Abstract
Salinity is a widespread adverse environmental problem globally, and significantly limits crop production. In this study, the possibility of enhancing salinity stress tolerance of Swiss chard (Beta vulgaris L. var. cicla) by 5-aminolevulinic acid (ALA) foliar application was investigated. The Swiss chard plants were grown in hydroponic culture. Twelve-week-old uniform seedlings were treated by 0 and 40 mM saline regimes generated by the mixture of sodium chloride and sodium sulfate (molar ratio NaCl:Na2SO4 = 9:1), and were foliar-sprayed with 0 and 60 μM L−1 ALA (every 3 days) for 6 days; then the plants were treated for another 7 days (every 3 day) with increased concentration of salinity and ALA, 80 mM and 120 μM L−1. Salinity without ALA application significantly decreased plant growth [43 % in shoot dry weight (DW), 21 % in root DW, 24 % in relative growth rate (RGR), 43 % in leaf area (LA)], water uptake [20.8 % in relative water content (RWC), 47.9 % in osmotic potential (OP)], chlorophyll (Chl) a content (10 %), Pn (36 %), Gs (72 %) and Tr (59 %) compared with those in control plants; however, under saline conditions, ALA foliar application improved plant growth (49.7 % in shoot DW, 27 % in root DW, 42.3 % in RGR, 72.1 % in LA) and increased RWC (12 %), Chl a content (10 %) and photosynthetic parameters (27 % in Pn, 28 % in Gs, 14 % in Tr) compared with those in untreated plants. Salinity significantly increased Na+ content, resulting in the reduction of Mg2+ and K+ contents. ALA foliar application alleviated ionic toxicity through the reduction of Na+ content and Na+/K+ ratio. On the other hand, it increased total nitrogen and glycine betaine (GB) content. ALA foliar application slightly reduced malondialdehyde (MDA) content, indicating that ALA has the potential to alleviate oxidative stress in salinity-stressed Swiss chard.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity is a widespread adverse environmental problem globally and is being aggravated by agricultural practices. Plant growth responds to salinity in two phases: a rapid, osmotic phase that inhibits the growth of young leaves, and a slower, ionic phase that accelerates the senescence of mature leaves (Munns and Tester 2008). As a consequence of these two primary phases, secondary stresses such as oxidative damage often occur (Zhu 2001). Vegetables are rich and important sources of nutrients for humans. However, their production can be significantly limited by salinity with consequent negative effects on food security. Therefore, it is very important to study the possibility of enhancing the salinity tolerance of crop species.
There are three approaches to develop stress-tolerant plants: breeding, genetic engineering and the use of plant growth regulators (PGRs). However, it is a very long process and requires a large amount of resources to improve salt tolerance through breeding, and it is difficult to identify genes for tolerance to a specific stress factor in crop species through genetic engineering (Korkmaz 2012). Therefore, plant growth substances are widely applied to agricultural crops for improving crop production and plant stress resistance. 5-aminolevulinic acid (ALA) is naturally found in plants, animals, algae and photosynthetic bacteria, and it is known as the precursor of all porphyrin compounds, such as vitamin B12, chlorophyll (Chl), heme and phytochrome (Korkmaz 2012). It has attracted substantial attention lately as a substance for improving plant growth and for stress amelioration at low concentration. Previous studies reported that low concentrations of ALA promoted the growth and yield of several crops and vegetables (Hotta et al. 1997; Hara et al. 2011), and that it has the potential to improve the stress tolerance during plant growth stages: Exogenous ALA application promoted the seed germination of pakchoi (Wang et al. 2005), leaf water relationships, chlorophyll content and mineral accumulation in oilseed rape seedlings (Naeem et al. 2010, 2011), photosynthetic gas exchange capacity in oilseed rape and spinach seedlings (Nishihara et al. 2003; Naeem et al. 2010), reduced Na+ uptake in cotton and oilseed rape seedlings (Watanabe et al. 2000; Naeem et al. 2012), and also reduced oxidative stress in spinach, rice, cucumber and oilseed rape seedlings and in potato microtubers under saline conditions (Nishihara et al. 2003; Zhang et al. 2006; Wongkantrakorn et al. 2009; Zhen et al. 2012; Naeem et al. 2012).
Sodium chloride is the most soluble and abundant salt released through weathering of parental rock, and to a lesser extent, sulfates and carbonates (Munns and Tester 2008). In many areas, such as in India and China, the salinity problem is mainly due to sulfates and chlorides, and many studies have focused on the effect of salinity stress caused by the mixture of NaCl and Na2SO4 on plant growth and physiology (Li et al. 2009, Zhang and Mu 2009, Liu et al. 2013). Na+, Cl− and SO4 2− in high concentration in the external solution are potentially taken up at high rate, and lead to excessive accumulation in the plant tissue. Those ions may affect membrane selective permeability and interfere with the uptake of other ions, thus altering the contents of a range of tissue element (Hu and Schmidhalter 2005, Colla et al. 2012). In addition, Na+ is often accompanied by Cl− and (or) SO4 2− in naturally saline areas (Renault et al. 2001), and the main difference between saline stress caused by Na2SO4 from that induced by NaCl is anion toxicity. Previous studies reported that SO4 2− was more inhibitory than Cl− at iso-osmotic concentration in Prosopis strombulifera plant (Sosa et al. 2005). On the contrary, Cl− was more phytotoxic to black spruce, white spruce and jack pine seedlings than the same molar SO4 2− (Nguyen et al. 2006). Hence it is contemplated to investigate the effects of SO 24 and Cl− on the physiology of vegetables. Low concentrations of ALA have the potential to promote the plant growth, and all previous studies focused on the potential of ALA to enhance plant salt tolerance under saline conditions generated by Na+ and Cl−, there have been no reports about the potential of ALA to promote plant salt tolerance under saline conditions generated by the mixture of Na+, Cl− and SO4 2−. Therefore, it is basically required to investigate whether low concentration of ALA would enhance the salt tolerance of vegetables to different types of salinity.
Swiss chard (Beta vulgaris L. subsp. cicla) is a glycophytic member of the Chenopodiaceae, and is distributed all over the world as a green vegetable. The leaf of Swiss chard contains nutritionally significant concentrations of magnesium, calcium and phosphorus, and is a good source of natural antioxidants for the protection of humans against several chronic diseases (Pyo et al. 2004, Maynard and Hochmuth 2007). However, its production is significantly reduced by NaCl stress (Keeling and Ireland 2009) as well as by the stress caused by 100 mM of a neutral salt mixture consisting of NaCl and Na2SO4 (9:1 molar ratio) (Liu et al. 2013). Those literatures showed that the Cl− based solution has considerably more inhibitory effect than the mixture of SO4 2− and Cl− based solution does on Swiss chard plant growth at the same concentration, indicating that the Cl− is more phytotoxic to Swiss chard than the same molar SO4 2−.
Therefore, the main objectives of the current investigation were to study the salinity tolerance of Swiss chard imparted by ALA foliar application through determining the influence of salinity and ALA foliar application on leaf water uptake, photosynthetic capacity, and the uptake of Na+ and other mineral nutrients in seedling growth under saline conditions, and to show the effects of ALA foliar application on oxidative stress and endogenous ALA content.
Materials and methods
Plant materials
Swiss chard (B. vulgaris L. subsp. cicla), the seeds of which were obtained from Utane Seed Co., Ltd. (Utsunomiya, Japan), was cultivated in a greenhouse of Hiroshima University at a maximum temperature of 31 ºC during the day and minimum temperature of 24 ºC during the night. The seeds were sown in seedbeds filled with soil mixture and watered using tap water. After germination, uniform seedlings were collected and grown under a hydroponic system. The basal nutrient solution contained 1.6 mM NO3 −–N, 0.1 mM NH4 +–N, 152.4 μM P, 838.0 μM K, 152.4 μM Mg, 381.0 μM Ca, 2.0 μM Mn, 4.0 μM B, 4.6 μM Fe, 38.1 nM Cu, 114.3 nM Zn and 30.5 nM Mo.
Stress treatments
Twelve-week-old uniform seedlings were transplanted into 70 L pots filled with basal nutrient solution. Each pot contained four plants and one plant considered as one replicate. After 7 days of acclimatization, seedlings were treated by 0 and 40 mM salinity (NaCl:Na2SO4 = 9:1 molar ratio), and foliar-sprayed with 0 and 60 μM−1 L ALA (Nacalai Tesque, Inc., Kyoto, Japan) on the 1st and 4th days. The volume of sprayed ALA solution was 5 mL per plant. From the 7th day, salinity and ALA were increased to 80 mM and 120 μM−1 L, respectively. ALA was foliar-sprayed on the 7th and 10th days, and the volume of sprayed solution was 10 mL per plant. After 12 days of treatment, sampling was initiated (four replicates per treatment).
Plant harvesting and growth measurements
The plants were divided at the position just above cotyledon and washed using tap water at first, and then using distilled water. After removing moisture with tissue paper, plant tissue was carefully separated into leaf, stalk and root, and then frozen directly in liquid nitrogen, the freeze-dried, and the dry weight (DW) measured. The youngest fully expanded leaves were freeze-dried for the evaluation of other parameters. The leaf area (LA) was measured using a LA meter (AMM-5 type leaf area meter, Hayashi-Denko, Tokyo, Japan). Relative growth rate (RGR) was calculated using the method according to Hunt (1990). The relative water content (RWC) was measured as described by Turner (1981). The osmotic potential (OP) of the cell sap was measured using a Wescor 5500 vapor pressure osmometer (Wescor Inc., Logan, UT, USA).
Physiological index analysis
The apparent photosynthetic rate (Pn), stomatal conductance (Gs) and transpiration rate (Tr) of the youngest fully expanded leaves were measured using a portable photosynthesis system equipped with a leaf chamber and a portable open flow infrared gas analyzer (LI-6400, LI-COR Biosciences, Lincoln, NE, USA) at 10:00–12:00 AM on the 12th day of treatment. Photosynthetically active radiation was applied at 1,000 μmol m−2 s−1, the relative humidity was 80 % and the ambient CO2 concentration was set at 400 μmol mol−1. The temperature of the leaf surfaces was 25 °C and the flow of air was 500 mL s−1. Chl a and b were determined following the measures described by Arnon (1949).
For cations, around 20 mg of finely ground powder was digested using nitric acid-hydrogen peroxide. The Na+ and K+ concentrations were determined using a flame photometer (ANA-135, Eiko Instruments Inc., Tokyo, Japan), the Mg2+ concentration was determined using an atomic absorption spectrophotometer (U-3310 Hitachi Co. Ltd., Tokyo, Japan) and N concentration was determined by the Kjeldahl method after digestion with sulfuric acid.
Glycine betaine (GB) was purified by Dowex-1-Cl− ion-exchange chromatography and Dowex-50-H+ ion-exchange chromatography from the leaf methanol extraction after separation using chloroform and water, and eluted from the column by Dowex-50-H+ ion-exchange chromatography using 6 M NH4OH. The dried NH4OH eluant was extracted with 10 mL of acetonitrile:methanol (20:1, v/v) to remove protein (Yang et al. 1995), and then GB was analyzed by HPLC (Gulliver system, Jasco Co., Tokyo, Japan) using a Shodex NH2P-40 4E column (Saneoka et al. 2004). Proline was determined following the ninhydrin method described by Bates et al. (1973) using l-proline as a standard.
The malondialdehyde (MDA) content was determined by employing the thiobarbituric acid (TBA) reaction as described by Fu and Huang (2001). Endogenous ALA content was measured following the method described by Aarti et al. (2007). Fresh samples were homogenized with 50 mM K2HPO4/KH2PO4, pH 6.8 using liquid nitrogen and then centrifuged. Ethylacetoacetate was subsequently added to an aliquot of the supernatant, which was boiled for 10 min and cooled on ice for 5 min. An equal volume of modified Ehrlich’s reagent was added to develop the color. After thorough vortexing, the mixture was centrifuged and its color development was measured at 553 nm using a spectrophotometer.
Statistical analysis
All data were examined by one-way ANOVA using Statistical Package for the Social Sciences (SPSS Version 21.0) for Windows. Values are given as mean ± standard deviation (SD) and multiple comparisons of means of data between different salinity treatments within the plants were performed using Duncan’s test at the 5 % significance level.
Results
Plant growth
The effects of saline condition and exogenous ALA foliar application on plant growth in terms of DW, RGR and LA are shown in Fig. 1. Saline stress clearly inhibited the plant growth of Swiss chard (43 % in shoot DW, 21 % in root DW, 24 % in RGR, 43 % in LA). The ALA foliar application improved the plant salinity tolerance: shoot DW increased by 49.7 %, root DW increased by 27 %, RGR increased by 42.3 % and LA increased by 72.1 %. ALA foliar application under control conditions did not affect the shoot DW, RGR and LA, and increased root DW by 14.5 % compared to those without ALA application.
Water relationship
To understand how exogenous ALA foliar application counters salt-induced changes in water relationship, RWC and OP were determined (Fig. 2). Without ALA foliar application, salinity seriously decreased RWC and OP (by 20.8 and 47.9 %, respectively), however under saline stress, the application of ALA increased RWC and OP (by 12.0 and 16.9 % respectively) compared to plants without application. Similarly ALA foliar application under control condition also increased OP by 16.9 % compared with untreated plants, but it had no effect on RWC (Fig. 2).
Chlorophyll content and photosynthesis
Chlorophyll content in topmost fully expanded leaves is shown in Fig. 3. Chl a content was decreased by 10 % by salinity compared with the control plants, however, this decrease was almost removed by the ALA application. Chl b contents were not affected by salinity alone or combination with ALA compared to control plants. At the same time, under control conditions, Chl a and Chl b contents were increased by 6.9 and 12.7 %, respectively, in ALA treated plants compared with those in untreated plants (Fig. 3). The influence of ALA on photosynthetic capacity was examined. It was shown in Table 1 that Pn, Tr and Gs decreased (36, 59 and 72 %, respectively) by salinity. In contrast, Pn, Tr and Gs were increased in plants with ALA application compared with those in plants without ALA application; these increase were 27.3, 14.0 and 27.7 %, respectively, under control conditions and were 41.4, 44.3 and 55.6 %, respectively, under saline conditions.
Mineral accumulation
Plant responses to salinity vary depending on the degree of salt stress and the stage of plant growth. To clarify the mechanism of adaptation of 12-week-old seedlings to saline stress promoted by ALA foliar application, changes in mineral contents of leaves were determined (Table 2). The results showed that Mg2+ and K+ contents were decreased, while Na+ content and Na+/K+ ratio were increased by salinity; In the presence of ALA under saline conditions, K+ content was not affected, Mg2+ content was slightly increased and Na+ content was reduced compared with those in plants untreated with ALA. Salinity stress generated by the mixture of NaCl and Na2SO4 slightly increased total N content compared with that under control conditions alone. ALA foliar application significantly increased total N content under both control and saline conditions compared with that without ALA foliar application (Table 2).
Osmotic adjustments, MDA content and endogenous ALA content
GB and proline are two major osmotic adjustment factors that accumulate in a variety of plant species in responses to environmental stresses (Ashraf and Foolad 2007). From the data illustrated in Table 3, it can be seen that proline and GB contents increased under saline conditions. Proline content was also changed by ALA application, it was increased under control with ALA conditions but was decreased under saline sress. At the same time, ALA foliar application under saline conditions produced a increase in GB content compared with that without ALA application.
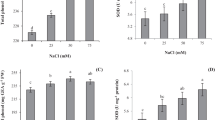
The level of lipid peroxidation was expressed in terms of MDA. The changes induced by treatments of ALA and salinity on MDA content were shown in Fig. 4. In comparison with plants under control condition without ALA application, MDA content was increased by 20 % in plants treated with salinity alone, however it was only slightly increased in plants under both treatments, control and salinity stress, they were 10 and 7.5 %, respectively.
ALA production is the primary determinant and the most sensitive step in the pathway of chlorophyll biosynthesis. The effect of exogenous ALA foliar application on endogenous ALA contents was shown in Fig. 5. Salinity and/or ALA application had almost no considerable effect on endogenous ALA content.
Discussion
Salinity is a major abiotic stress in plant agriculture worldwide, and this has led to research into salt tolerance with the aim of improving crop plants (Zhu 2001). Recently, ALA has attracted substantial attention as a plant growth substance for improving plant growth and stress amelioration at low concentration. Some studies reported that a low concentration of exogenous ALA promoted the salt tolerance of cotton, spinach and oilseed rape seedlings (Watanabe et al. 2000; Nishihara et al. 2003; Naeem et al. 2010). In this study, plant growth in terms of shoot DW, root DW, RGR and LA was greatly reduced by salinity, and treatment of ALA significantly improved these parameters (shoot DW: 49.7 %, root DW: 27 %, RGR: 42.3 % and LA: 72.1 %) under saline conditions compared with those under saline conditions alone (Fig. 1), suggesting that ALA foliar application can produce recovery of Swiss chard growth from the harmful effects of salinity (NaCl and Na2SO4 mixture) and improve its salinity tolerance.
Plant responses to salinity can occur in two phases over time: the osmotic phase and the ionic phase. The osmotic phase starts immediately after the salt concentration around the roots increases to a threshold level, resulting in the inhibition of young leaf growth and a significant reduction in the rate of shoot growth (Munns and Tester 2008). The RWC and OP in leaves of Swiss chard were significantly reduced under saline conditions, indicating that the cell expansion in root tips and young leaves of Swiss chard was reduced by salinity, resulting in the reduction of plant biomass. Osmotic stress tolerance is one distinct type of plant adaptation to salinity, and increased osmotic tolerance is evident mainly by the increased ability to continue producing new leaves (Munns and Tester 2008). In the present study, exciting results were obtained that the increase in OP in leaves of Swiss chard was induced by ALA foliar application under saline conditions, suggesting that it was possible for Swiss chard to exhibit positive turgor for cell extension, and then enhanced ability to continue producing new leaves by ALA foliar application under saline conditions.
Salinity produces important declines in the chlorophyll content of leaves and results in the oldest leaves starting chlorosis and falling with a prolonged period of salinity stress (Parida and Das 2005), while ALA has a variety of plant physiological effects on chlorophyll synthesis (Hotta et al. 1997). Previous studies reported that chlorophyll content was significantly decreased by salt and water stresses in oilseed rape seedlings, and increased by ALA application (Naeem et al. 2010; Liu et al. 2011). In this study, Chl a contents were increased by ALA foliar application under control and saline conditions, suggesting that ALA might enhance chlorophyll biosynthesis and Chl a content through its foliar application. Chl b content was increased under control conditions by ALA foliar application, suggesting that the conversion from Chl a to Chl b is activated by ALA foliar application. However, Chl b content was not affected under saline conditions with ALA foliar application compared with that under saline conditions alone, maybe because chlorophyllide a oxygenase (CAO) had been damaged by the salinity.
The degree of salt-induced reduction in photosynthetic capacity depends on the area of the photosynthesizing tissue (leaf area), photosynthetic pigments (Chl a and b), and stomatal and non-stomatal factors that affect CO2 assimilation (Dubey 2005). Salinity causes leaf cells to lose water, then immediately and transiently affects stomatal conductance (Munns and Tester 2008). As time passes, osmotic stress leads to reductions in cell elongation and cell division, so leaves are smaller and thicker (Munns and Tester 2008). Under saline conditions alone, the reduction of RWC in leaves of Swiss chard resulted in the reduction of stomatal conductance, and then reductions of cell elongation, cell division and finally LA occurred with time. Reductions of both stomatal conductance and LA led to the reduction of photosynthetic capacity. The improvement of water uptake from roots by ALA foliar application under saline conditions reduced stomatal limitation of carbon dioxide uptake and photosynthetic gas exchange, and then improved the net photosynthetic rate. Chlorophyll functions as a pigment for light energy harvesting and transfer to the reaction center in photosynthesis (Masuda and Fujita 2008). Therefore, the enhancement of Chl content by ALA foliar application boosted the light-harvesting capacity and improved the net photosynthetic rate under saline conditions. Under salinity stress, metabolic limitations of photosynthesis resulted from increased concentration of Na+ in the leaf tissue (Chaves et al. 2009). In this study, Na+ content in leaf tissue was significantly increased being toxic for photochemistry, while ALA foliar application reduced the ionic toxicity through the reduction of Na+ content and improved the photosynthetic capacity.
Intercellular ionic homeostasis is important for the activities of many cytosolic enzymes, and for maintaining membrane potential and an appropriate osmoticum for cell volume regulation (Zhu 2003). However, salinity may cause nutrient deficiencies or imbalances due to the competition of Na+ with nutrients such as K+, Ca2+ and Mg2+ (Hu and Schmidhalter 2005). In this study, salinity increased Na+ content, decreased Mg2+ and K+ contents, and then resulted in injury regarding Swiss chard growth under saline conditions. The cytosolic K+/Na+ ratio has been repeatedly named as a key determinant of plant salt tolerance, and the optimal cytosolic K+/Na+ ratio can be maintained by either restricting Na+ accumulation in plant tissue or preventing K+ loss from the cell (Shabala and Cuin 2008). In this study, the improvement of plant growth under saline conditions due to ALA foliar application was associated with reduced Na+ uptake and maintenance of lower Na+/K+ ratio in the leaves of Swiss chard. These results agree with previous studies that reported the improvement of salt tolerance associated with reduced accumulation of Na+ in cotton seedlings (Watanabe et al. 2000), and that ALA foliar application reduced Na+ uptake, leading to a significant decrease in Na+/K+ ratio in oilseed rape seedlings (Naeem et al. 2010, 2012). Therefore, exogenous ALA has the potential to manipulate the uptake of Na, and overcome the injurious effects of salt stress on some plants. Mg2+ is the central atom of the chlorophyll molecule, and fluctuations in its levels in the chloroplast regulate the activity of key photosynthetic enzymes (Shaul 2002). Hu and Schmidhalter (2005) suggested that Mg2+ supplementation could play a role in increasing plant resistance when this nutrient is available at low levels. Mg2+ content in leaves of Swiss chard was significantly reduced by salinity and slightly increased by ALA foliar application under saline conditions, and the induction of Mg2+ might help Swiss chard to improve its salt resistance through improvement of the biosynthesis of chlorophyll.
N is the mineral element in plants required in the largest amounts and is a constituent of many plant cell components, including amino acids and nucleic acids; nitrogen deficiency rapidly inhibits plant growth (Hu and Schmidhalter 2005). Previous studies reported that salt stress reduces N uptake in many plants, attributed to the antagonism between NO3 − and Cl− (Yousif et al. 2010), and an ALA-based fertilizer significantly increased the concentration of N in the leaves of treated date palm plant (Awad 2008); in addition, foliar application of ALA enhanced the concentration of all mineral macronutrients in oilseed rape seedlings treated with salinity compared with that under saline treatment alone (Naeem et al. 2010). However, in this study, the total N content showed no reduction in the leaves of Swiss chard under saline treatment alone, and was significantly increased by ALA foliar application under control and saline conditions. Fertilizer N will not increase yield without sufficient water being available to the plant, and increasing soil–water availability will not increase production without adequate N supply (Hu and Schmidhalter 2005), suggesting that there is a positive relationship between N content and water availability in growing plants. Therefore, the increase of total N content by ALA foliar application under saline conditions in leaves of Swiss chard might have been due to the increase of photosynthetic capacity and water uptake in this plant, and ALA foliar application improved the Swiss chard growth under saline conditions with the increase of total N content in its leaves.
It is well known that plants can avoid severe injury by the accumulation of osmotic adjustments such as in the levels of free amino acids, proline and GB under saline conditions. A previous study reported that osmoregulation due to the accumulation of some free amino acids and GB was one of the predominant strategies used by spinach plants to tolerate saline stress, and the increase of GB balanced the decline in free amino acids in the osmotic adjustment of the cells of spinach plants (Martino et al. 2003). In this study, proline and GB contents were increased under saline conditions, suggesting that Swiss chard has the capacity to maintain water accumulation for plant growth under saline conditions. However, proline content was decreased while GB content was increased by ALA foliar application under saline conditions compared with those without ALA foliar application.
Proline is an organic osmolyte that accumulates in a variety of plant species in response to environmental stresses (Ashraf and Foolad 2007). However, the significance of proline accumulation in osmotic adjustment is still debated and varies according to the species (Meloni et al. 2004). Previous studies reported that proline does not seem to have an important role in the mechanism of salt tolerance in legume (Meloni et al. 2004), and proline accumulation is a symptom of salt-stress injury in rice (Lutts et al. 1999). In addition, Demiral and Türkan (2006) reported that exogenous GB treatment caused a decrease in the level of proline accumulation in rice plant (IR-28), and Hu et al. (2012) reported that the application of GB reduced MDA and proline content in salt-stressed perennial ryegrass plant. In this study, the decrease of proline content under saline with ALA conditions might have been due to the increase of GB by ALA foliar application, and the decrease of proline content might have been a symptom of salt tolerance in Swiss chard plant. Averina et al. (2010) reported that the stimulation of ALA formation at high salinity redirects the glutamic acid metabolism from the proline synthesis pathway to the Chl and heme synthesis pathway, which suppresses proline formation in barley plants. Therefore, exogenous ALA might suppress proline formation through the stimulation of Chl synthesis pathway under saline conditions in Swiss chard. At the same time, the decrease of proline content by exogenous ALA agrees with previous studies, which reported that 30 mg L−1 ALA treatment resulted in the lowest proline accumulation under 0.5 % NaCl stress in potato microtubers (Zhang et al. 2006).
It is well known that oxidative stress is induced under environmental stresses through the production of reactive oxygen species, which are highly toxic, damage many important cellular components (Fu and Huang 2001; Bor et al. 2003) and cause leakage of the cell membrane. The measurement of MDA is widely used as an indicator of lipid peroxidation, which is used as an indicator of oxidative stress in cells and tissues. In this study, MDA content in leaves was almost the same under control and saline conditions without ALA foliar application, suggesting that the lipid peroxidation of cell membrane in leaves of Swiss chard was not induced by salinity. In addition, plant responses to salinity vary depending on the degree of salt stress, the stage of plant growth and the plant species. The formation of oxidative stress is followed by stomatal closure caused by water deficit. Therefore, the lipid peroxidation of the cell membrane in leaves of Swiss chard was possibly not induced due to the stage of plant growth being old before treatments, and the degree of salt stress was insufficient to result in the oxidative stress in Swiss chard. However, the ALA foliar application decreased MDA content by 10.3 and 7.5 % under control and saline conditions compared with that without ALA foliar application, respectively, suggesting that ALA foliar application functions as a protectant against oxidative damage of membranes (Zhang et al. 2006). Hu et al. (2012) reported that the application of GB reduced MDA content in salt-stressed perennial ryegrass plant. In this study, the decrease of MDA content by ALA foliar application might have been due to the increase of GB content.
ALA biosynthesis proceeds by two distinct pathways: the Shemin pathway from succinyl-CoA and glycine, and the Beale pathway from glutamate (Sasaki et al. 2002), and the rate-limiting step in chlorophyll biosynthesis is ALA formation, which depends primarily on enzymes involved in the synthesis and breakdown of ALA (Korkmaz 2012; Akram and Ashraf 2013). In this study, ALA foliar application did not enhance endogenous ALA contents under control and saline conditions, it did enhance chlorophyll contents, suggesting that ALA has the capacity to improve chlorophyll content and chlorophyll biosynthesis through its foliar application, thus the foliar-applied ALA could be used for improving the chlorophyll contents in Swiss chard. GB contents increased by ALA foliar application under saline conditions, these results suggested that the foliar-applied ALA may have regulatory effects on GB synthesis.
Abbreviations
- ALA:
-
5-Aminolevulinic acid
- RGR:
-
Relative growth rate
- Pn :
-
Apparent photosynthetic rate
- Tr :
-
Transpiration rate
- Gs :
-
Stomatal conductance
- MDA:
-
Malondialdehyde
- DW:
-
Dry weight
- Chl:
-
Chlorophyll
- RWC:
-
Relative water content
- GB:
-
Glycine betaine
- OP:
-
Osmotic potential
- LA:
-
Leaf area
References
Aarti D, Tanaka R, Ito H, Tanaka A (2007) High light inhibits chlorophyll biosynthesis at the level of 5-aminolevulinate synthesis during de-etiolation in cucumber (Cucumis sativus) cotyledons. Photochem Photobiol 83:171–176
Akram NA, Ashraf M (2013) Regulation in plant stress tolerance by a potential plant growth regulator, 5-aminolevulinic acid. J Plant Growth Regul. doi:10.1007/s00344-013-9325-9
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Averina NG, Gritskevich ER, Vershilovskaya IV, Usatov AV, Yaronskaya EB (2010) Mechanisms of salt stress tolerance development in barley plants under the influence of 5-aminolevulinic acid. Rus J Plant Physiol 57:792–798
Awad MA (2008) Promotive effects of 5-aminolevulinic acid-based fertilizer on growth of tissue culture-derived date palm plants (Phoenix dactylifera L.) during acclimatization. Sci Hortic 118:48–52
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-studies. Plant Soil 39:205–207
Bor M, Özdemir F, Türkan I (2003) The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritima L. Plant Sci 164:77–84
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Colla G, Rouphael Y, Rea E, Cardarelli M (2012) Grafting cucumber plants enhance tolerance to sodium chloride and sulfate salinization. Sci Hortic 135:177–185
Demiral T, Türkan I (2006) Exogenous glycinebetaine affects growth and proline accumulation and retards senescence in two rice cultivars under NaCl stress. Environ Exp Bot 56:72–79
Dubey RS (2005) Photosynthesis in plants under stressful conditions. In: Pessarakli M (ed) Handbook of photosynthesis, 2nd edn. CRC Press, New York, pp 717–718
Fu JM, Huang BR (2001) Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ Exp Bot 45:105–114
Hara M, Takahashi I, Yamori M, Tanaka T, Funada S, Watanabe K (2011) Effects of 5-aminolevulinic acid on growth and amylase activity in the radish taproot. Plant Growth Regul 64:287–291
Hotta Y, Tanaka T, Takaoka H, Takeuchi Y, Konnai M (1997) Promotive effects of 5-aminolevulinic acid on the yield of several crops. Plant Growth Regul 22:109–114
Hu YC, Schmidhalter U (2005) Drought and salinity: a comparison of their effects on mineral nutrient of plants. J Plant Nutr Soil Sci 168:541–549
Hu LX, Hu T, Zhang XZ, Pang HC, Fu JM (2012) Exogenous glycine betaine ameliorates the adverse effect of salt stress on perennial ryegrass. J Am Soc Hortic Sci 137:38–46
Hunt R (1990) Basic growth analysis: plant growth analysis for beginners. Unwin Hyman, London
Keeling C, Ireland CR (2009) Characterization of saline tolerance in Swiss chard (Beta vulgaris spp. cicla L.): a potential novel crop for production on saline soils. Comp Biochem Physiol Part A 153:S217–S218
Korkmaz A (2012) Effects of exogenous application of 5-aminolevulinic acid in crop plants. In: Ahmad P, Prasad MNV (eds) Abiotic stress responses in plants. Springer, Berlin, pp 215–234
Li XY, Liu JJ, Zhang YT, Lin JX, Mu CS (2009) Physiological responses and adaptive strategies of wheat seedlings to salt and alkali stresses. Soil Sci Plant Nutr 55:680–684
Liu D, Pei ZF, Naeem MS, Ming DF, Liu HB, Khan F, Zhou WJ (2011) 5-Aminolevulinic acid activates antioxidative defence system and seedling growth in Brassica napus L. under water-deficit stress. J Agron Crop Sci 197:284–295
Liu L, Ueda A, Saneoka H (2013) Physiological responses of white Swiss chard (Beta vulgaris L. subsp. cicla) to saline and alkaline stresses. Aust J Crop Sci 7:1046–1052
Lutts S, Majerus V, Kinet JM (1999) NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol Plant 105:450–458
Martino CD, Delfine S, Pizzuto R, Loreto F, Fuffi A (2003) Free amino acids and glycine betaine in leaf osmoregulation of spinach responding to increasing salt stress. New Phytol 158:455–463
Masuda T, Fujita Y (2008) Regulation and evolution of chlorophyll metabolism. Photochem Photobiol Sci 7:1131–1149
Maynard DN, Hochmuth GJ (2007) Vegetables and vegetable industry. Knott’s handbook for vegetable growers, 5th edn. Wiley, Hoboken, pp 1–53
Meloni DA, Gulotta MR, Martínez CA, Oliva MA (2004) The effects of salt stress on growth, nitrate reduction and proline and glycinebetaine accumulation in Prosopis alba. Braz J Plant Physiol 16:39–46
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Naeem MS, Jin ZL, Wan GL, Liu D, Liu HB, Yoneyama K, Zhou WJ (2010) 5-Aminolevulinic acid improves photosynthetic gas exchange capacity and ion uptake under salinity stress in oilseed rape (Brassica napus L.). Plant Soil 332:405–415
Naeem MS, Rasheed M, Liu D, Jin ZL, Ming DF, Yoneyama K, Takeuchi Y, Zhou WJ (2011) 5-Aminolevulinic acid ameliorates salinity-induced metabolic, water-related and biochemical changes in Brassica napus L. Acta Physiol Plant 33:517–528
Naeem MS, Warusawitharana H, Liu HB, Liu D, Ahmad R, Waraich EA, Xu L, Zhou WJ (2012) 5-Aminolevulinic acid alleviates the salinity-induced changes in Brassica napus as revealed by the ultrastructural study of chloroplast. Plant Physiol Biochem 57:84–92
Nguyen H, Calvo Polanca M, Zwiazek JJ (2006) Gas exchange and growth responses of ectomycorrhizal Picea mariana, Picea glauca, and Pinus banksiana seedlings to NaCl and Na2SO4. Plant Biol 8:646–652
Nishihara E, Kondo K, Parvez MM, Takahashi K, Watanabe K, Tanaka K (2003) Role of 5-aminolevulinic acid (ALA) on active oxygen-scavenging system in NaCl-treated spinach (Spinacia oleracea). Plant Physiol 160:1085–1091
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Pyo YH, Lee TC, Logendra L, Rosen R (2004) Antioxidant activity and phenolic compounds of Swiss chard (Beta vulgaris subspecies cycla) extracts. Food Chem 85:19–26
Renault S, Croser C, Franklin JA, Zwiazek JJ (2001) Effects of NaCl and Na2SO4 on red-osier dogwood (Cornus stolonifera Michz) seedlings. Plant Soil 233:261–268
Saneoka H, Moghaieb REA, Premachandra GS, Fujita K (2004) Nitrogen nutrition and water stress effects on cell membrane stability and leaf water relations in Agrostis palustris Huds. Environ Exp Bot 52:131–138
Sasaki K, Watanabe M, Tanaka T, Tanaka T (2002) Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid. Appl Microbiol Biotechnol 58:23–29
Shabala S, Cuin TA (2008) Potassium transport and plant salt tolerance. Physiol Plant 133:651–669
Shaul O (2002) Magnesium transport and function in plants: the tip of the iceberg. Biometals 15:309–323
Sosa L, Llanes A, Reinoso H, Reginato M, Luna V (2005) Osmotic and specific ion effects on the germination of Prosopis strombulifera. Ann Bot-Lond 96:261–267
Turner NC (1981) Technique and experimental approaches for the measurement of plant water status. Plant Soil 58:339–366
Wang LJ, Jiang WB, Liu H, Liu WQ, Kang L, Hou XL (2005) Promotion by 5-aminolevulinic acid of germination of pakchoi (Brassica campestris ssp. chinensis var. communis Tsen et Lee) seeds under salt stress. J Integr Plant Biol 47:1084–1091
Watanabe K, Tanaka T, Hotta Y, Kuramochi H, Takeuchi Y (2000) Improving salt tolerance of cotton seedlings with 5-aminolevulinic acid. Plant Growth Regul 32:99–103
Wongkantrakorn N, Sunohara Y, Matsumoto H (2009) Mechanism of growth amelioration of NaCl-stressed rice (Oryza sativa L.) by δ-aminolevulinic acid. J Pestic Sci 34:89–95
Yang WJ, Nadolska-Orczyk A, Wood KV, Hahn DT, Rich PJ, Wood AJ, Saneoka H, Premachandra GS, Bonham CC, Rhodes JC, Joly RJ, Samaras Y, Goldsbrough PB, Rhodes D (1995) Near-isogenic lines of maize differing for glycinebetaine. Plant Physiol 107:621–630
Yousif BS, Nguyen NT, Fukuda Y, Hakada H, Okamoto Y, Masaoka Y, Saneoka H (2010) Effect of salinity on growth, mineral composition, photosynthesis and water relations of two vegetable crops; new Zealand spinach (Tetragonia tetragoniodes) and water spinach (Ipomoea aquatic). Int J Agric Bio 12:211–216
Zhang JT, Mu CS (2009) Effects of saline and alkaline stresses on the germination, growth, photosynthesis, ionic balance and anti-oxidant system in an alkali-tolerant leguminous forage Lathyrus quinquenervius. Soil Sci Plant Nutr 55:685–697
Zhang ZJ, Li HZ, Zhou WJ, Takeuchi Y, Yoneyama K (2006) Effect of 5-aminolevulinic acid on development and salt tolerance of potato (Solanum tuberosum L.) microtubers in vitro. Plant Growth Regul 49:27–34
Zhen A, Bie ZL, Huang Y, Liu ZX, Fan ML (2012) Effects of 5-aminolevulinic acid on the H2O2-content and antioxidantive enzyme gene expression in NaCl-treated cucumber seedlings. Biol Plant 56:566–570
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–444
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, L., Nguyen, N.T., Ueda, A. et al. Effects of 5-aminolevulinic acid on Swiss chard (Beta vulgaris L. subsp. cicla) seedling growth under saline conditions. Plant Growth Regul 74, 219–228 (2014). https://doi.org/10.1007/s10725-014-9913-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-014-9913-0