Abstract
Pre-harvest sprouting (PHS) of wheat commonly occurs in rainy or humid weather conditions before harvest. It not only affects the yield but also decreases the wheat quality and seed value. As the most important grain crop in the world, reducing PHS and improving the yield and quality of wheat is essential. In this study, a new compound of methyl jasmonate (MeJA) analog, N-(2, 4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide, was synthesized and its effect on PHS tested. Concentration screening of the new compound showed that [1500 mg L−1 N-(2, 4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide + 1% dimethyl sulfoxide (DMSO) + 0.1% penetrant (T2)] was suitable for inhibiting PHS. Exogenously applied T2 effectively inhibited seed germination and increased abscisic acid (ABA) and decreased gibberellin (GA) concentration. These changes in endogenous hormones led to a significant reduction in α-amylase activity. The results of RNA-Seq and quantitative real-time PCR indicated that expression of genes encoding biosynthesis and catabolism of ABA, GA and α-amylase had a consistent trend with the results of hormone concentrations and α-amylase activity. In conclusion, application of T2 can delay PHS by 2 to 3 days through changing of endogenous hormones to significantly decrease the α-amylase activity and ensure the vigor of wheat seed. These findings can provide some help for the development of a retardant to prevent PHS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pre-harvest sprouting (PHS) of wheat grain is one of the worldwide hazards which affects wheat yield and quality (Groos et al. 2002; Biddulph et al. 2008; Mares and Mrva 2014), including yield loss of 6–10% and the reduction of economic benefits by 20–50% (Simsek et al. 2014). PHS also leads to significant degeneration of starch physicochemical properties due to high α-amylase activity in wheat (Simsek et al. 2014). Sprouted wheat cannot be used for wheat seed or flour production and can only be used to make feed (Simsek et al. 2014). As a worldwide agricultural problem, many studies over the past few decades have focused on factors inducing PHS (Sarah et al. 2013; Mares and Mrva 2014; Kashiwakura et al. 2016). PHS is induced by internal factors such as seed behavior, enzymes, hormones, α-amylase activity, and external factors such as water, temperature, and soil nutrition (Shorinola et al. 2016; Deng et al. 2017). Among these various factors, the activity of α-amylase is a key factor as the germination of wheat seed depends on the supply of nutrients, and the supply of nutrients mainly depends on the activity of amylase to degrade starch (Masojc and Milczarski 2009; Stobrawa et al. 2017). Storage substances in the grains are first decomposed for PHS, and the ɑ-1, 4-glucoside bonds are hydrolyzed in starch molecules, and cut into short-chain dextrins and small amounts of low-molecular sugars (Shahpiri et al. 2015; Qisen and Chengdao 2017). The seed germination rate was positively correlated with α-amylase activity, which indicated that the process and result of spike germination were closely related to endogenous α-amylase activity (Sarah et al. 2013). Therefore, inhibiting or reducing endogenous α-amylase activity is a key factor to prevent PHS of wheat grains.
Molecular breeding, cultivation or chemical methods are used to reduce PHS in wheat grains (Kulwal et al. 2010; Xiao et al. 2012; Liu et al. 2013). Although some achievements have been made, PHS-resistant varieties obtained through breeding methods are far from meeting the production needs, and PHS cannot be effectively overcome at present. There are some chemical retardants such as penicillin, uniconazole for reducing PHS, but they cannot meet the specific needs of current agricultural production and the application range is limited. Therefore, development of new products with new uses has great agricultural application.
Plant hormones as the main substances that regulate plant growth and development and they have been used as plant growth regulators (PGRs) in agriculture for many years (Yang et al. 2020). ABA and GA as main plant hormones are closely related to seed dormancy. ABA is a positive regulator of dormancy and is involved in the maintenance of dormancy. It can also prevent α-amylase activity and transcription, the absorption of Ca2+ by aleurone cells and induce specific proteins such as α-amylase inhibitors (Derera 1989). GA is correlated with stem elongation and growth of leaves, and is also essential for dormancy release and germination increase. Gibberellin-deficient mutation of Arabidopsis and tomato seeds do not germinate without exogenous GA applied (Simsek et al. 2014). These findings suggest that GA is essential to the germination of these seeds. In gramineous plants, α-amylase is newly synthesized during seed germination. This process depends on GA secreted by the embryo, while ABA can inhibit the induction process of GA (Shitani et al. 1997; Cheng et al. 2015). However, ABA is unstable under natural ultraviolet light as well as expensive to purchase, thus restricting its use in agricultural production. MeJA as a plant growth regulator is widely present in plants, and some of its effects are considered similar to ABA (Creelman and Mellut 1997). MeJA also inhibits seed germination and growth (Tsai et al. 1997; Yang et al. 2018). Therefore, the aim of the present study was to synthesize a MeJA analogue and tests its effect on reducing PHS in wheat and elucidating its potential modes of action.
Materials and methods
Plant materials and growth conditions
Fanmai NO. 8, as a widely cultivated wheat variety, was selected as the experimental material. It was planted on a farm (114°26' E, 34°45' N) in Kaifeng, Henan Province, China. The field management method was the same as commercial production method during the growing season at 2018–2019. Wheat spikes were reaped at maturity with 30–40 cm stem.
Synthesis of N-(2, 4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide
A new compound of MeJA analogue, N-(2, 4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide (Fig. 1), was synthesized according to synthesis Scheme 1. The steps for the synthesis of the new compound are as follows.
Step 1 Preparation of dihydrojasmonic acid. The commercially available methyl dihydrojasmonate (5 g, 0.022 mol) and NaOH (2 g, 0.05 mol) in ethanol (40 mL) were stirred at room temperature overnight. At the end of the reaction, the reaction solution was desolvated and 100 mL water was added, then 1 mol L−1 hydrochloric acid was added to adjust the pH value to 3–4. The solution was extracted with 3 × 100 mL ethyl acetate, and the organic phase was combined. After drying with anhydrous sodium sulfate, the target compound 3.00 g was obtained.
Step 2 Preparation of 2-(3-oxo-2-pentylcyclopentyl) acetyl chloride. A mixture of dihydrojasmonic acid (2.04 g, 0.0096 mol) and oxalyl chloride (2 mL) and 3 drops of DMF in dichloromethane (50 mL) was stirred overnight at room temperature to obtain the target compound, which was directly used for the next step reaction.
Step 3 Preparation of N-(2, 4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide. A mixture of 2-(3-oxo-2-pentylcyclopentyl) acetyl chloride (2.59 g, 0.011 mol), ethyl acetate (50 mL) and 2, 4-dimethoxyaniline (1.61 g, 0.0105 mol) was reacted for 3 h. The organic phase was dried with anhydrous sodium sulfate and desolvated, and the target compound 3.10 g was obtained by column chromatography with a yield of 85.1%.
Concentration Screening of N-(2, 4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide and MeJA
Harvested spikes dried at room temperature for 4 days were soaked for 4 h under different concentrations of N-(2,4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide with 1% DMSO dissolved, and six different treatments were designed: the distilled water (CK); 1% DMSO + 0.1% Penetrant (D + P); 1000 mg L−1 N-(2,4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide + 1% DMSO + 0.1% Penetrant (T1); 1500 mg L−1 N-(2,4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide + 1% DMSO + 0.1% Penetrant (T2); 3000 mg L−1 N-(2,4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide + 1% DMSO + 0.1% Penetrant (T3); 4000 mg L−1 N-(2,4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide + 1% DMSO + 0.1% Penetrant (T4). After soaking for 4 h, the wheat spikes were immediately washed with distilled water. Then, they were placed upright in triangular flasks and cultured for 48 h in a light incubator (25 °C, 90% Relative humidity), and sprayed with distilled water four times per day to simulate the rainy weather in the field. The concentration screening of MeJA (200 mg L−1, 400 mg L−1, 800 mg L−1, 1200 mg L−1, 1500 mg L−1) was the same as the above steps Three biological replicates for each treatment were measured.
Analysis of seed germination rate
Based on the results of the concentration screening, an experiment to determine the germination rate was conducted. Five treatments were designed: distilled water (CK); 0.1% Penetrant (P); 1% DMSO + 0.1% Penetrant (D + P); 1200 mg L−1 MeJA + 1% DMSO + 0.1% Penetrant (MeJA); 1500 mg L−1 N-(2, 4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide + 1% DMSO + 0.1% Penetrant (T2). Specific experimental steps were the same as the concentration screening experiment. Germination rate was counted at 0 h, 1 h, 6 h, 12 h, 24 h, 36 h, 48 h, 60 h, 72 h, 84 h, 96 h, 120 h, 168 h after treatment of the wheat spikes (Each wheat spike has 47–53 wheat seeds). The seed germination standard was carried out as described by Gao et al. (2012) with little modification. A wheat grain is considered as germinated when the radicle root of the seed is as long as the seed, and the new bud is at least 1/2 of the seed length (Son et al. 2016). Three biological replications were measured.
Determination of the activity of α-amylase
Wheat grains (ca. 0.5 g FW) from each treatment were weighed and ground in 2 mL of distilled water. The homogenates standing at room temperature for 20 min with shaking every 5 min were centrifuged at 8000 g for 10 min, and the supernatant was diluted to 10 mL. The obtained amylase solution was heated in a water bath at 70 °C for 15 min to inactivate the β-amylase. Then the activity of α-amylase was determined as protocol of the kit (Nanjing Jiancheng, China) with a reaction mixture (0.6 mL) of 0.5 mL substrate buffer and 0.1 mL sample solution. The mixture was incubated for 7.5 min at 37 °C, then 0.5 mL iodine solution and 3.0 mL distilled water were added. Soluble starch was determined as absorbance at 660 nm. One unit of amylase activity is defined as the amount of tissue (1 g) that will hydrolyse 1 mg starch in 1 min at 37 °C. Three biological replications were measured.
Quantification of ABA and GA3 contents
The endogenous hormone, GA3 and ABA, were determined as described by Yoshimoto et al. (2009) with some modifications. The germinated seeds (Ca.0.5 g FW) were added to the precooled mortar and ground to a homogenate with 5 mL 80% precooled chromatographic methanol (V/V) in the ice bath, and extracts were centrifuged at 4 °C for 10 min at 10,000 g. Then 2 mL 80% precooled chromatographic methanol was added to the sample residue to extract for 12 h and centrifuged. The extracted was repeated twice as above. 0.2 g Polyvinylpolypyrrolidone cross-linked (PVPP) was added to the collected supernatant to adsorb phenols and pigments, and shaken for 60 min (4 °C) and centrifuged as above. The concentrated extract was loaded onto a Bond Elut C18 (Agilent, USA) and the cartridge was washed with 5 mL 80% methanol. The liquid was collected in a 25 mL beaker and freeze-dried by using a Lyophilizer (Thermo Fisher Scientific Inc., Waltham, MA, USA) in the dark. After that, 2 mL 80% chromatographic methanol was used to dissolve the above. Extracts were filtered with 0.22 µm (Millipore, USA) and eluted into 1.5 mL autosampler-vials (Agilent, USA), then measured by HPLC–MS (Agilent 6490, USA) HPLC programme was as follows: column: SB C18 column (100 mm × 2.1 mm × 1.8 µm), mobile phase: distilled water with 0.1% formic acid (A) methanol with 0.1% formic acid (B), gradient: 0–0.2 min liner from 5 to 48% B, 0.2–7.0 min liner from 48 to 57% B, flow rate: 0.3 mL min−1, column temperature: 30 °C, injection volume: 2 µL. The eluent was introduced to the MS interface using the electrospray ionization in the negative ion mode. Three biological replications were measured.
RNA-seq analysis
In order to explore the changes of genes under exogenously applied T2, RNA-Seq analysis was performed. Three differently treated groups of wheat seeds were carried out RNA-Seq analysis. As germination rates of wheat seed began to apparently different at 48 h under exogenously applied T2 and MeJA relative to control, samples taken from 48 h were used for RNA-Seq analyses.
T2 treated and untreated wheat seeds (200–300 mg) were frozen in liquid nitrogen and immediately stored at −80 °C. Total RNA was extracted using an RNA extraction kit (Huayueyang, China) according to the manufacturer’s protocol. RNA degradation and contamination was monitored on 1% agarose gels and RNA purity was checked using the Nano Photometer® spectrophotometer (IMPLEN, CA, USA). RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, CA, USA). A total amount of 1 µg RNA per sample was used as input material for the RNA sample preparations. Sequencing libraries were generated using NEBNext® UltraTM RNA Library Prep Kit, PCR amplified, and sequenced on an Illumina® (NEB, USA) system.
Analysis of differentially expressed genes (DEGs) was performed using DESeq2 R package (1.16.1). The genes with an absolute fold change (FC) > 2 and a False Discovery Rate (FDR) < 0.05 were considered as differentially expressed. Principal component analysis (PCA) was also performed in order to assess the consistency among replicates. Three biological replications were measured.
Verify differential expression DEGs by quantitative real-time PCR
Accuracy of expression of DEGs selected from RNA-Seq was verified by quantitative real-time PCR. Total RNA was extracted by using TRIzol reagent (Invitrogen, San Diego, CA) and was reverse transcribed into cDNA synthesis according to the instruction of the Super Smart cDNA Synthesis Kit (Takara, PrimeScript™ RT reagent Kit with gDNA Eraser). Quantitative real-time PCR was performed on a CFX Connect1M Real-Time System (Bio-Rad, USA) using a SYBR Green I Master mix kit (Takara) following the manufacturer’s protocols. The 2−△△CT method was used to calculate relative expression levels with β-actin as internal controls. The gene-specific primers used in this study were as follows: for α-Amy1, ACAGGTCATGCAGGGATAG and CTGCGGGTCCTGATTGAC; for α-Amy2, 5'-TCCACTGATCCACCATTCGA-3' and 5'-CGTATGGCTCATCATTTTGGG-3'; for TaNCED1, 5'-CCCAGCACTAATCGATTCC-3' and 5'-CCGCTAACTGTATCCATGC-3'; for TaCYP707A1, 5'-GCCAGGAAGCGGAACAAG-3' and 5'-AAGAGGTGCGCCTGAGTA-3'; for TaGA3ox2, 5'-GACTCGGGCTTCTTCACCTT-3' and 5'-TGGTGAGGATCTGGAAGAGG-3'; for TaGA20ox, 5'-TTCACGCAGAAGCACTACC-3' and 5'-GAGCAGAGCAATCCATCATGTC-3' and Ta-β-actin (5'-GCTGGAAGGTGCTGAGGGA-3' and 5'-TGGTGAGGATCTGGAAGAGG-3') as the internal control. Three biological replications were measured.
Statistical analysis
A randomized design was adopted in this study with three replications. The statistical analysis was done using Excel 2013 (Microsoft) and among which the volcano plot and the principal component analysis (PCA) were made using R Language (ggplot 2 package). The results were presented as means ± standard deviations.
Results
Concentration screening of different concentrations of N-(2, 4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide
Different responses occurred after soaking the seeds with different concentrations of the N-(2, 4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide (Fig. 2). Compared to the CK, treatments of D + P showed no significant differences, thus demonstrating that these treatments had no significant effect on seed germination. However, there was a significant effect on germination in the wheat spikes soaked in N-(2, 4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide and MeJA solutions. In the treatments of T3 and T4, germination was inhibited due to the higher concentration of N-(2, 4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide. The germination rate of T2 (1.8%) treatment was significantly lower than T1 (14.4%), but it also showed a certain extent of germination capacity, indicating that germination of wheat grains was delayed under T2 treatment (Fig. 2a) and maintain the vigor of the seeds. Treatment of M4 (Fig. 2b) showed the same results.
Effect of grain germination on the soaked wheat spikes under different concentration of N-(2, 4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide in winter wheat (a). CK: the distilled water; D + P: 1% DMSO + 0.1% Penetrant; T1:1000 mg L−1 N-(2, 4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide + 1% DMSO + 0.1% Penetrant; T2: 1500 mg L−1 N-(2, 4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide + 1% DMSO + 0.1% Penetrant; T3: 3000 mg L−1 N-(2, 4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide + 1% DMSO + 0.1% Penetrant; T4: 4000 mg L−1 N-(2, 4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide + 1% DMSO + 0.1% Penetrant, respectively. Effect of grain germination on the soaked wheat spikes under different concentration of MeJA in winter wheat (b). CK: the distilled water; D + P: 1% DMSO + 0.1% Penetrant; M1:200 mg L−1 MeJA + 1% DMSO + 0.1% Penetrant; M2: 400 mg L−1 MeJA + 1% DMSO + 0.1% Penetrant; M3: 800 mg L−1 MeJA + 1% DMSO + 0.1% Penetrant; M4: 1200 mg L−1 MeJA + 1% DMSO + 0.1% Penetrant; M5: 1500 mg L−1 MeJA + 1% DMSO + 0.1% Penetrant, respectively. Three independent replicates were measured
The germination rate of wheat grains after ripening
Germination rates of CK, P and P + D treated seeds were similar, and this suggested that the germination of wheat seeds was unaffected by penetrant and DMSO (Fig. 3). The treatments of CK, P and P + D led to approximately 90% germination rate of wheat seeds within 96 h of inducing. By contrast, exogenously applied T2 and M4 significantly reduced the germination rate where the germination rate was 34.43% and 6.12% respectively after 168 h. Compared to the control, the T2-treated wheat seeds had a delayed germination time of about 60 h, but retained seed vigor. The germination rate of M4-treated wheat seeds was significantly decreased but most of the seeds lost vigor.
Effect of different chemical compounds on germination rate of winter wheat. CK: the distilled water; P: 0.1% Penetrant; D + P: 1% DMSO + 0.1% Penetrant; M4:1200 mg L−1 MeJA + 1% DMSO + 0.1% Penetrant; T2: 1500 mg L−1 T2 + 1% DMSO + 0.1% Penetrant, respectively. Three independent replicates were measured
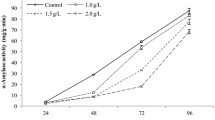
Determination of α-amylase activity after treatment of T2
With an increase in the induced seed germination time, α-amylase activity of each treatment increased except in the M4 treatment (Fig. 4). The increase range of α-amylase activity in the CK, P and P + D treated seeds was significantly greater than that of T2 and M4 treated seeds. The α-amylase activity of CK, P and P + D increased significantly while there was no significant increase in the α-amylase activity of T2 treated seeds before 48 h. After 48 h, there was a slow increase in the α-amylase activity in the T2 treated seeds. The α-amylase activity did not change after treatment with M4 which indicated that the seeds may have lost their vigor.
Quantification of ABA and GA3 contents.
Application of T2 led to an increase in endogenous ABA and a significant decrease in GA3 compared to the control. Exogenously applied T2 increased the ABA concentration before 24 h and then it slowly decreased (Fig. 5a). GA3 content sharply decreased to almost undectable levels by 24 h and then slowly increased (Fig. 5b).
RNA-Seq analysis under exogenously applied T2 at 48 h
In total, 43.04–50.02 million raw reads were generated by high-throughput sequencing for each sample, and 40.71–49.55 million clean reads were obtained after low quality regions removed. According to false discovery rate (FDR) < 0.05 and fold change > 2 differently expressed genes (DEGs), 23,137 up-regulated and 32,200 down-regulated genes were identified in T2 treated sample compared to the control (Fig. 6). Thus application of T2 led to apparent changes in gene expression.
Three biological replicates of each samples clustered together demonstrating the high level of similarity in gene expression among these replicates (Fig. 7). The PCA also showed how genes separate according to different treatments. Wide dispersion of samples indicated that there were large changes in gene expression levels under different treatment (Fig. 7). Compared with CK, the samples treated with T2 and M4 were widely dispersed, however, T2 and M4 are not very dispersed. This showed that T2 and M4 had a relatively consistent effect on the genetic changes of wheat seeds.
Validation of expression genes encoding biosynthesis and catabolism of ɑ-amylase, ABA and GA by quantitative real-time PCR
Quantitative real time PCR was carried out to verify the differentially expressed genes (DEGs) identified by RNA-Seq. Six genes related to the PHS of wheat, α-Amy1, α-Amy2, TaGA3ox2, TaGA20ox, TaNCED1, and TaCYP707A1 were selected to do qRT-PCR. The correlation between qPCR and RNA-Seq data was highly coefficient, which means reliability of the RNA-Seq data.
The expression genes of encoding α-amylase biosynthesis, α-Amy1 and α-Amy2 significantly down-regulated after exogenously applied T2. Expression of GA biosynthesis gene, TaGA3ox2 and TaGA20ox, was apparently down-regulated. ABA-related gene, both expression genes of biosynthesis and catabolism, TaNCED1 and TaCYP707A1 were up-regulated (Fig. 8).
Discussion
In order to clarify how the new chemical compound effects on the germination of wheat seeds, α-amylase activity, RNA-Seq analysis, genes expression related to the seed germination together with endogenous hormones were examined. The present investigation showed that the germination of wheat seeds was delayed 2 to 3 days after exogenously applied T2 and induced the expression of genes related to ABA, GA, and α-amylase. This caused changes in the concentration of ABA, GA and α-amylase activity which decreased the seed germination rate.
Germinating wheat seeds contain many kinds of enzymes, and the content and activity level of various hydrolases are greatly increased during the germination (Gale et al. 2002). The activity of α-amylase is correlated to the degree of seed dormancy, which accounts for 84% of seed germination (Gale and Ainsworth 1984). As a main biosynthesis gene of α-amylase, overexpression of α-Amy1 is likely to cause high concentration of α-amylase (Gao et al. 2013). In the present study, the expression of the α-Amy1 and α-Amy2 was tested, together with α-amylase activity. After treatment with T2, α-amylase activity significantly decreased compared to the control. Data from RNA-Seq and quantitative real-time PCR showed that expressions of both α-Amy1 and α-Amy2 was down-regulated. Obvious reduction in the expression of α-Amy1 and slight decrease in the expression of α-Amy2 were observed. Previous study reported that α-Amy2 peaked in the beginning of kernel development, while α-Amy1 increased towards harvest maturity (Sarah et al. 2013). These results indicated that induction of α-amylase activity in this study may be mainly induced by the expression of α-Amy1.
Decreasing the α-amylase activity may also relate to the hormone balance in seeds (Gazzarrini and Tsai 2015). The endogenous hormones which have the most effect on PHS are GA and ABA (Vanstraelen and Benková 2012). The antagonistic relationship and the ratio between these two hormones regulate the transition from embryogenesis to seed germination (Weiss and Ori 2007).
The expression level of α-Amy1 and α-Amy2 could be regulated by GA3 (Chen and An 2006). Significant expression of α-Amy is induced by the external usage of GA3, which results high α-amylase activity (Chen and An 2006). GA also up-regulates transcription factor GAMYB, and which can positively control expression of α-Amy by binding on the GARE box present in the promoter region of the α-Amy (Gomez-Cadenas et al. 2001). In the GAMYB-deficient barley seeds, the expression of α-Amy is repressed by blocking GA induction (Gomez-Cadenas et al. 2001). Consistent with these functions, seed germination ability is reduced in the mutations of GA3ox1 deficient plants (Mitchum et al. 2006; Holdsworth et al. 2008). All these results taken together, the genes related with biosynthesis of GA have direct effect on α-Amy expression and seed germination. To clarify whether exogenously applied T2 affected seed dormancy and germination via GA, the expression of GA biosynthesis genes (GA3ox2 and GA20ox) was determined by quantitative real-time PCR and RNA-Seq in this study. Expression of GA3ox2 and GA20ox significantly decreased after applied T2. Both of the lowest expression appeared at 48 h and began to increase at 72 h. Consistent with related gene expression, GA concentration also obviously decreased at the beginning of the T2 application and slightly increased after 48 h. These results corresponded with the germination rate of wheat seeds, which may can explain GA effectively regulated the α-amylase activity to affect germination rate of wheat seed.
Expression of Nine-cis-epoxycarotenoid dioxygenase (NCED) (ABA biosynthesis gene) and abscisic acid 8’-hydroxylase (ABA catabolism gene) is tightly related to ABA content (Thompson et al. 2000, 2007), and ABA concentration is a balance between biosynthesis and catabolism (Nambara and Marion-Poll 2005). Endogenous ABA levels increased in the over-expression of AtNCED in transgenic Arabidopsis (Iuchi et al. 2001). Instead, ABA level is reduced by over-expression of the key ABA catabolism gene (CYP707A) (Millar et al. 2006). The expressions of TaNCED1 and TaCYP707A1 both up-regulated and the highest expressions both appeared at 48 h after treated by T2. The concentration of ABA increased compared with control after treated by T2 and the highest concentration occurred at 24 h. High expression of ABA catabolism gene decreased the ABA content after 24 h. Above results showed that the concentration of ABA and gene expression related with ABA had similar tendency.
Exogenously applied MeJA leads to increase of ABA concentration and decrease of GA concentration in wheat cob and root (Ma et al. 2017). In this study, the PCA showed application of T2 led to obvious changes in the expressions of genes compared with control, however, relatively less changed between T2 and M4. Application of MeJA analog solution (T2) sharply increased the endogenous ABA content at 24 h and after that decreased steadily, while, GA content in wheat seeds sharply decreased at 24 h and then slowly increased. The contents of endogenous hormones tendency were in accordance with the seed germination. Usage of T2 inhibited the seeds germination through synergistic effect by increasing the ABA content and decreasing the GA content at the first stage. Increase of GA content and decrease of ABA content after 24 h as well as starting of seeds germination from 72 h indicated that application of this new compound inhibited the seeds germination without losing seed vigor. Above results demonstrated that effect of the new chemical compound on PHS may similar with MeJA, however, it can slowly recovery the seed vigor some days later. Thus, exogenous application of T2 increased concentration of ABA and significantly decreased concentration of GA by regulating the gene expression related with ABA and GA biosynthesis and catabolism. A decrease in GA content inhibited the synthesis of α-amylase, and increase of ABA content also inhibited GA-induced process of α-amylase synthesis. These synergies reduced the α-amylase activity and led to the reduction of PHS.
Although more tests are needed to demonstrate the mechanism of T2 inhibition on germination, the results suggest that exogenously applied T2 can delay seed germination by 2 to 3 days by decreasing α-amylase activity through increasing ABA content as well as decreasing GA content. The new synthesized compound T2 on PHS-resistant was effective in not only delaying seed germination but also maintaining seed vigor. These results indicate that T2 can be considered as a retardant of PHS, however, whether there is a chemical residue and the safety of food need to be further studied.
Conclusion
In conclusion, the results of the study indicated that exogenously applied T2 [1500 mg L−1 N-(2, 4-dimethoxyphenyl)-2-(3-oxo-2-pentylcyclopentyl) acetamide + 1% DMSO + 0.1% Penetrant] could delay seed germination by 2 to 3 days compared to the control. This is likely due to inhibit the seed germination of wheat grain through changing endogenous hormone, ABA and GA, to sharply decrease the activity of α-amylase. To clarify how T2 effect on PHS, expression of main genes (α-Amy1, α-Amy2, TaNCED, TaCYP707A, TaGA3ox, TaGA20ox) related to biosynthesis and catabolism of α-amylase, GAs, and ABA were tested and the expression level results showed the same tendency to the ABA and GA contents and α-amylase activity. These findings should helpful to development of PHS-resistant growth retardant.
References
Biddulph TB, Plummer JA, Setter TL et al (2008) Seasonal conditions influence dormancy and pre-harvest sprouting tolerance of wheat (Triticum aestivum L.) in the field. Field Crop Res 107:116–128
Chen KG, An YQC (2006) Transcriptional responses to gibberellin and abscisic acid in barley aleurone. J Integr Plant Biol 48:591–612
Cheng W, Cheng Y, Wang L et al (2015) Physiological characteristics and related gene expression of after-ripening on seed dormancy release in rice. Plant Biol 17:1156–1164
Creelman RA, Mellut JE (1997) Biosynthesis and action of jasmonates in plant. Annu Rev Plant Physiol Plant Mol Biol 48:355–381
Deng M, Qian H, Chen L et al (2017) Influence of pre-harvest red light irradiation on main phytochemicals and antioxidant activity of Chinese kale sprouts. Food Chem 222:1–5
Derera NF (1989) Pre-harvest field sprouting in cereals. CRC Press
Gale MD, Ainsworth CC (1984) The relationship between α-amylase species found in developing and germinating wheat grain. Biochem Genet 22:1031–1036
Gale MD, Flintham JE, Devos KM (2002) Cereal comparative genetics and preharvest sprouting. Euphytica 126:21–25
Gao F, Jordan MC, Ayele BT (2012) Transcriptional programs regulating seed dormancy and its release by after-ripening in common wheat (Triticum aestivum L.). Plant Biotechnol J 10:465–476
Gao X, Hu CH, Li HZ et al (2013) Factors affecting pre-harvest sprouting resistance in wheat (Triticum aestivum L.). J Animal Plant Sci 23:556–565
Gazzarrini S, Tsai AYL (2015) Hormone cross-talk during seed germination. Essays Biochem 58:151–164
Groos C, Gay G, Perretant MR et al (2002) Study of the relationship between pre-harvest sprouting and grain color by quantitative trait loci analysis in a white × red grain bread-wheat cross. Theor Appl Genet 104:39–47
Holdsworth MJ, Bentsink L, Soppe WJJ (2008) Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol 179:33–54
Iuchi S, Kobayashi M, Taji T (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J Cell Mol Biol 27:325–333
Kulwal PL, Mir RR, Kumar S et al (2010) QTL analysis and molecular breeding for seed dormancy and pre-harvest sprouting tolerance in bread wheat. J Plant Biol 37:59–74
Liu S, Sehgal SK, Li J et al (2013) Cloning and characterization of a critical regulator for pre-harvest sprouting in wheat. Genetics 195:263–273
Ma C, Feng Y, Zhang J et al (2017) Effect of exogenous methyl jasmonate on endogenous hormones and yield formation in wheat after anthesis under drought stress. Plant Physiol J 53:1051–1058
Mares DJ, Mrva K (2014) Wheat grain pre-harvest sprouting and late maturity α-amylase. Planta 240:1167–1178
Masojc P, Milczarski P (2009) Relationship between QTLs for pre-harvest sprouting and α-amylase activity in rye grain. Mol Breeding 23:75–84
Millar AA, Jacobsen JV, Ross JJ et al (2006) Seed dormancy and ABA metabolism in Arabidopsis and barley: the role of ABA 8′-hydroxylase. Plant J 45:942–954
Mitchum MG, Yamaguchi S, Hanada A et al (2006) Distinct and overlapping roles of two gibberellin 3-oxidases in Arabidopsis development. Plant J 45:804–818
Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56:165–185
Qisen Z, Chengdao L (2017) Comparisons of copy number, genomic structure, and conserved motifs for α-amylase genes from barley, rice, and wheat. Front Plant Sci 8:180–188
Sarah DL, Jan DR, Ingeborg S et al (2013) α-amylase gene expression during kernel development in relation to pre-harvest sprouting in wheat and triticale. Acta Physiol Plant 35:2927–2938
Shahpiri A, Talaei N, Finnie C (2015) Spatio-temporal appearance of α-amylase and limit dextrinase in barley aleurone layer in response to gibberellic acid, abscisic acid and salicylic acid. J Sci Food Agric 95:141–147
Shitani A, Kato H, Minamikawa T (1997) Hormonal regulation of expression of two cysteine endopeptidase gene in rice seedlings. Plant Cell Physiol 38:1242–1248
Shorinola O, Bird N, Simmonds J et al (2016) The wheat Phs-A1 pre-harvest sprouting resistance locus delays the rate of seed dormancy loss and maps 0.3 CM distal to the PM19 genes in UK germplasm. J Exp Bot 67:4169–4178
Simsek S, Ohm JB, Lu H et al (2014) Effect of pre-harvest sprouting on physicochemical changes of proteins in wheat. J Sci Food Agric 94:205–212
Son SH, Chitnis VR, Liu A et al (2016) Abscisic acid metabolic genes of wheat (Triticum aestivum L.): identification and insights into their functionality in seed dormancy and dehydration tolerance. Planta 244:429–447
Stobrawa J, Rdzawski Z, Głuchowski W et al (2017) New insight in cereal starch degradation: identification and structural characterization of four α-amylases in bread wheat. Amylase 1:35–49
Thompson AJ, Jackson AC, Symonds RC et al (2000) Ectopic expression of a tomato 9-cis-epoxycarotenoid dioxygenase gene causes over-production of abscisic acid. Plant J 23:363–374
Thompson AJ, Mulholland BJ, Jackson AC et al (2007) Regulation and manipulation of ABA biosynthesis in roots. Plant Cell Environment 30:67–78
Tsai F-Y, Lin C-C, Kao C-H (1997) Acomparative study of the effects of abscisic acid and methyl jasmonate on seedling growth of rice. Plant Growth Regul 21:37–42
Vanstraelen M, Benková E (2012) Hormonal interactions in the regulation of plant development. Ann Rev Cell Dev Biol 28:463–487
Weiss D, Ori N (2007) Mechanisms of cross talk between gibberellin and other hormones. Plant Physiol 144:1240–1246
Xiao SH, Zhang HP, You GX et al (2012) Integration of marker-assisted selection for resistance to pre-harvest sprouting with selection for grain-filling rate in breeding of white-kernelled wheat for the Chinese environment. Euphytica 188:85–88
Yang N, Guo X, Wu Y et al (2018) The inhibited seed germination by ABA and MeJA is associated with the disturbance of reserve utilizations in Astragalus membranaceus. J Plant Interact 13:388–397
Yang Z, Zhu L, Tian H et al (2020) Design, synthesis and biological activities of novel urea derivatives with superior plant growth-inhibiting activity. Plant Growth Regul 6:1680–1692
Yoshimoto K, Jikumaru Y, Kamiya Y et al (2009) Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senes-cence and the innate immune response in Arabidopsis. Plant Cell 21:2914–2927
Kashiwakura Y, Kobayashi D, Jikumaru Y et al (2016) Highly sprouting –tolerant wheat grain exhibits extreme dormancy and cold imbibition-resistant accumulation of Abscisic acid. Plant Cell Physiol 57:715–732
Acknowledgements
This work was supported by the grants from the National Key Research and Development Program of China (No. 2017YFD0201300).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflicts of interest to this work.
Additional information
Communicated by Shubpriya Gupta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cui, S., Mao, Z., Hou, X. et al. Effect of a newly synthesized compound on delaying pre-harvest sprouting in winter wheat (Triticum aestivum L.). Plant Growth Regul 97, 203–213 (2022). https://doi.org/10.1007/s10725-021-00719-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-021-00719-3