments significantly delayed, but did not completely inhibit, the germination of rice seeds. After the seed germination test, shorter root length and shoot height were observed in the four combination treatments compared with the control. This phenomenon might be related to the inhibitory effect of uniconazole on GA biosynthesis, which leads to slower growth and stocky seedlings (Saito et al. 2006). In this study, no abnormal seedlings were observed, suggesting that the selected range of MH concentrations might be acceptable for safe application. In the PHS laboratory simulation, the four combination treatments produced a lower sprouting rate and sprouting index than the control.
During grain seed germination, α-amylase plays a prominent role in hydrolyzing endosperm starch into metabolizable sugars and providing energy for radicle protrusion (Sithichoke et al. 2005; Akazawa and Hara-Nishimura 1985; Beck and Ziegler 1989). Unlike other amylolytic enzymes, it is responsible for activating carbohydrate reserves by breaking α-1,4-linked polyglucans and initiating starch breakdown (Janeček et al. 2014). In the present study, α-amylase activity was almost undetectable when seeds were imbibed in water for 12 h; however, it increased markedly with increasing imbibition times. The application of exogenous inhibitors significantly postponed the synthesis of α-amylase. This result might be closely related to the prolonged mean germination time, lower germination index, shorter root length and shoot height, and lower sprouting rate and sprouting index observed after treatment with the combinations of inhibitors. However, this phenomenon still warrants further study. In addition, relatively stronger inhibitory effects on seed germination and α-amylase enzyme activities were observed for combinations 8 and 14, which might be due to the higher MH concentrations used in these combinations. Compared with the α-amylase activity data, several genes involved in α-amylase synthesis had different expression patterns. Among the four tested genes, expression levels of OsAMY3B and OsAMY3E were significantly inhibited in the four combinations compared with the control; the levels of these genes declined dramatically from 24 to 48 h of seed imbibition and reached the maximum difference at 48 h. These results were consistent with the lower α-amylase activities in combination treatments than in the control from 48 to 72 h. The results also suggested that the changes in the protein content resemble the changes in gene expression, but changes of protein content came after gene expression, which was consistent with changes in gene transcription being followed by protein translation. In addition, OsAMY1A and OsAMY3A expression levels showed no consistent change among the four combination treatments, possibly due to the existence of complex regulation of the α-amylase activity level during seed imbibition.
In the field trial, the rice ears sprayed with combinations of inhibitors showed significantly lower sprouting rates and indexes than those treated with water. The sprouting rates and sprouting indexes observed under the four combination treatments were similar, and these values were approximately 52–62 and 54–62 %, respectively, of the control. The germination test of seeds freshly harvested from the hybrid line treated with the combinations of inhibitors showed a delayed mean germination time and lower germination index than the control, which might have resulted from the sustained inhibitory effect of the combination treatments. The differences in the seed germination parameters between the combination treatments and the control decreased after 1 month of storage, indicating that the inhibitory effect of the combination treatments on seed germination gradually weakened with the seed storage time. In addition, combination 10 appeared to have a slightly stronger inhibitory effect on grain sprouting in field production, and seeds harvested after treatment with combination 10 showed a similar germination performance compared with the other three combinations. These results are slightly inconsistent with the performance that was observed under treatment with combination 10 in the laboratory; this phenomenon might be related to the properties of the chemicals and of the different treatment conditions.
In our preliminary study, MH had a strong inhibitory effect on seed germination and a negative effect on seedling growth. The inhibitory effect of eugenol was weaker than that of MH; however, eugenol is an environmentally friendly compound that may promote chemical permeation. In the present study, the tested chemical combinations were designed to achieve a better product that showed both a strong inhibitory effect on rice PHS and no negative effect on seed quality. Further studies on the improvement of inhibitory combinations and the associated molecular and physiological mechanisms involved in PHS inhibition are still required.
References
Akazawa T, Hara-Nishimura I (1985) Topographic aspects of biosynthesis, extracellular secretion, and intracellular storage of proteins in plant cells. Annu Rev Plant Physiol Plant Mol Biol 36(1):441–472
Beck E, Ziegler P (1989) Biosynthesis and degradation of starch in higher plants. Annu Rev Plant Biol 40(1):95–117
Cervenka L, Peskova I, Pejchalova M, Vytrasova J (2008) Inhibition of Arcobacter butzleri, Arcobacter cryaerophilus, and Arcobacter skirrowii by plant oil aromatics. J Food Prot 71(1):165–169
Chono M, Honda I, Shinoda S, Kushiro T, Kamiya Y, Nambara E, Kawakami N, Kaneko S, Watanabe Y (2006) Field studies on the regulation of abscisic acid content and germinability during grain development of barley: molecular and chemical analysis of pre-harvest sprouting. J Exp Bot 57(10):2421–2434
Darabi HR, Mohandessi S, Balavar Y, Moghaddam MM, Aghapoor K, Mohsenzadeh F, Nourinia AA (2011) Clove bud oil: an efficient, economical and widely available oil for the inhibition of wheat seed germination. Environ Chem Lett 9(4):519–524
DePauw RM, Clarke FR, Fofana B, Knox R, Humphreys G, Cloutier S (2009) RL4137 contributes preharvest sprouting resistance to Canadian wheats. Euphytica 168(3):347–361
Duan XM, Fan LJ, Ma HS (1996) Study on PHS inhibition of Hybrid rice shanyou 63 (1996) Hybrid Rice 1:37–39 (In Chinese with English abstract)
Fletcher RA, Hofstra G (1990) Improvement of uniconazole-induced protection in wheat seedlings. J Plant Growth Regul 9(1–4):207–212
Groos C, Gay G, Perretant MR, Gervais L, Bernard M, Dedryver F, Charmet G (2002) Study of the relationship between pre-harvest sprouting and grain color by quantitative trait loci analysis in a white-red grain bread-wheat cross. Theor Appl Genet 104:39–47
Gubler F, Millar AA, Jacobsen JV (2005) Dormancy release, ABA and pre-harvest sprouting. Curr Opin Plant Biol 8(2):183–187
Hu WM, He CX, Ma HS (2002) Genetic effects of pre-harvest sprouting in hybrid rice (Oryza sativa L.). Chin J Rice Sci 3:76–79 (In Chinese with English abstract)
Izumi K, Nakagawa S, Kobayashi M, Oshio H, Sakurai A, Takahashi N (1988) Levels of IAA, cytokinins, ABA and ethylene in rice plants as affected by a gibberellin biosynthesis inhibitor, Uniconazole-P. Plant Cell Physiol 29(1):97–104
Janeček Š, Svensson B, MacGregor EA (2014) α-Amylase: an enzyme specificity found in various families of glycoside hydrolases. Cell Mol Life Sci 71:1149–1170
Li HS (2000) Principle and technology of plant physiological and biochemical experiments. Higher Education Press, Beijing, pp 34–67
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408
Maclean JL, Dawe DC, Hardy B, Hettel GP (2002) Rice almanac, 3rd edn. IRRI, WARDA, CIAT and FAO, Philippines
Marcano L, Carruyo I, Del Campo A, Montiel X (2004) Cytotoxicity and mode of action of maleic hydrazide in root tips of Allium cepa L. Environ Res 94:221–226
Paterson DR, Wittwer SH, Weller LE (1952) The effect of preharvest foliar sprays of maleic hydrazide on sprout inhibition and storage quality of potatoes. Plant Physiol 27:101–107
Saito S, Okamoto M, Shinoda S, Kushiro T, Koshiba T, Kamiya Y, Hirai N, Todoroki Y, Sakata K, Nambara E, Mizutani M (2006) A plant growth retardant, uniconazole, is a potent inhibitor of ABA catabolism in Arabidopsis. Biosci Biotech Bioch 70(7):1731–1739
Sithichoke T, Maliwan N, Chinae T (2005) Isolation and characterization of an α-amylase gene in cassava (Manihot esculenta). Plant Physiol Bioch 43(9):821–827
Tan HJ, Tao LX, Wang X, Yang CD, Dong WZ, Ji MR (2006) Inhibitory Effects of Suiyake on pre-harvest sprouting of female plant in Hybrid Rice. China Rice 4:30–32 (In Chinese with English abstract)
Tso TC, Steffens GL, Engelhaupt ME (1965) Tobacco sucker control, inhibition of tobacco axillary bud growth with fatty acid methyl esters. J Agr Food Chem 13(1):78–81
Ullrich SE, Lee H, Clancy JA (2009) Genetic relationships between preharvest sprouting and dormancy in barley. Euphytica 168(3):331–345
Valipour M (2014) Future of agricultural water management in africa. Arch Agron Soil Sci 52(7):245–268
Valipour M (2015) A comprehensive study on irrigation management in asia and oceania. Arch Agron Soil Sci 61(9):1247–1271
Valipour M, Ahmadi MZ, Raeini-Sarjaz M, Sefidkouhi MAG, Shahnazari A (2014) Agricultural water management in the world during past half century. Arch Agron Soil Sci 61(5):1–22
Walsh SE, Maillard JY, Russell AD, Catrenich CE, Charbonneau DL, Bartolo RG (2003) Activity and mechanisms of action of selected biocidal agents on Gram-positive and -negative bacteria. J Appl Microbiol 94:240–247
Wang X, Tao XL, Huang XL, Yu MY (2000) Effects of exogenous ABA on panicle sprouting of F1 in hybrid rice seed production. Acta Agron Sin 26(1):59–64 (In Chinese with English abstract)
Wang YW, Zhang Y, Jin XB, Yi Wei, Zhu JQ (2008) The selection of inhibitors combination to inhibit pre-harvest sprouting in rice and study on eletrophoretic analysis. Seed 27(8):18–21 (In Chinese with English abstract)
Wang Y, Hu J, Qin GC, Cui HW, Wang QT (2012) Salicylic acid analogues with biological activity may induce chilling tolerance of maize (Zea mays L.) seeds. Botany 90(9):845–855
Yang Y, Zhao XL, Xia LQ, Chen XM, Xia XC, Yu Z, He ZH, Röder M (2007) Development and validation of a Viviparous-1 STS marker for pre-harvest sprouting tolerance in Chinese wheats. Theor Appl Genet 115(7):971–980
Yurdakok B, Baydan E, Okur H, Gurcan IS (2014) Cytotoxic effects of etephon and maleic hydrazide in Vero, Hep2, HepG2 cells. Drug Chem Toxicol 37:459–465
Zhang S, Hu J, Zhang Y, Xie XJ, Allen K (2007) Seed priming with brassinolide improves lucerne (Medicago sativa L.) seed germination and seedling growth in relation to physiological changes under salinity stress. Crop Pasture Sci 58(8):811–815
Zhong M, Yuan Y, Shu S, Sun J, Guo S, Yuan R, Tang Y (2015) Effects of exogenous putrescine on glycolysis and Krebs cycle metabolism in cucumber leaves subjected to salt stress. Plant Growth Regul 12:1–12
Zhou SB, Lin W, He LJ, Xiao LT, Zhang XQ (2011) Studies on the physiological changes of preharvest sprouting in rice. Hybrid Rice 26(4):68–71 (In Chinese with English abstract)
Zhu G, Ye N, Zhang J (2009) Glucose-induced delay of seed germination in rice is mediated by the suppression of ABA catabolism rather than an enhancement of ABA biosynthesis. Plant Cell Physiol 50:644–651
Acknowledgments
This research was supported by the Special Fund for Agro-Scientific Research in the Public Interest (No. 201203052), National Natural Science Foundation of China (No. 31371708, 31201279), Zhejiang Provincial Natural Science Foundation (LZ14C130002), Project of Science and Technology Department of Zhejiang Province (No. 2013C02005, 2013C32023), Jiangsu Collaborative Innovation Center for Modern Crop Production and Fundamental Research Funds for the Central Universities (No. 2015QNA6019), People’s Republic of China.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, Q., Fu, Y., Guan, Y. et al. Inhibitory effect of chemical combinations on seed germination and pre-harvest sprouting in hybrid rice. Plant Growth Regul 80, 281–289 (2016). https://doi.org/10.1007/s10725-016-0165-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-016-0165-z