Abstract
Heavy metals such as Cd are considered to be the most important pollutants in soil contamination. Cd is a non-essential element adversely affecting plant growth and development, and it has caused some physiological and molecular changes. Metallothioneins (MTs) are low molecular weight, cysteine-rich, and metal binding proteins. In this study, we aimed to evaluate the MT gene expression levels and minerals uptake in the tissues of Solanum lycopersicum exposed to Cd. The transcriptional expression of the MT genes was determined by real-time quantitative PCR. The MT genes were regulated by the Cd and the mineral elements uptake changed tissue type and applied doses. The MT1 and MT2 transcript levels increased in the roots, the leaves and the fruits of the tomato. The MT3 and MT4 transcript pattern changed according to the tissue types. The Cd treatment on the growth medium increased the Mg, Ca, and Fe content in both the leaves and fruits of the tomato. However, the Cd affected the mineral levels in the roots depending on the mineral types and doses. Also, the Cd content increased in the roots, the leaves, and the fruits of the tomato, respectively. The results presented in this study show that Cd has synergistic and/or antagonistic effects on minerals depending on the tissue types. These results indicate that the MT1 and MT2 expression pattern increased together with the Mg, Ca, and Fe content in both the leaves and the fruits of the tomato.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are the main abiotic stress factors for living organisms that cause environmental pollution, and their distribution into the environment is increasing (Maksymiec 2007; Rascio and Navari-Izzo 2011). Plants are in continuous interaction with their natural habitats. They are exposed to stress depending on the adaptability of the environment in which the plants can survive in unsuitable conditions. Stress occurs in plants when environmental conditions change, and it adversely affects their normal growth and development (Büyük et al. 2012). Heavy metals are considered to be the most important pollutants in soil contamination. They give rise to many problems such as changes in microbial activity, soil productivity, biodiversity, and product yield. Soil contamination from heavy metals can be the result of both industrial and agricultural activities (Kavamura and Esposito 2010). Soil acts as a natural buffer which controls the transfer of chemical elements and substances into the hydrosphere and biota, as well as being one of the most important components of the biosphere (Kabata-Pendias and Pendias 2001). Heavy metals are the main abiotic stress agents for organisms because of accumulation, toxicity, and a longer half-life (Bertoli et al. 2012). They adversely affect the natural environment, soil fertility remaining in the soil for a long time, and human health via the food chain (Gratao et al. 2008). Plants have developed advanced molecular and biochemical mechanisms for the uptake, the mobilization, and the regulation of the intracellular concentration of heavy metals. Also, they synthesize low molecular weight proteins, chelating compounds to remove the heavy metals, and membrane transporters for accumulating them in the vacuole. One of the plant strategies for the detoxifying of heavy metals is to synthesize the chelators to minimize the metal ions, and MTs play crucial roles as a tolerance mechanism (Hossain and Komatsu 2012).
Cadmium is a non-essential element adversely affecting plant growth and development. It is considered to be a very important contaminant because of its high toxicity and water solubility. Cd changes the uptake of useful minerals in the soil and reduces the population of soil microorganisms (Benavides et al. 2005). It affects the absorption of Ca, Mg, P, K, and water intake and usage (Balestrasse et al. 2003). Elements accumulate more in plant roots because they are initially absorbed in the root. Cd enters the roots and reaches the xylem in apoplastic or symplastic ways, and finally reaches the aerial parts of the plants. Cadmium is in competition with nutrients such as K, Ca, Mg, Fe, Mn, Cu, Zn, and Ni, passing through the cell membrane because they have used the same carriers (Benavides et al. 2005). Cd inhibits carriers associated with the translocation of the minerals in the plant tissues (Nazar et al. 2012).
Metallothioneins (MT) with their low molecular weight are characterized by being rich in cysteine amino acids, and being devoid of aromatic amino acids. MTs bind easily to heavy metals with high affinity because of their high cysteine content, so they are thought to play a protective role in the cells and organelles exposed to heavy metal (Brewer and Marcus 2007; Shi and Chance 2008). The MT gene expressions in plants are stimulated when exposed to heavy metals. Therefore, MTs are used as a biological indicator in the assessment of the stress in organisms, and for the monitoring of various metal pollutants such as cadmium (Canpolat and Lynes 2001; Dabrio et al. 2002). The metallothionein gene expression in plants can be considered as a tolerance for stress recovery. The syntheses of metal-binding proteins such as metallothionein increase in plants exposed to toxic levels. The toxic level of heavy metals can induce visible injuries and physiological disorder such as the reduced chlorophyll content in plants (Mudgal et al. 2010; Nagajyoti et al. 2010; Singh et al. 2011). MTs play a role in biological and physiological reactions by binding the metal ions in cells and organelles (Jia et al. 2012; Ryvolova et al. 2012). Metallothioneins are stress response proteins with a low molecular weight (10.7 kDa), cysteine-rich (30 %), devoid of aromatic amino acids, and bind heavy metals with a high affinity. These proteins have been subdivided into four categories based on the arrangement of the cysteine residues in their N-and C-terminal regions as MT1, MT2, MT3, and MT4. These genes are induced by various heavy metals and expressed at different levels in plant tissues. An accumulation of heavy metals in plants can cause structural changes such as physiological, biochemical, and mineral element intake (Cobbett and Goldsbrough 2002). MTs are expressed in different tissues such as the roots, the stems, the leaves, the fruits and the seeds (Kohler et al. 2004). Type 1 MT genes are predominantly expressed in both the leaves and the roots, and type 2 MT genes are expressed primarily in the leaves, the stems, and the developing seed. Type 3 MT genes are expressed in the leaves or in the ripening fruits, and type 4 MT genes are not only detected in the developing seed, but also in the reproductive organs and vegetative tissues (Yang et al. 2015). The expression of the MT genes in plants is regulated by a variety of stimuli including heavy metals and oxidative stress (Huang et al. 2012).
Solanum lycopersicum is appropriate for the tracking of cadmium and mineral elements in the roots, the leaves and the fruits of the plant, which are an important source of nutrients. Although several studies on MT gene expression in various plants have been conducted, the relationships of MT gene expression and composition of the plant element are still poorly known in the tomato. In the current study, we investigated the expression of MT isoforms (MT 1, 2, 3 and 4) in tomato tissues and focused on tissue differences. We studied Cd accumulation and the effect of Cd on some mineral elements composition of the tomato roots, the leaves and the fruits. This research combines the relationship between the gene expressions and mineral elements contents in the tomato understand the effect of Cd.
Materials and methods
Plant material and growth conditions
Tomato (S. lycopersicum cv. çiko F1) seedlings were grown in pots containing a 10 kg mixture of peat and garden soil (1:1) under greenhouse conditions with 16:8 photoperiods, at 24–27 °C. 150 ppm N, 80 ppm P, 100 ppm K, and other nutrients (Ca, Mg, Zn and B) were applied in 20 ppm for plant growth. After 3 weeks of acclimatization, the seedlings were exposed to 10, 20, and 50 ppm CdCl2 as a heavy metal, and the application was carried out 3 times with an interval of 2 days. After the seedlings were approximately 40 cm in size, they were allowed to grow vertically in order to support the body of tomatoes on the ropes. The tomato leaves were harvested 2 weeks after the heavy metal application. The fruits were harvested after the beginning of ripening, and then the roots were sampled. All the samples were stored at −80 °C until the RNA isolation and element analysis.
RNA isolation and RT-qPCR analysis
The total RNA from the roots, the leaves, and the fruits, was extracted by using the Plant RNA Mini-Preps Kit (Bio Basic) according to the manufacturer’s instructions. The isolated RNA was dissolved in RNA-free water and stored at −80 °C. The RNA integrity was confirmed on the agarose gel, and the RNA concentration was calculated by using a μDrop plate (Thermo Scientific).
The cDNA synthesis and RT-qPCR was performed by using the one-step QuantiTect SYBR Green RT-PCR Kit (Qiagen) according to the suggestions of the manufacturer’s procedures. The QuantiTect SYBR Green RT-PCR master mix 10 µl, primers 2 × 2 µl, RT mix 0,3 µl, template RNA 3 µl were added in the capillary tubes, and the final volume was brought up to 20 µl with RNase-free water. The sequences for the tomato were obtained from the NCBI (http://www.ncbi.nlm.nih.gov/nucleotide) databank. The primer sequences used for the amplifications were designed by using a primer3 (version 4.0) except the MT2 and actin’s primers which were obtained from Goupil et al. (2009). The GC % and Tm values of the primers were confirmed with an oligonucleotide properties calculator, which is an OligoCalc web-based program (http://www.basic.northwestern.edu/biotools/oligocalc.html). The following oligonucleotides were used as a primers: MT1 5′-CTAGCTGCAAGTGCGACAAC-3′(forward), 5′-ACCCCAAGCACCAAAGTCTC-3′(reverse), MT2 5′-GCTGTGGATCTA GCTGCAAGTGCG-3′(forward), 5′-AAGGGTTGCACTTGCAGTCAGATCC-3′ (reverse), MT3 5′-ATGTCTTGCTGTGGTGGAAG-3′(forward), 5′-TAGCAATTGCAAGGGTCACA-3′(reverse), MT4 5′-TGTGGGATGTACCCCGACTT-3′ 3′(forward), 5′-TCTGTTGCTTTCTCAGCCACT-3′(reverse), Actin 5′-GGGATGGAGAAG TTTGGTGGTGG-3′ (forward), 5′-CTTCGACCAAGGGATGGTGTAGC-3′ (reverse). The MTs nucleotide sequences have been deposited in the EMBL nucleotide sequence database under accession number Z68138, Z68185, Z68309 and Z68310 for MT1, MT2, MT3 and MT4, respectively (Giritch et al. 1998).
The real-time qPCR was performed under the following conditions of the Qiagen protocols with minor modifications: the reverse transcription step 20 min 50 °C, the PCR initial activation step 15 min 95 °C, followed by 50 cycles, denaturation 15 s 94 °C, annealing 30 s 56 °C, extension 30 s 72 °C, cooling 20 s 40 °C by using the Roche LightCycler 1.5 PCR machine.
Analysis of Cd and mineral elements
The plant tissues were dried at 65 °C in the oven until they reached a constant weight. The dried samples were ground, and burned in the oven. The temperature was increased gradually 550 ± 50 °C for 6 h. After the cooling, the samples were treated with concentrated HCI, and 2 N nitric acid. The sample temperature was brought to room temperature and pure water was added to the filter. Ca, Mg, Zn, Fe, Mn, Cu, and Cd were determined by a 7700-X model Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) (Tokyo, Japan) using an external calibration method (Döker et al. 2014).
Statistical analysis
The relative expression of the MT gene expression levels was determined using the 2−ΔΔCT method (Livak and Schmittgen 2001). The data were submitted to analysis of the variance ANOVA by using the SPSS 20.0 program package. Significant difference between the treated group and the control group was accepted if P < 0.05 and the data were expressed as mean ± SD.
Results
The expression pattern of metallothionein genes
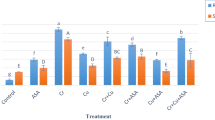
The application of cadmium to the tomato affected the metallothionein transcript expressions depending on the tissue types. After the RT-qPCR amplification, the MT2 gene in the leaves was separated and checked on 2.5 % agarose gel (Fig. 1). The effect of the Cd on MT1 gene expression in the roots, the leaves, and the fruits of the tomato subjected to concentrations of Cd are shown in Fig. 2a. The MT1 gene expression significantly increased in all parts of the S. lycopersicum whose growth medium contained Cd. The greatest expression pattern of MT1 was seen in the leaves of the plant which were exposed to 50 ppm CdCI2 compared to the control groups. The MT2 gene expression levels are shown in Fig. 2b. The application of cadmium significantly induced the MT2 transcript in the roots, the leaves, and the fruits. When the tomato plants were exposed to cadmium, the transcript level of the MT3 gene had no significant change in the roots ant the fruits, but it increased at 10 and 50 ppm Cd treatment in the leaves compared to the control groups (Fig. 2c). The treatment of cadmium on the growth medium significantly induced the level of the MT4 gene expression in the leaves, but slightly increased in the roots. However, the S. lycopersicum treated with all Cd concentrations did not show any significant expression pattern in the MT4 in the fruits of the tomato plants (Fig. 2d).
The expression of MT1, MT2, MT3 and MT4. The relative quantities of MT genes in different tissues of tomato under 0 (control), 10, 20, 50 ppm CdCI2. The gene was quantified by RT-qPCR and normalized with the housekeeping actin transcript. Bars represent means values ± SD from Ct values of independents experiments. Bars marked with the asterisk are significantly (P < 0.05) different from control group
Cd content and its effect on the mineral element in the tomato
Mineral elements such as Ca, Mg, Zn, Fe, Mn and Cu, and cadmium content were determined in the roots, the leaves, and the fruits of the tomato. According to the Cd analysis, it was absorbed by the plant roots, and reached the leaves and the fruits (Fig. 3). The Cd changed the mineral content showing synergistic and/or antagonistic effects depending to the tissue types and minerals.
Mineral element contents in the roots
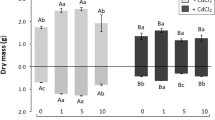
The effect of the Cd on minerals is shown in Fig. 4. The Cd caused a slight increase in the Ca content in all doses of Cd. The Zn level was significantly increased when 10, 20 ppm Cd was added to the growth medium while it decreased at 50 ppm. Moreover, the Cu content increased at 20, 50 ppm application, but the Mn level decreased in the same applications. There was no significant correlation between the amount of Mg and Fe in the roots of the tomato with the increasing treatment of Cd in any of the doses.
Mineral element contents in the leaves
When the tomato plant was exposed to Cd, the content of the mineral elements is shown in Fig. 5. The content of the Mg, Ca, Fe, and Zn were increased depending on the mineral types. However, the Cu and Mn levels decreased with the increasing doses of Cd.
Mineral element content in the fruits
The content of the Mg, Ca, Fe, and Cu, increased with treatment of the Cd on the growth medium. The Zn and Mn levels were decreased by using Cd. These changes depended on the elements and applications. All the results are shown in Fig. 6.
Discussion
Plant growth and developing have been decelerated as results of biochemical changes and physiology processes in contaminated soils. Heavy metals have changed mineral distribution in plants and biological properties of soil (Chibuike and Obiora 2014). The Cd is one of the most toxic elements, because it displaces some essential elements such as zinc, manganese, and calcium, and reacts with sulfur in amino acid side chains (Clemens et al. 2002). Plants have developed a series of complex mechanism to control the element uptake, transportation, and detoxification. One of the mechanisms of heavy metal detoxification is their chelation in the cytosol. Metallothioneins have rich cysteine residues so it plays a role as metal chelators (Shanker and Venkateswarlu 2011). MTs family of genes is regulated in different ways and it is essential for cellular metal detoxification. The expression levels of MT have varied according to plant tissue and its environmental agents.
In the present study, Cd has increased MT1 gene levels in the roots, leaves and ripening fruits of tomato depending on applied doses. It was reported that cadmium induced MT1 gene expression in the Ziziphus jujuba applied 100 mM CdCI2 (Yang et al. 2015). The expression of MT1 increased in the roots, shoots and leaves of poplar cultivated in growth medium contained zinc (Castiglione et al. 2007). In our results, MT2 gene expression level induced in all tissue of tomato. The most expression pattern was seen at 50 ppm Cd application comparing to controls. Similar results were declared in different plants. MT2 transcript level increased in Avicennia germinans (Gonzalez-Mendoza et al. 2007), Arachis hypogaea (Quan et al. 2007) planted in contaminated with cadmium. While MT2 gene level induced by arsenic, chromium did not cause a significant change in the roots and shoots of tomato (Goupil et al. 2009). Tombuloglu et al. (2012) stated that MT2 gene expression increased at doses of 160–320 µM, but it decreased in high and lower doses comparing to control in tomato nourished with boron. MT3 gene expression slightly increased in roots and fruits of tomato; however 50 ppm Cd application caused important to increase the MT3 gene levels in leaves. Kohler et al. (2004) reported that Cd did not change MT3 gene level while Zn slightly increased in roots of Populous alba. Also, they declared Fe and Cu slightly suppressed MT3 gene, while Mn and Zn slightly increased in root tips of tomato. MT4 gene expression levels changed according to tissue types, that is, Cd slightly induced in roots, significantly increased in leaves, and did not change in fruits. Giritch et al. (1998) noticed that Fe, Mn, and Cu did not induce MT4 gene expression, while Zn induced in tomato. Our results show that MT gene expression pattern has changed tissue type and cadmium concentration. These transcript changes may be associated with physiological functions occurred by plants (Huang and Wang 2009). These responses depend on the development stage, tissue type, heavy metal concentration and uptake, and hormonal status of the tissues (Castiglione et al. 2007).
Mineral elements have essential roles in plant growth and development. Mineral composition of plants shows changes depending on the genetic structure of the plant, the chemical composition of soil, climatic factors, plant age and other abiotic factors. Heavy metals such as Cd have changed mineral element composition of plants (Rao et al. 2006). The Cd has changed the plant nutrition levels displacing with essential elements such as Zn, Fe, and Mn which cofactor for some enzymes (López-Millán et al. 2009). In this study, Cd reached to the aerial tissues such as leaves and fruits by uptake from roots of tomato. Cd limited Mn content at 20 and 50 ppm doses. Moreover, Zn amount increased at 10 and 20 ppm, while it decreased at 50 ppm in roots. Cadmium treatment showed synergistic effect increasing on Mg, Ca, Fe and Zn levels in leaves. Also, it showed the similar effect on Mg, Ca, Fe and Cu content at fruits, but it decreased Mn and Zn content with antagonistic effect. Bertoli et al. (2012) indicated in tomato that Cd decreased Ca, Mn and Zn content at aerial parts. Also, they showed that Cd increased Cu and Fe content, and decreased Mn content at roots of tomatoes. However, Mg level did not show significant change at all parts of tomato. While CdCI2 (10 µM) increased Mg content in roots and did not cause a significant change on Ca content at all part of tomatoes cultivated in hydroponics culture (López-Millán et al. 2009). At the same study, Mn content decreased at root, but it did not change at shoots and leaves. Cd application decreased Fe, Zn Cu, Ca and Mg contents in wheat genotypes (Eker et al. 2013). It was reported that Cd decreased Fe, Zn, Cu and Mg contents in the first sampling of roots and leaves in rice. However, Cd increased Fe and Mn content, decreased Zn, Cu and Mg content in roots of ripening plants. Moreover, while it decreased Fe, Zn, Mn, and Mg content, increased Cu content in leaves of ripened rice (Liu et al. 2003).
These results showed that heavy metals affect the content of Ca, Mg, Mn, Fe, and Zn, depending on the tissue type, and application doses in the tomato. This interaction may be associated with the synergistic or antagonistic effects of the Cd and mineral elements used in the same transporters. Some factors such as the plant types, the cultivation method and season, and the sampling method and climatic factors could influence the mineral contents (Suarez et al. 2007). It can be seen in our results that Cd reached the fruits. This situation can endanger human health from the food chain. When farming in soils containing heavy metals such as Cd, it should be taken into account that these metals may be transported into the edible parts of the plants.
In conclusion, we have demonstrated that Cd affected the MT gene expression and mineral elements in tomato tissues. These findings could help improve our understanding of the interaction mechanism. The elements found in the plant growth medium can affect various genes. Mineral elements can interact with each other through nutrient transporters in the cell membrane. Thus, the effect of nutrients and heavy metals can be revealed on gene expressions that are responsible for the transportation of elements and cases between each other.
References
Balestrasse KB, Benavides MP, Galego SM, Tomaro ML (2003) Effect of cadmium stress on nitrogen metabolism in nodules and roots of soybean plants. Func Plant Biol 30:57–64
Benavides MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plants. Braz J Plant Physiol 17:21–34
Bertoli AC, Cannata MG, Carvalho R, Bastos ARR, Freitas MP, Augusto AS (2012) Lycopersicon esculentum submitted to Cd-stressful conditions in nutrition solution: nutrient contents and translocation. Ecotoxicol Environ Saf 86:176–181
Brewer TM, Marcus RK (2007) Separation and determination of iron containing proteins via liquid chromatography, particle beam, hollow cathode, optical emission spectroscopy. Anal Chem 79:2402–2611
Büyük İ, Soydam-Aydın S, Aras S (2012) Molecular responses of plants to stress conditions. Turk Hij Den Biyol Derg 69:97–110
Canpolat E, Lynes MA (2001) In vivo manipulation of endogenous metallothionein with a monoclonal antibody enhances a T-dependent humoral immune response. Toxicol Sci 62:61–70
Castiglione S, Franchin C, Fossati T, Lingua G, Torrigiani P, Biondi S (2007) High zinc concentrations reduce rooting capacity and alter metallothionein gene expression in white poplar (Populus alba L. cv. Villafranca). Chemosphere 67:1117–1126
Chibuike GU, Obiora SC (2014) Heavy metal polluted soils: effect on plants and bioremediation methods. Appl Environ Soil Sci 2014:1–12
Clemens S, Palmgreen MG, Kramer U (2002) A long way ahead: understanding and engineering plant metal accumulation. Trends Plant Sci 7:309–315
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Plant Biol 53:159–182
Dabrio M, Rodrigueez AR, Bordin G, Bebianno MJ, Leu M, Sestakova I, Vasak M, Nordberg M (2002) Recent developments in quantification methods for metallothionein. J Inorg Biochem 88:123–134
Döker S, Aydemir O, Uslu M (2014) Evaluation of digestion procedures for trace element analysis of Cankiri, Turkey honey by inductively coupled plasma mass spectrometry. Anal Lett 47:2080–2094
Eker S, Erdem H, Yazıcı MA, Barut H, Heybet EH (2013) Effects of cadmium on growth and nutrient composition of bread and durum wheat genotypes. Fresenius Environ Bull 22:1779–1786
Giritch A, Ganal M, Stephan UW, Baumlein H (1998) Structure, expression and chromosomal localisation of the metallothionein like gene family of tomato. Plant Mol Biol 37:701–714
Gonzalez-Mendoza D, Morenob AQ, Zapata-Perez O (2007) Coordinated responses of phytochelatin synthase and metallothionein genes in black mangrove, Avicennia germinans, exposed to cadmium and copper. Aquat Toxicol 83:306–314
Goupil P, Souhuir D, Ferjani E, Faure O, Hitmi A, Ledoigt G (2009) Expression of stress related genes in tomato plants exposed to arsenic and chromium in nutrient solution. J Plant Physiol 166:1446–1452
Gratao PL, Monterio CC, Antunes AM, Peres LEP, Azevedo RA (2008) Acquired tolerance of tomato (Lycopersicon esculantum cv. Micro-tom) plants to cadmium-induced stress. Ann Appl Biol 153:321–333
Hossain Z, Komatsu S (2012) Contribution of proteomic studies towards understanding plant heavy metal stress response. Front Plant Sci 3:310
Huang GY, Wang YS (2009) Expression analysis of type 2 metallothionein gene in mangrove species (Bruguiera gymnorrhiza) under heavy metal stress. Chemosphere 77:1026–1029
Huang GY, Wang YS, Ying GG, Dang AC (2012) Analysis of type 2 metallothionein gene from mangrove species (Kandelia candel). Trees 26:1537–1544
Jia D, Li Y, Hao L (2012) Advances in metallothionein studies in forest trees. Plant Omics J 5:46–51
Kabata-Pendias A, Pendias H (2001) Trace elements in soil and plant. CRC Press, New York
Kavamura VN, Esposito E (2010) Biotechnological strategies applied to the decontamination of soils polluted with heavy metals. Biotechnol Adv 28:61–69
Kohler A, Blaudez D, Chalot M, Martin F (2004) Cloning and expression of multiple metallothioneins from hybrid poplar. New Phytol 164:83–93
Liu J, Li K, Xu J, Liang J, Lu X, Yang J, Zhu Q (2003) Interaction of Cd and five mineral nutrients for uptake and accumulation in different rice cultivars and genotypes. Field Crop Res 83:271–281
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:02–408
López-Millán AF, Sagardoy R, Solanas M, Abadía A, Abadía J (2009) Cadmium toxicity in tomato (Lycopersicon esculentum) plants grown in hydroponics. Environ Exp Bot 65:376–385
Maksymiec W (2007) Signaling responses in plants to heavy metal stress. Acta Physiol Plant 29:177–187
Mudgal V, Madaan N, Mudgal A (2010) Heavy metals in plants: phytoremediation: plants used to remediate heavy metal pollution. Agric Biol J North Am 1:10–46
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Nazar R, Iqbal N, Masood A, Khan MIR, Syeed S, Khan NA (2012) Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am J Plant Sci 3:1476–1489
Quan XQ, Shan L, Bi YP (2007) Cloning of metallothionein genes from Arachis hypogaea and characterization of AhMT2a. Russ J Plant Physiol 54:669–675
Rao KRM, Raghavendra AS, Reddy JK (2006) Physiology and molecular biology of stress tolerance in plants. Springer, Dordrecht
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180:169–181
Ryvolova M, Adam V, Kizek R (2012) Analysis of metallothionein by capillary electrophoresis. J Chromatogr 1226:31–42
Shanker AK, Venkateswarlu B (2011) Abiotic stress in plants-mechanisms and adaptations. InTech, Croatia
Shi W, Chance MR (2008) Metallomics and metalloproteins. Cell Mol Life Sci 65:3040–3048
Singh R, Gautam N, Mishra A, Gupta R (2011) Heavy metals and living systems: an overview. Indian J Pharmacol 43:246–253
Suarez MH, Rodriguez EM, Romero CD (2007) Mineral and trace element concentrations in cultivars of tomatoes. Food Chem 104:489–499
Tombuloğlu H, Semizoğlu N, Sakcali S, Kekec G (2012) Boron induced expression of some stress-related genes in tomato. Chemosphere 86:433–438
Yang M, Zhang F, Wang F, Dong Z, Cao Q, Chen M (2015) Characterization of a type 1 metallothionein gene from the stresses-tolerant plant Ziziphus jujuba. Int J Mol Sci 16:16750–16762
Acknowledgments
The authors thankfully acknowledge support from the Scientific Research Projects Commission, Gaziosmanpaşa University, Tokat, Turkey (Project No: 2013/129).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kısa, D., Öztürk, L. & Tekin, Ş. Gene expression analysis of metallothionein and mineral elements uptake in tomato (Solanum lycopersicum) exposed to cadmium. J Plant Res 129, 989–995 (2016). https://doi.org/10.1007/s10265-016-0847-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-016-0847-7