Abstract
Selenium (Se), one of the most widely distributed elements of the earth’s crust, is required in trace amounts for normal growth and development of biological activity but its increasing level in soil poses productivity problems in many crops including sugarcane. In the present investigation, a promising line of sugarcane (CoLk 94184) was used to assess the impact of selenium on growth, physio-biochemical attributes vis-à-vis expression of metallothionein (MT) gene. Single bud setts of sugarcane (Saccharum spp. hybrids) was planted with differential levels of selenium (sodium selenite) viz., 0, 10, 50 and 100 ppm under soil tray culture conditions. At higher concentrations (50 and 100 ppm Se), symptoms of metal toxicity as stunted growth, reduced plant height, vigor, root, shoot weight and leaf chlorosis were observed. Biochemical analysis revealed reduction in content of chlorophylls, carotenoids, proline and induction of lipid peroxidation in terms of malondialdehyde content and higher activity of peroxidase enzyme. qRT-PCR analysis indicated increase in expression of MT gene in leaf tissue with an increase in Se supply and highest expression was observed at 50 ppm Se. At 100 ppm supply, adverse effect of Se was very severe and a minor increase in expression of MT gene was observed. Results suggest that MT gene is related to the Se homeostasis which in turn helps in tolerance to Se toxicity in sugarcane.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Every organism has an ability to withstand a specific quantity of essential and non-essential elements present in the environment and utilize them for their growth processes. However, the same elements, when present in high quantities can be toxic (Bradshaw et al. 1990) and exhibit symptom akin to stress such as disturbances in photosynthesis, respiration and reduced biomass production. The increased industrial activities, indiscriminate use of inorganic/organic fertilizers, pesticides and disposal of industrial effluents enhance heavy metals toxicity in agro-ecosystem and environment. Cultivation of sugarcane adjacent to industries, further the application of municipal wastes and phosphatic fertilizers enhance the problem of heavy metal toxicity in sugarcane crop.
Selenium is one of the most widely distributed elements of the Earth’s crust. It is generally associated with sulphide minerals (Germ et al. 2007). The presence/absence of Se in any soil depends on the composition of the ground material and on leaching or other processes subsequent to soil formation that have added Se (Shamberger 1981). Selenium is an essential microelement for animals, humans and microorganisms (Rotruck et al. 1973). It has three levels of biological activity: (1) trace concentrations are required for normal growth and development, (2) moderate concentrations can be stored to maintain homeostatic functions and (3) elevated concentrations can result in toxic effects (Hamilton 2004). Selenium has not been classified as an essential element for plants, although its role has been considered to be beneficial in plants capable of accumulating large amounts of the element (Shanker 2006). Hartikainen et al. (2000) reported Se at concentrations of 0.1 and 1.0 mg kg−1 acted as an antioxidant, inhibiting lipid peroxidation in ryegrass (Lolium perenne). Selenium delayed the senescence and promoted the growth of ageing seedlings by preventing the reduction of tocopherol concentration and enhancing superoxide dismutase activity (Hartikainen and Xue 1999; Xue et al. 2001). Some plant species viz., Morinda reticulata and Neptunia amplexicaulis accumulate very high concentrations of Se (up to 4000 mg Se kg−1 dry matter), when these are grown on seleniferrous soils and eventually become Se-tolerant. However, most plants are Se non-accumulators (contain less than 25 mg Se kg−1 dry matter) and are sensitive to Se (Terry et al. 2000; Ellis and Salt 2003; Tinggi 2003). The content of Se in plants can be increased in different ways; by addition of Se to soil, soaking seeds in Se solution, hydroponic and aeroponic cultivation in a nutrient solution containing Se and foliar application of plants with Se solution.
Metallothioneins (MTs) are cysteine rich, low molecular weight metal binding proteins reported in a wide variety of organisms including animals, plants, cyanobacteria and fungi. Plant’s MTs are extremely diverse and have been classified into four subfamilies (MT1 to MT4) based on the arrangement of Cys-residues. Recently the role of MT genes in heavy metal tolerance mechanism and phytoremediation has been demonstrated in several plant species (Hamer 1986; Zhou et al. 2006; Hossain et al. 2012). Sugarcane is one of the important plant species that contain all four types of MTs and considered as most potential phytoremediation species having ability to produce high biomass and metal enrichment capacity (Guo et al. 2013). Present study was aimed to evaluate the physiological and biochemical responses of sugarcane to graded concentration of Se by analyzing various growth parameters vis-à-vis changes in photosynthetic pigments, free proline content, peroxidase activity and degree of membrane degradation (lipid peroxidation). In order to determine tolerance in sugarcane, semi-quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed to investigate the expression of MT gene in leaf tissues when plants were exposed to various levels of Se.
Materials and methods
Plant materials and growth conditions
Single bud setts of sugarcane (Saccharum spp. hybrids) variety/cultivar CoLk 94184 were planted in soil tray culture condition at Indian Institute of Sugarcane Research, Lucknow in year 2014. Graded levels of selenium were applied at the time of planting by mixing selenium in soil @ 0, 10, 50 and 100 ppm Se as sodium selenite. These trays were kept under net house conditions at natural photoperiod of 12–13 h and ambient temperature (day temperature 28–31 °C; night temperature 15.6–19.7 °C).
Experimental design and parameters analyzed
Present experiment was carried out in three replications and each replication contains ten setts. Sprouting of buds was recorded at 10, 12 and 14 days after planting (DAP). At 15 DAP, plant height was recorded and fresh leaves were collected for biochemical and molecular analysis.
Determination of photosynthetic pigments
Photosynthetic pigments viz., chlorophyll a, b, total and carotenoids were determined in fresh leaves of treated and control plants by the method of Arnon (1949). Hundred milligram of leaf tissues were ground in 10 ml acetone (80 %) (v/v) with a pinch of CaCO3. The homogenate was centrifuged at 10,000 rpm at room temperature for 10 min and supernatants were collected. The absorbance of the supernatants was measured at 663, 645 and 470 nm. The chlorophyll a, b, total and carotenoids contents were calculated using formulas:
The results were expressed in mg per g fresh weight.
Determination of lipid peroxidation
Level of lipid peroxidation in leaf tissues was determined by estimating the malondialdehyde (MDA) content as described by Heath and Packer (1968). Fresh leaves (200 mg) were ground in 5 % TCA solution and centrifuged at 6000 rpm for 10 min. Two ml supernatant was mixed with 2 ml 5 % TBA and incubated for 30 min at 100 °C. The mixture was cooled rapidly in an ice bath and then it was centrifuged for 10 min at 10,000 rpm. After centrifugation, absorbance (A) was measured at 532 and 600 nm. Lipid peroxidation was expressed as µmol g−1 using the formula = [(A530 − A600)/155].
Quantification of proline content
Proline was determined in fresh leaves by the method of Bates et al. (1973). Leaf tissues (200 mg) were extracted in 2 ml of 3 % (w/v) sulphosalicylic acid. Extract was centrifuged at 6000 rpm and supernatant was used for estimation of proline. In 1 ml aliquot, 2 ml ninhydrin reagent and 2 ml acetic acid were added and then mixture was heated for 30 min in boiling water bath. After cooling, color was extracted in 5 ml toluene by vortex mixing and the upper (toluene) phase was collected in a dry glass tube and the absorbance was measured at 520 nm. Results were expressed in µg proline per 100 mg fresh weight.
Assay of peroxidase enzyme
Fresh leaf tissues were ground in liquid nitrogen and powder was suspended in 5 ml 0.1 M phosphate buffer (pH 7.5) containing 0.5 mM EDTA. Homogenate was centrifuged at 12,000 rpm, 4 °C for 10 min and the supernatant collected was used as enzyme extract. For peroxidase assay, the reaction mixture containing 5 ml 0.1 M phosphate buffer (pH 6.0), 1 ml 0.01 % H2O2, and 1 ml 0.5 % p-phenylenediamine and enzyme extract (0.1 ml) was incubated for 5 min at 25 °C and then the reaction was stopped by adding 2 ml 5 N H2SO4 as described by Luck (1963). The color developed was measured at 485 nm and the activity was expressed as change in OD per mg protein.
Estimation of soluble protein
Protein was estimated in 0.1 ml of enzyme extracts, according to Lowry et al. (1951). The intensity of blue color was measured at 640 nm using bovine serum albumin as the calibration standard.
RNA extraction and qRT-PCR reactions
Total RNA was isolated from leaf tissues of control and treated plants using QIAGEN RNeasy plant Mini kit following the manufacturer’s instructions. Total RNA was then treated with RNase-free DNase I (QIAGEN) to remove any genomic DNA contamination. The quantity and quality of RNA was checked using Picodrop spectrophotometer and on 0.8 % agarose gel. The purified RNA was stored at −20 °C. Nucleotide sequences for candidate MT gene (gene accession no. EU760482.1) and for internal control Actin (gene accession no. 53759188) was retrieved from National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/). For qRT-PCR expression analysis, oligonucleotide primers were designed using Prime3 Output software. Primer sequences were, actin forward: GGACATCCAGCCTCTTGT, actin reverse: GCAAGATCCAAACGAAGAATG, MT forward: AGATGTACCCAGACATGAGC and MT reverse: AGGGTTACACTTGCAGTCAG. qRT-PCR was performed in PCT-200 Thermal cycler (BioRad, USA) using equal amounts of RNA (200 ng). Amplification was carried out using the QIAGEN one step qRT-PCR kit as follows: 50 °C for 30 min for reverse transcription reaction, 94 °C for 15 min and 30 cycles of 94 °C for 1 min, 49 °C for 1 min, 72 °C for 1 min and final extension at 72 °C for 10 min. Amplification products (12.5 µl) were electrophoresed on 1.6 % agarose gel. Gel was stained with ethidium bromide and visualized on gel documentation system (AlPha Innotech, USA) after agarose gel electrophoresis. Three biological replicates were assayed for each treatment and each reaction was performed in duplicate. Gene expression in terms of integrated density value (IDV) was determined using AlphaEase software supplied along with gel documentation system (Alpha Innotech, USA). IDV thus obtained was divided by 1000 for ease of writing on the y axis; thus, the IDV had to be multiplied by 1000 to get the original value of gene expression.
Statistical analysis
The experiment was conducted in a completely randomized design (CRD) with three replications. The data were analyzed by one-way analysis of variance, according to Cochran and Cox (1957). The mean values were compared using post hoc least significant difference (LSD) test and the term significant has been used to indicate differences for which p < 0.05.
Results and discussion
Bud sprouting and plant vigour
In general selenium application decreased bud sprouting, plant height and vigour; the highest decrease was obtained at 100 ppm Se (Fig. 1). Reduction in bud sprouting might be due to poor growth of setts roots which adversely affected the availability of moisture and other essential nutrients. Reduction in plant vigour might be due to reduced plant height (50 % reduction over control) and lower bud sprouting indicating phytotoxicity of selenium for sugarcane at 100 ppm concentration. 50 ppm Se level showed a moderate effect probably by maintaining homeostatic functions.
Shoot and root weight
Selenium treated plants exhibited stunted growth, reduced leaf area, root number, length and leaf chlorosis at early stage. Fresh weight of both root and shoot reduced by excess Se in growing medium; root growth was affected more (85 %) than shoot (58.6 %) at 100 ppm Se supply (Fig. 1). Effect of Se on sett roots was maximum at 100 ppm, which in turn adversely affected the shoot growth. After about 30 DAP, when setts roots were dead and new shoot roots start emerging, plant growth recovered relatively both at 10 and 50 ppm Se. Higher Se also reduced plant growth in cabbage (Wu et al. 2009). These results showed inhibition of root and shoot growth at higher levels in sugarcane, as selenium in the form of selenate is more soluble and easily translocated to shoots resulting in oxidative stress due to reactive oxygen species (ROS) formation which causes alterations in cell membrane damage.
Photosynthetic pigments
Both chlorophyll and carotenoid contents were comparatively lower in Se treated plants as compared to control; highest decrease was obtained at 100 ppm Se (Fig. 2). Reduction in photosynthetic pigments might be due to poor availability of essential nutrients to metabolically active leaf tissues, resulting in leaf chlorosis. Similar results were reported due to heavy metals (Cr, Ni, Cd) toxicity in sugarcane (Jain et al. 2000, 2004; Yadav et al. 2010) and many other plants (Cenkci et al. 2010). An excess of metals has deleterious effects on the content and functionality of the photosynthetic pigments (Broadley et al. 2007). This may be caused by the inhibition of pigment synthesis (Prasad and Prasad 1987), the formation of metal-substituted chlorophylls of reduced functionality (Küpper et al. 1996), direct oxidative damage to the pigments (Oláh et al. 2010) or through the substitution of the central Mg ion (Cenkci et al. 2010; Pourraut et al. 2011).
Malondialdehyde (MDA)
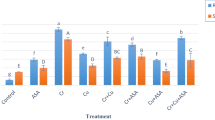
Lipid peroxidation was determined in terms of MDA in leaf tissues of both treated and control plants. It was significantly increased due to Se in growing medium; maximum content was observed at 100 ppm Se level (77.3 % increase over control) (Fig. 3). Similar results were also reported by Wu et al. (2009) in cabbage exposed to higher dose of Se. At higher concentration, Se acts as a pro-oxidant enhancing the accumulation of lipid peroxidation products (Hartikainen et al. 2000). The level of MDA content has been considered as an indicator of oxidative stress. MDA is the decomposition product of polyunsaturated fatty acids of bio-membranes and its increase shows that plants are under antioxidant stress. Thus increased MDA content shows the generality of oxidative stress and this may be one of the potential mechanisms by which toxicity due to heavy metals is manifested in plant tissues (Gupta et al. 2009).
Proline content
Proline content increased significantly at 100 ppm Se (99.2 % increase over control) (Fig. 3). Proline plays an important role to stabilize protein structures, DNA, as well as membranes and sub-cellular structures against denaturation (Kavi Kishor et al. 1995). It is well known that proline accumulates in plants during the adaptation to various types of environmental stresses such as drought, high temperature, nutrient deficiency and exposure to heavy metals (Oncel et al. 2000). Stress-inducible proline accumulation in sugarcane plants under selenium stress acts as a component of antioxidative defense system rather than as an osmotic adjustment mediator.
Peroxidase activity and soluble protein content
Gradual increase in peroxidase activity was observed when plants were exposed from 10 to 100 ppm Se (Fig. 3). Similar results have been reported with rye grass (Hartikainen et al. 2000), suggesting antioxidative effect of Se application. Protein content decreased due to Se application. Decreased protein content reduced the integrity of membrane causes death of the cells due to higher dose of Se (Fig. 3). Increased activity of antioxidant enzyme protects sugarcane plants from membrane damage caused by free radicals generated by Se exposure.
MT gene expression analysis
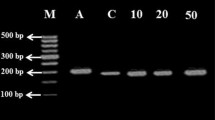
Expression of MT gene increased in leaf tissue of plants at all the levels of selenium (10, 50 and 100 ppm Se); increase was about 24.5 and 96.8 % over control at 10 and 50 ppm Se supply, respectively (Fig. 4). At 100 ppm Se, a minor increase (6.2 %) in expression of MT gene was observed, may be due to its lethal effect. MT transcripts might have accumulated in leaf tissues due to Se in growing medium which is in accordance with the results reported by Teixeira et al. (2013) by heavy metals. Result indicated that MT gene plays important role in Se tolerance in sugarcane.
Expression pattern of MT gene in leaves of Saccharum spp. hybrid CoLK 94184 plants exposed to graded levels of selenium. The amount of total RNA used for the qRT-PCR procedures corresponding to 200 ng of RNA loaded and separated by 0.8 % (w/v) agarose gel electrophoresis. An equal quantity of RNA in each reaction was verified by amplifying a constitutively expressed actin. To calculate IDV, 12.5 µl PCR products were separated on 1.2 % agarose gel, stained with ethidium bromide and quantified using AlphaEase software supplied along with gel documentation system (Alpha Innotech, USA). IDV thus obtained was divided by 1000 for ease of writing on the y axis; thus, the IDV needs to be multiplied by 1000 to get the original value of gene expression. The vertical bars indicate ±SE. Mean values with different letters indicate significant (p ≤ 0.05) differences between treatments
Heavy metals are one of the serious environmental constrains for optimal growth and yield of sugarcane. Higher rate of stalk mortality, low relative growth rate, reduced cane yield and juice quality loss are major effects of heavy metals. The quantification of membrane damage in terms of MDA content, carotenoids and free proline levels in plants exposed to graded levels of selenium (Se) provides indications of the damaging effects caused by such exposure and the degree of stress imposed to sugarcane plants. The increase in free proline content in leaves of Se treated plants may be the response to metal uptake, indicating the involvement of proline in homeostasis of heavy metals in plants, as suggested earlier in sugarcane and other plants (Mehta and Gaur 1999; Pandey and Sharma 2003; Jain et al. 2000). Results of present investigation indicated increased proline and MDA contents and peroxidase activity in leaf tissues with an increase in selenium doses, 100 ppm Se showed higher accumulation of lipid peroxidation products and proline contents than other treatments. Contrast to this, chlorophyll, carotenoids and soluble protein contents decreased gradually with an increase in Se level; highest decrease was obtained at 100 ppm Se treatment. Decrease in chlorophyll content in plants leads to decreased photosynthetic activity and increase in chlorosis with change in leaf colour as pale yellow or yellow white. Upregulation of MT gene may help sugarcane plants to tolerate Se toxicity up to certain concentrations (50 ppm Se) and at very high dose, 100 ppm Se, it exhibited lethal effects.
Abbreviations
- MT:
-
Metallothionein
- MDA:
-
Malondialdehyde
- mRNA:
-
Messenger ribonucleic acid
- TCA:
-
Trichloroacetic acid
- TBA:
-
Thiobarbutaric acid
- DAP:
-
Days after planting
References
Arnon DI (1949) Copper enzymes in isolated chloroplasts: polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bradshaw AD, McNeilly T, Putwain PD (1990) The essential qualities. In: Shaw AJ (ed) Heavy metal tolerance in plants: evolutionary aspects. CRC Press, Boca Raton, pp 323–334
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173:677–702
Cenkci S, Ciererch IH, Yildiz M, Ozay C, Bozdag A, Terzi H (2010) Lead contamination reduces chlorophyll biosynthesis and genome template stability in Brassica rapa L. Environ Exp Bot 67:467–473
Cochran WG, Cox GM (1957) Experimental designs. Wiley, New York
Ellis DR, Salt DE (2003) Plants, selenium and human health. Curr Opin Plant Biol 6:273–279
Germ M, Stibilj V, Kreft I (2007) Metabolic importance of selenium for plants. Euro J Plant Sci Biotechnol 1:91–97
Guo J, Xu L, Su Y, Wang H, Gao S, Xu J, Que Y (2013) ScMT 2-1-3, a metallothionein gene of sugarcane, plays an important role in the regulation of heavy metal tolerance/accumulation. BioMed Res Inter. doi:10.1155/2013/904769
Gupta DK, Nicoloso FT, Schetinger MRC, Rossato LV, Pereira LB, Castro GY, Srivastava S, Tripathi RD (2009) Antioxidant defence mechanism in hydroponically grown Zea mays seedlings under moderate lead stress. J Hazard Mater 172(1):479–484
Hamer DH (1986) Metallothionein. Annu Rev Biochem 55:913–951
Hamilton SJ (2004) Review of selenium toxicity in the aquatic food chain. Sci Total Environ 326:1–31
Hartikainen H, Xue T (1999) The promotive effect of selenium on plant growth as trigged by ultraviolet irradiation. J Environ Qual 28:1272–1275
Hartikainen H, Xue T, Piironen V (2000) Selenium as an antioxidant and pro-oxidant in ryegrass. Plant Soil 225:193–200
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. Arch Biochem Biophys 125:189–198
Hossain MA, Piyatida P, da Silva JAT, Fujita M (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot. doi:10.1155/2012/872875
Jain R, Srivastava Sangeeta, Madan VK (2000) Influence of chromium on growth and cell division of sugarcane. Indian J Plant Physiol 3:228–231
Jain R, Shrivastava AK, Srivastava S (2004) Heavy metals in industrial wastes and their effect on sugarcane. Inter Sugar J 22:23–27
Kavi Kishor PB, Hong Z, Miao GH, Hu CA, Verma DPS (1995) Overexpression of Δ1-pyrroline-5-carboxilate synthase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108:1387–1394
Küpper H, Küpper F, Spiller M (1996) Environmental relevance of heavy metal-substituted chlorophylls using the example of water plants. J Exp Bot 47:259–266
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–375
Luck H (1963) Peroxidase. In: Bergmeyer HU (ed) Methods in enzymatic analysis. Academic Press, New York, pp 895–897
Mehta SK, Gaur JP (1999) Heavy-metal-induced proline accumulation and its role in ameliorating metal toxicity in Chlorella vulgaris. New Phytol 143:253–259
Oláh V, Lakatos G, Bertok C, Kanalas P, Szollosiz E, Kis J, Meszaros I (2010) Short-term chromium (VI) stress induces different photosynthetic responses in two duckweed species, Lemna gibba L. and Lemna minor L. Photosynthetica 48:513–520
Oncel I, Keles Y, Ustun AS (2000) Interactive effects of temperature and heavy metal stress on the growth and some biochemical compounds in wheat seedlings. Environ Pollut 107:315–320
Pandey N, Sharma CP (2003) Chromium interference in iron nutrition and water relations of cabbage. Environ Exp Bot 49:195–200
Pourraut B, Shahid M, Dumat C, Winterton P, Pinelli E (2011) Lead uptake, toxicity and detoxification in plants. Rev Environ Contam Toxicol 213:113–136
Prasad DPH, Prasad ARK (1987) Effects of lead and mercury on chlorophyll synthesis in mungbean seedlings. Phytochemistry 26:881–884
Rotruck JT, Pope AL, Ganther HE, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Shamberger RJ (1981) Selenium in the environment. Sci Total Environ 17:59–74
Shanker AK (2006) Countering UV-B stress in plants: does selenium have a role? Plant Soil 282:21–26
Teixeira J, Ferraz P, Almeida A, Verde N, Fidalgo F (2013) Metallothionein multigene family expression is differentially affected by chromium (III) and (VI) in Solanum nigrum L. plants. Food Energy Secur 2:130–140
Terry N, Zayed AM, de Souza MP, Tarun AS (2000) Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol 51:401–432
Tinggi U (2003) Essentiality and toxicity of selenium and its status in Australia: a review. Toxicol Lett 137:103–110
Wu XP, Liang D, Bao J, Xue R (2009) Effects of different concentrations of selenate and selenite on growth and physiology of Chinese cabbage. Acta Scientiae Circumstantiae 29:2163–2171
Xue T, Hartikainen H, Piironen V (2001) Antioxidative and growth-promoting effect of selenium in senescing lettuce. Plant Soil 237:55–61
Yadav DV, Jain R, Rai RK (2010) Impact of heavy metals on sugar cane. In: Sherameti I, Verma A (eds) Soil heavy metals: soil biology series, vol 19. Springer, Berlin, pp 339–367
Zhou G, Xu Y, Li J, Yang L, Liu JY (2006) Molecular analyses of the metallothionein gene family in rice (Oryza sativa L.). J Biochem Mol Biol 39:595–606
Acknowledgments
We would like to thank Director, Indian Institute of Sugarcane Research (ICAR Unit), Lucknow for his encouragement and support. We are also thankful to Dr. Mani Ram Verma (Chief Technical Officer) for statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jain, R., Verma, R., Singh, A. et al. Influence of selenium on metallothionein gene expression and physiological characteristics of sugarcane plants. Plant Growth Regul 77, 109–115 (2015). https://doi.org/10.1007/s10725-015-0042-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-015-0042-1