Abstract
A number of studies have established that plant growth and development in oilseed rape (Brassica napus L.) are hampered by salinity stress. Nowadays, researchers have focused on the use of plant growth regulators to increase plant tolerance against salinity. An experiment was performed to evaluate the effects of 5-aminolevulinic acid (ALA, 30 mg l−1) on Brassica napus L. (cv. ‘ZS 758’) plants under NaCl (100, 200 mM) salinity. Data presented here were recorded on two different leaf positions (first and third) to have a better understanding of the ameliorative role of ALA on NaCl-stressed oilseed rape plants. Results have shown that increasing salinity imposed negative impact on relative growth rate (root and shoot) and leaf water relations (osmotic potential and relative water content), whereas enhanced the level of relative conductivity, malondialdehyde (MDA) content, osmolytes (soluble sugar, soluble protein, free amino acid and proline) concentration, reactive oxygen species (ROS), and enzymatic (ascorbate peroxidase, guaiacol peroxidase, catalase and superoxide dismutase) and non-enzymatic (reduced glutathione and ascorbate) antioxidants activity in two different leaf position samples. Foliar application of ALA improved relative growth rate (root and shoot) and leaf water relations (osmotic potential and relative water content), and also triggered the further accumulation of osmolytes (soluble sugar, soluble protein, free amino acid and proline) as well as enzymatic (ascorbate peroxidase, guaiacol peroxidase, catalase and superoxide dismutase) and non-enzymatic (reduced glutathione and ascorbate) antioxidants activity in both leaf samples, whereas decreased the membrane permeability, MDA content and ROS production. Our results also indicate that osmolytes are preferentially accumulated in younger tissues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity is one of the major abiotic stress factors which affects the growth and productivity of oilseed rape and many other crops. About 20% of the world’s cultivated land area and 50% of all irrigated lands are adversely affected by salinity (Rhoades and Loveday 1990). High salinity is extremely toxic to cell metabolism, causing osmotic imbalance, membrane disorganization, growth reduction, and inhibition of cell division and expansion, and has deleterious effects on the functions of some enzymes (Niu et al. 1995).
Salt stress, in addition to the known components of osmotic stress and ion toxicity, is also manifested as an oxidative stress, and all these factors contribute to its deleterious effects (Hernandez and Almansa 2002). All these adverse conditions increase the rate of reactive oxygen species (ROS) like superoxide radical (O2 −), hydrogen peroxide (H2O2), and hydroxyl radical (OH−) via enhanced leakage of electrons to oxygen. These ROS are responsible for the damage to membranes and other essential macromolecules such as proteins, lipids, and nucleic acids. However, the degree of damage to these molecules depends upon the balance between the formation of ROS and their removal by the antioxidative scavenging systems (Yeo 1998).
It is the need of the time that some quick and effective measures to be worked out to resolve salinity problem. One effective but time consuming way is to breed salinity stress adaptive cultivars. Another promising approach is the proper use of plant growth regulators (PGRs) to increase plant tolerance against salinity (Chakrabarti and Mukherjee 2003). PGRs improve productivity and alter the growth and production of plants by triggering physiological (hormonal) responses. In fact, PGRs are often used to increase the stress resistance of plants (El-Tayeb 2005; Zhang et al. 2008; Zhou and Xi 1993).
5-Aminolevulinic acid (ALA) is one of those PGRs being used for stress amelioration (Hotta et al. 1997a) and an essential precursor for the biosynthesis of tetrapyrroles such as heme and chlorophyll. ALA is found in all plants and animals. Recently, it was found that low concentrations of ALA had a promotive effect on growth and yield of several crops and vegetables (Hotta et al. 1997a, b; Watanabe et al. 2000). ALA at low concentrations also increased cold resistance in rice, salt tolerance in potato in vitro and alleviated herbicide toxicity in oilseed rape seedlings (Hotta et al. 1998; Zhang et al. 2006, 2008).
However, the growth promotive mechanism of ALA against biotic and abiotic stress has yet to be elucidated. The information available regarding exogenous application of ALA at low concentration is very much limited. Keeping in view the beneficial effects of ALA on the growth of other crops, a study was planned to investigate the alleviating role of ALA regarding the salinity-induced morphological, metabolic, osmotic and antioxidant activity changes in Brassica napus L. cv. ‘ZS 758’.
Materials and methods
Plant materials and treatments
Healthy seeds of Brassica napus L. cv. ‘ZS 758’ were obtained from the College of Agriculture and Biotechnology, Zhejiang University, and were sown in the field. Thirty days after sowing, morphologically uniform seedlings were selected and plugged in plate holes on plastic pots (five plants per pot) containing a modified half-strength Hoagland nutrient solution (Arnon and Hoagland 1940), aerated continuously with an air pump, in the greenhouse. The pH of the solution was adjusted to 6.0. The seedlings were grown under light intensity in the range of 250–350 μmol m−2 s−1. The temperature was in the range of 16–20°C, and the relative humidity was approximately 55–60%. Each treatment was replicated three times. The solution was renewed every 5 days.
After a 2-week acclimatization period, solutions were adjusted to the desired salinities (0, 100 or 200 mM NaCl) and plants were simultaneously treated with an aqueous solution of 5-aminolevulinic acid (ALA, provided by Cosmo Oil Co. Ltd., Japan) at a concentration of 0 or 30 mg l−1 by foliar spray. Following were the treatment combinations viz. (A0N0, control; A0N1, 100 mM NaCl; A0N2, 200 mM NaCl; A1N0, 30 mg l−1 ALA, A1N1, 100 mM NaCl + 30 mg l−1 ALA; A1N2, 200 mM NaCl + 30 mg l−1 ALA). The concentrations of NaCl and ALA were determined based on our preliminary studies. Three biological replicates were used.
Lower as well as upper leaf surface was sprayed until wetted with a handheld atomizer, as it was reported that absorption by the lower leaf surface is rapid and effective (Hull et al. 1975). A subsequent application was made 5 days after first spray. Plants sprayed with distilled water served as the control. Leaves were numbered basipetally such that leaf 1 designated as the youngest fully expanded leaf. Ten days after treatment, first and third leaves of all treated plants were separately harvested, and then frozen and stored in liquid nitrogen for subsequent analysis, with three technical replicates.
Relative growth rate (RGR)
Just prior to treatment application, randomly six plants per treatment were harvested in order to measure the dry weight of the shoots and roots. After 10 days of stress, again randomly chosen six plants were collected. Relative growth rates (RGR) were then estimated separately for roots and shoots as reported by Venus and Causton (1979). Relative growth rate (RGR): (lnW 2 − lnW 1)/t 2 − t 1 where W 2 is the dry weight of the considered organ at the end of treatment exposure and W 1 is the dry weight at the time of treatment imposition.
Osmotic potential and relative water content
The leaves (first and third) for the measurement of leaf osmotic potential (ψ s ) were frozen in liquid nitrogen and stored at −80°C. The samples were thawed for 30 s, which provided 10 μl of sap that was used for the determination of osmotic potential according to Gucci et al. (1991) using a vapor pressure osmometer (Wescor Inc., Logan, UT, USA).
The relative water content (RWC) was determined in fresh leaves (first and third), excluding midrib. Samples were weighed quickly and immediately floated on double distilled water, in Petri dishes to saturate them with water for the next 24 h, in dark. The excessive water was blotted and turgor weight was taken. Dry weight of these samples was obtained, after dehydrating them at 70°C for 48 h. Relative water content was calculated by placing the observed values in the following formula (Jones and Turner 1978):
RWC = [(fresh weight − dry weight)/(turgid weight − dry weight)] × 100.
Since both osmotic potential and RWC are interdependent variables, the linear regression was fitted with RWC as a dependent variable.
Cell membrane injury and lipid peroxidation assay
Cell membrane injury of the leaves was measured as relative conductivity according to the method of Blum and Ebercon (1981). Three leaf discs 9 mm in diameter for each (first and third) leaf were rinsed with distilled water separately and immersed in 6 mL of distilled water for 12 h. The conductivity of the solution (R1) was measured with a conductivity meter (Model DDS-11A, Shanghai Leici Instrument Inc., Shanghai, China). Samples were then heated in boiling water for 20 min and then cooled down to room temperature. The conductivity of the solution (R2) was again measured. The results were expressed as relative conductivity [(R1/R2) × 100].
The level of lipid peroxidation was estimated in terms of malondialdehyde (MDA) content and was determined as 2-thiobarbituric acid (TBA) reactive metabolites, as previously described by Zhou and Leul (1998). Plant fresh tissues (0.5 g) were homogenized and extracted in 10 ml of 0.25% TBA made in 10% trichloroacetic acid (TCA). The extract was heated at 95°C for 15 min and then quickly cooled on ice. After centrifugation at 5,000g for 10 min, the absorbance of the supernatant was measured at 532 nm. Correction of non-specific turbidity was made by subtracting the absorbance value taken at 600 nm. The level of lipid peroxidation was expressed as nmol g−1 fresh weight by using an extinction coefficient of 155 mM cm−1.
Soluble protein and proline contents
The soluble protein contents contained in the supernatant (extracted for antioxidant enzymes assay) were measured by the method of Coomassie blue according to Bradford (1976) using bovine serum albumin as a standard, as described by Gong et al. (2008).
The proline content in fresh leaves (first and third) was determined by adopting the method of Bates et al. (1973). Samples were extracted with sulfosalicylic acid. In the extract an equal volume of glacial acetic acid and ninhydrin (1.25 g ninhydrin, 30 ml of glacial acetic acid, 20 ml of 6 M H3PO4) solutions were added. The samples were incubated at 100°C for 40 min, to which 3 ml of toluene was added. The absorbance of the toluene layer was read at 520 nm, on a spectrophotometer. The proline concentration was determined using a standard curve.
Soluble sugar and free amino acid contents
The soluble sugars content was estimated as reported by Zhang et al. (2006). Fresh leaves (first and third) were boiled in distilled water in water bath for 30 min and then centrifuged at 2,000×g for 15 min. The supernatants were used for the sugar analyses by using spectrophotometric method. The soluble sugar concentration was determined using a standard curve.
Total free amino acids were estimated according to the method of Yemm and Cocking (1955). One milliliter aliquot of the acidified extract was mixed with 2 ml sodium acetate buffer (pH 6.5) and 1 ml freshly prepared ninhydrin reagent. The resulting color was then read at 570 nm with spectrophotometer. Leucine was used as a standard.
Superoxide (O2 −) radical and hydrogen peroxide (H2O2) contents
O2 − was measured as described by Elstner and Heupel (1976) by monitoring the nitrite formation from hydroxylamine in the presence of O2 −, with some modifications. 0.2 g of frozen leaf segments were homogenized with 4 ml of 65 mM potassium phosphate buffer (pH 7.8) and centrifuged at 5,000×g for 10 min. The incubation mixture contained 0.9 ml of 65 mM phosphate buffer (pH 7.8), 0.1 ml of 10 mM hydroxylamine hydrochloride, and 1 ml of the supernatant. After incubation at 25°C for 20 min, 17 mM sulfanilamide and 7 mM α-naphthylamine were added to the incubation mixture. After reaction at 25°C for 20 min, trichloromethane in the same volume was added and centrifuged at 1,500×g for 5 min. The absorbance in the aqueous solution was read at 530 nm. A standard curve with NO2 − was used to calculate the production rate of O2 − from the chemical reaction of O2 − and hydroxylamine.
The content of H2O2 was estimated by reading the titanium-peroxide complex at 415 nm as reported by Brennan and Frenkel (1977). Absorbance values were calibrated to a standard curve generated with known concentrations of H2O2.
Activities of non-enzymatic antioxidants
To determine non-enzymatic antioxidants (reduced glutathione and ascorbate) activity, fresh leaves (0.4 g) were ground with a mortar and pestle in 4 mL of 5% (v/v) trichloroacetic acid (TCA). The homogenates were centrifuged at 10,000×g for 15 min at 4°C. The supernatants were used for the assays of reduced glutathione (GSH) and ascorbate (ASC) contents.
GSH content was measured according to the method of Ellman (1959). Supernatant (0.2 mL) was added to 2.6 mL of 150 mM NaH2PO4 (pH 7.4). Two hundred microliters of 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), was then added. The mixture was incubated at 30°C for 5 min. Absorbance was determined at 412 nm and the GSH concentration was calculated by comparison to a standard curve.
ASC content was determined according to the method of Law et al. (1983). Supernatant (0.4 mL) was combined with 0.2 mL of 150 mM NaH2PO4 (pH 7.7). To this mixture 0.4 mL of 10% (v/v) TCA, 0.4 mL of 44% (v/v) H3PO4, 0.4 mL of 4% (w/v) bipyridyl (in 70% alcohol), and 0.2 mL of 3% FeCl3 (w/v) was successively added. The mixture was incubated at 37°C for 1 h. Absorbance was determined at 525 nm. ASC concentration was calculated by comparison to a standard curve.
Activities of antioxidant enzymes
Antioxidants enzymes (ascorbate peroxidase, guaiacol peroxidase, catalase and superoxide dismutase) activity was determined according to Zhang (1992) with some modifications. Leaf (first and third) samples were ground with a mortar and pestle under the chilled condition in the homogenization buffer. The homogenate was centrifuged at 10,000×g for 20 min at 4°C, and the supernatants were used for enzyme assays (Leul and Zhou 1999).
Ascorbate peroxidase (APX, EC1.11.1.11) activity was carried out in a reaction mixture of 3 ml containing 100 mM phosphate (pH 7.0), 0.1 mM EDTA-Na2, 0.3 mM ascorbic acid, 0.06 mM H2O2 and 100 μl enzyme extract. The change in absorption at 290 nm was recorded 30 s after addition of H2O2 (Nakano and Asada 1981).
Guaiacol peroxidase (POD, EC1.11.1.7) activity was measured with guaiacol as the substrate in a total volume of 3 ml. The reaction mixture consisted of 50 mM potassium phosphate buffer (pH 6.1), 1% guaiacol, 0.4% H2O2 and enzyme extract. Increase in the absorbance due to oxidation of guaiacol was measured at 470 nm as reported by Zhou and Leul (1999).
Superoxide dismutase (SOD, EC1.15.1.1) activity was assayed by using the photochemical NBT method. The samples (0.5 g) were homogenized in 5 ml extraction buffer consisting of 50 mM phosphate, pH 7.8. The assay mixture in 3 ml contained 50 mM phosphate buffer, pH 7.8, 26 mM l-methionine, 750 μM NBT, 1 μM EDTA, and 20 μM riboflavin. The photoreduction of NBT (formation of purple formazan) was measured at 560 nm and an inhibition curve was made against different volumes of extract. One unit of SOD is defined as being present in the volume of extract that causes inhibition of the photoreduction of NBT by 50% (Zhou et al. 1997).
Catalase (CAT, EC1.11.1.6) activity was determined by following the consumption of H2O2 (extinction coefficient 39.4 mM−1 cm−1) at 240 nm for 3 min (Aebi 1984). The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 10 mM H2O2 and 200 μl of enzyme extract in a 3 ml volume.
Statistical analysis
All data are presented are mean values. Measurements were performed on three replicates, of each treatment. Data were analyzed using the statistical program SAS and the analysis of variance (ANOVA) was followed by Fisher’s protected LSD test to identify homogenous groups within the means. Significant differences among treatments were considered at the P ≤ 0.05 level.
Results
Relative growth rate
The RGR of the root slightly decreased in response to 100 mM NaCl while that of shoot was not affected (Fig. 1), thus confirming the tolerant nature of Brassica napus L. cv. ‘ZS 758’ towards low salinity. RGR decreased significantly as compared to that of control plants at higher stress intensity (200 mM) for both organs. However, the foliar application of ALA promoted growth and also partially ameliorated the toxic effects caused by high salinity in both root and shoot. The plants under 100 mM sodium chloride salinity were completely recovered by ALA application; the RGR values are, therefore, at par with that of the control plants. The highest RGR was observed with ALA treatment alone whereas 200 mM NaCl substantially reduced it.
Effect of 5-aminolevulinic acid on salinity-induced changes in relative growth rate (root and shoot) of Brassica napus cv. ‘ZS 758’. RGR was estimated on dry weight basis after 10 days of treatment. Each value represents the mean of three replicates of each treatment and means followed by same small letters are not significantly different by the LSD test at P ≤ 0.05. Vertical bars represent SE
Osmotic potential and relative water content
Leaf osmotic potential decreased with an increase in NaCl concentration in both leaf position samples (Fig. 2a). However, the application of ALA helped the plants to adjust osmotic potential, plants treated with 100 mM NaCl recovered completely, and those treated with 200 mM NaCl also significantly recovered. The lowest osmotic potential (−1.82 MPa) was observed in the first leaf samples of the plants treated with 200 mM NaC1. The osmotic potential of the first leaf was lower than the third leaf irrespective of the salinity or ALA treatments.
Effect of 5-aminolevulinic acid on salinity-induced changes in a osmotic potential and b relative water content of Brassica napus cv. ‘ZS 758’. Each value represents the mean of three replicates of each treatment and within a specific leaf position, means followed by same small letters are not significantly different by the LSD test at P ≤ 0.05. Vertical bars represent SE
The relative water content (RWC) in the leaves decreased with the increasing concentration of NaCl (Fig. 2b). The ALA treatment significantly increased RWC over the control and also overcome the toxic effect caused by NaCl. The RWC of the first leaf was higher as compared to the third leaf irrespective of the treatment. Moreover, the RWC of the first leaf of plants treated with 200 mM NaC1 was even higher than the third leaf of the untreated plants. A regression analysis shows (Fig. 3) that there is a significant positive correlation between osmotic potential and relative water content of first leaf and third leaf (r = 0.98, P = 0.001; r = 0.92, P = 0.010), respectively.
Cell membrane injury and lipid peroxidation assay
Degree of cell membrane injury was indirectly assessed by solute leakage of salinity-treated plants in the presence or absence of ALA. To avoid differences in ion balance due to the salinity treatment, results are expressed as relative instead of absolute conductivity. Salinity induced a significant increase in the membrane injury in terms of relative conductivity (RC) in both first and third leaf position samples as compared to that of control. An almost twofold increase in electrolyte leakage was observed in plants treated with 200 mM NaCl compared to that of control plants in first as well as third leaf. ALA application showed a significant reduction in the RC values of the both types of leaf samples (Fig. 4a). However, the third leaf showed higher RC values, might be due to leaf aging.
Effect of 5-aminolevulinic acid on salinity-induced changes in a relative conductivity (%) and b malondialdehyde (MDA) content of Brassica napus cv. ‘ZS 758’. Each value represents the mean of three replicates of each treatment and within a specific leaf position, means followed by same small letters are not significantly different by the LSD test at P ≤ 0.05. Vertical bars represent SE
Malondialdehyde (MDA) contents in plants exposed to adverse environmental conditions is a reliable indicator of free radical formation in the tissues, and it is currently used as an index of lipid oxidation in biological systems. Figure 4b showed that MDA content in both leaf position samples increased as the salinity stress level raised and this increase was 78.62% in first leaf and 55.99% in third leaf as compared to that of control under 200 mM NaCl stress. The application of ALA significantly decreased the level of MDA contents in the stressed seedlings at 100 and 200 mM NaCl. On the other hand, no significant change was observed with ALA treatment alone.
Soluble protein and proline contents
Soluble protein and proline level was higher in the first leaf than the third leaf (Table 1). Concentration of both metabolites increased in both leaves as compared to that of respective control, if supplied with NaCl or ALA as individual treatments. Soluble proteins content decreased in the plants treated with NaCl and ALA together or ALA alone, as compared to those under the corresponding salinity stress levels and this reduction was 22.72% in first leaf and 32.96% in third leaf, when plants treated with ALA and 200 mM NaCl. On the other hand, the plants exposed to the ALA as well as salinity as an individual treatment possessed the highest quantities of proline which increased further with an increase in the level of NaCl. The highest level of proline was recorded in the plants that received NaCl (200 mM) and ALA treatment in combination. Here, the values were 81.94% in the first leaf and 60.53% in third leaf higher as compared to those under the corresponding salinity stress levels.
Soluble sugar and free amino acid contents
The plants treated with either NaCl or ALA alone, showed a significant increase in soluble sugar as well as free amino acids content in both leaf position samples (Table 2). Foliar application of ALA to the NaCl-treated plants has been shown to cause a further increase in soluble sugars and free amino acids content as compared to that of respective control leaf samples. Control plants possessed the minimum values while the highest level of soluble sugars (95.05%) and free amino acids content (75.53%) were recorded in the first leaf samples that received NaCl (200 mM) and ALA as compared to their respective control leaf samples.
Superoxide (O2 −) radical and hydrogen peroxide (H2O2) contents
Effect of two different concentrations of NaCl and ALA, alone or combined was investigated on the levels of H2O2 and O2 − in the first and the third leaf of Brassica napus L. plants (Table 3). It was observed that salinity induced while ALA alone or in combination with salinity reduced the level of H2O2 and O2 − in both leaves compared to the respective salt treated leaf samples. Treatment with 200 mM NaCl alone induced the highest concentration of H2O2 (56.36% in first leaf and 48.16% in third leaf) and O2 − (85.28% in first leaf and 90.29% in third leaf), where as ALA alone showed a lowest concentration as compared to their respective control samples. Foliar application of ALA to the plants treated with 100 mM NaCl helped them to adjust the level of H2O2 and O2 − at par with that of control in both leaf position samples and those treated with 200 mM NaCl also substantially recovered by ALA treatment. However, the overall level of H2O2 and O2 − in the first leaf was lower than those in the third leaf irrespective of the salinity or ALA treatments.
Activities of non-enzymatic antioxidants
Activities of two main non-enzymatic, water soluble antioxidants, viz. reduced glutathione (GSH) and ascorbate (ASC), against salinity and ALA alone or in combination are shown (Table 4). In the third leaf, the inherent GSH and ASC contents were higher (by 4 and 11.5%, respectively) than those of the first leaf. GSH contents significantly increased in response to 100 and 200 mM NaCl alone or in combination with ALA except ALA as a sole treatment in both leaf position samples as compared to the control leaf samples. The maximum GSH concentration (54.87% in first leaf and 57.28% in third leaf) was observed in the plants treated with 200 mM NaCl with ALA as compared to that of control. No significant change was observed in the ASC contents in the both leaf position samples from the plants treated with 100 mM NaCl, 200 mM NaCl or ALA as an individual treatment as compared to the control samples. However, the ASC level increased significantly and reached maximum in the first leaf as well as in the third leaf, when treated with salinity and ALA as a combined treatment. Maximum ASC concentration (80.32% in first leaf and 86.81% in third leaf) was observed in the plants treated with 200 mM NaCl and ALA together.
Activities of antioxidant enzymes
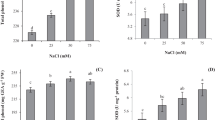
Figure 5 highlights the effect of salinity and ALA alone or in combination on four different antioxidant enzymes such as APX (Fig. 5a) and POD (Fig. 5b), the two key enzymes for the removal of H2O2 in the chloroplasts, CAT (Fig. 5c), the enzyme mainly responsible for eliminating H2O2 in the peroxisomes and SOD (Fig. 5d) the enzyme for catalyzing the dismutation of O2 − to O2 and H2O2. Control plants showed the minimum values. All four antioxidants activity tested, did not change significantly in the first and the third leaf of 100 mM NaCl-treated plants, whereas their activity increased significantly in the third and the third leaf of 200 mM NaCl-treated plants as compared to that of control plants. Moreover, the foliar application of ALA to the NaCl-treated plants had synergistic effect on the enzyme activity. The application of ALA with the highest concentration (200 mM) of NaCl was the most effective and the activity of APX was increased by 92.25 and 87.46%, POD by 90.33 and 79.37%, CAT by 96.09 and 83.05%, and SOD by 97.1% and 90.22% in the first leaf and the third leaf, respectively, as compared to their respective controls.
Effect of 5-aminolevulinic acid on salinity-induced changes in a ascorbate peroxidase (APX), b guaiacol peroxidase (POD), c catalase (CAT), and d superoxide dismutase (SOD) activities of Brassica napus cv. ‘ZS 758’. Each value represents the mean of three replicates of each treatment and within a specific leaf position, means followed by same small letters are not significantly different by the LSD test at P ≤ 0.05. Vertical bars represent SE
Discussion
This study is an effort to evaluate the role of 5-aminolevulinic acid (ALA) on growth, water relations (relative water content and osmotic potential), membrane damage, osmolyte biosynthesis, reactive oxygen species (ROS) production and enzymatic as well as non-enzymatic antioxidants activity in Brassica napus L., under salinity conditions generated by sodium chloride (NaCl). Reduction in RGR (root and shoot) reflects the increased metabolic energy cost and reduced carbon gain, which are associated with salt adaptation (Netondo et al. 2004). These findings agree with those of Yusuf et al. (2008), in Brassica juncea L. and El-Tayeb (2005) in barley under NaCl stress. However, foliar application of ALA enhanced the relative growth rate by improving salt tolerance; as reported by Watanabe et al. (2000) in cotton.
Salinity stress damage can also be attributed to the water stress or a kind of physiological drought generated by NaCl (Hopkins 1995), as evident from the decrease in osmotic potential (ψ s ) and relative water content (Fig. 2). The decline in osmotic potential (ψ s ) in the saline solution resulted from reduced tissue water content, and some entry of sodium (Na+) and chloride (Cl−) solutes with maintenance of K+ concentrations and increased production of organic solutes. Glenn (1987) found a similar response to NaCl stress in halophytic grasses compared with glycophytic grasses. ALA application induced an increase in osmotic potential (ψ s ) and relative water content of the stressed seedlings, which is more pronounced at the highest stress level of 200 mM NaCl (Fig. 2). Data values showed that older leaves displayed higher level of osmotic adjustment compared to the younger leaves as reported by Madan et al. (1994).
Sreenivasulu et al. (2000) reported that high salinity impairs cellular electron transport leading to the generation of reactive oxygen species (ROS) which triggers lipid peroxidation and membrane damage. Increasing relative conductivity and MDA contents with the increasing salinity presented in this work indicate the degree of membrane integrity impairment. Third leaf showed higher relative conductivity as well as MDA content compared to that of the first leaf probably due to leaf aging. Application of ALA reduced the relative conductivity and MDA content in plant leaves under salinity stress. Nishihara et al. (2003) and Zhang et al. (2008) reported that ALA at low concentration declined the MDA content under salinity and herbicide stress, respectively. However, response to exogenous ALA application varies among different plants, stage of plant development, timing, and applied concentration (Hotta et al. 1997b; Nishihara et al. 2003).
Proteins contents (Table 1) that accumulate in plants grown under saline conditions may provide a storage form of nitrogen that is re-utilized when stress is over and may play a role in osmotic adjustment. During characterization of salt-induced proteins in tobacco, a 26 kDa protein named as “Osmotin” was detected (Ashraf and Harris 2004).
The accumulation of compatible solutes at high concentrations is believed to facilitate “osmotic adjustment”, can reduce inhibitory effects of ions on enzyme activity and prevent dissociation of enzyme complexes (Delauney and Verma 1993). Proline, soluble sugars and free amino acids have been mentioned as important compatible solutes in osmoregulation and protect plants from stress through different mechanisms, including cellular osmotic adjustment, detoxification of reactive oxygen species, protection of membrane integrity and stabilization of proteins/enzymes (Ashraf and Foolad 2007; Sairan and Tyagi 2004). The accumulation of proline in the leaves might be involved in one or more of above processes and contributed to salinity tolerance. However, the actual role of proline as an osmo-regulator is controversial. Some researchers correlate the accumulation of proline under stress as stress tolerance (Nayyar and Walia 2003), while others consider the accumulation of proline as stress injury (de Lacerda et al. 2003). Our study favors the former school of thought, as our data shows that foliar application of ALA enhanced proline accumulation in the leaves under salinity stress (Table 1), as reported by Yusuf et al. (2008), in Brassica juncea seedlings.
Salinity enhances carbohydrates in some plant species (de Lacerda et al. 2003; Cha-um et al. 2009) or decrease in others (Agastian et al. 2000). In this work, carbohydrate content increased in leaves (Table 2) under salinity or in combination with ALA, contributing therefore to water status maintenance in leaves. Free amino acid (FAA) accumulation in plants under salt stress has often been attributed to alterations in biosynthesis and degradation processes of amino acids and proteins (Roy-Macauley et al. 1992). In this study, FAA content increased in leaves (Table 2) under salinity alone or in combination with ALA. So, we can hypothesize that ALA treatment may stimulate hydrolysis of proteins, providing a pool of compatible osmolyte, which is important in osmotic adjustment. This hypothesis could be supported by the observation that ALA increased FAA accumulation at the sake of proteins. The higher osmolytes (proline, soluble sugars and FAA) accumulation in first leaf than third leaf may be associated with declined translocation of these osmolytes from younger leaves to older leaves, and contribute towards osmotic adjustment.
Salinity stimulates hydrogen peroxide (H2O2) production (Hernandez et al. 1995; Hajlaoui et al. 2009), which plays a crucial role in the mechanism of salt injury (Singha and Choudhuri 1990). Salinity enhanced the level of H2O2 and superoxide anion (O2−) generation whereas ALA application helped the salinity-treated plants to detoxify both ROS by manipulating the antioxidant enzyme activities as reported by Nishihara et al. (2003) in spinach leaves. However, the production of hydrogen peroxide and superoxide has been shown to depend on the intensity of stress, repeated stress periods, species and age of the plants (Kanazawa et al. 2000), as highlighted by enhanced level of ROS in third leaf compared to the first leaf.
Plants have several mechanisms to defend themselves against salinity-induced stress in the form of non-enzymic scavengers (ascorbate, glutathione, etc.) and antioxidant enzymes (APX, POD, CAT, SOD, etc.) that act in concert to alleviate cellular damage under oxidative stress conditions (Foyer and Noctor 2000).
ALA application on salinity-treated plants enhances the activity of the ascorbate–glutathione cycle by increasing GSH and ASC levels. Nishihara et al. (2003) reported that ALA up-regulates the activities of reduced glutathione and ascorbate in spinach. The results suggest that the high ascorbate–glutathione pool would help plants to some extent in minimizing the salinity adverse effects by scavenging ROS in cytoplasm and enhance the metabolic process of plant to tolerate salinity. In our study, it is interesting to note that induction of the SOD activity coincided with changes in the activity of the enzymes of the ASC–GSH cycle. This fact is of some importance since the expected increase in H2O2 as a result of the SOD reaction was accompanied by an increased non-enzymatic antioxidants capacity to decompose it.
Several studies have demonstrated that salt-tolerant species increase their antioxidant enzyme activities in response to salt stress, while salt-sensitive species failed to do so (Shalata et al. 2001; Jbir et al. 2001). Our results showed an enhanced activity of enzymatic antioxidants (APX, POD, CAT, and SOD) in response to ALA application on salinity-treated plants. However, the intensity of this activity is higher in third leaf compared to first leaf; higher antioxidant activity in third leaf is due to higher production of ROS in older leaves as a matter of defense. Previously, Bor et al. (2003) and Yusuf et al. (2008) also reported an increase in antioxidants activity under salinity in sugar beet and Indian mustard, respectively. The probable reason behind the enhanced activities of antioxidants due to application of ALA could be: as we know that ALA is an essential precursor in the biosynthesis of heme, so its exogenous application boosts up the activity of heme-based biomolecules. Bhaya and Castelfranco (1985) reported that isolated chloroplast can synthesize heme when exogenously treated with ALA. Moreover, Castelfranco and Jones (1975) described the role of ALA in heme-based proteins accumulation in barley plants. Thus, we can say that exogenous ALA application alone or in combination with salinity enhance the activity of heme-based enzymatic antioxidants (APX, POD) and CAT activity induced by exogenous ALA application alone or in combination with salinity, to scavenge efficiently the ROS produced by oxygen metabolism under salinity conditions. However, the mechanism by which ALA enhances the SOD activity is still not clear.
In conclusion, ALA foliar application has potential to enhance tolerance against salt stress via decreasing the oxidative damage of plant membranes by promoting the enzymatic and non-enzymatic antioxidant activity, and/or compatible solutes for osmotic adjustment in Brassica napus L. plants. Our results also indicate that osmolytes are preferentially accumulated in younger tissues so we can deduce that a significant effect of salt may appear non-significant if the position of the leaves is not taken into account while sampling. Moreover, plants under 100 mM NaCl recovered completely compared to substantial recovery by 200 mM treated plants. However, it needs further study at molecular level that how ALA impart salinity tolerance to Brassica napus L. plants.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Agastian P, Kingsley SJ, Vivekanandan M (2000) Effect of salinity on photosynthesis and biochemical characteristics in mulberry genotypes. Photosynthetica 38:287–290
Arnon DI, Hoagland DR (1940) Crop production in artificial solution with special reference to factors affecting yield and absorption of inorganic nutrients. Soil Sci 50:463–485
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bhaya D, Castelfranco PA (1985) Chlorophyll biosynthesis and assembly into chlorophyll–protein complexes in isolated developing chloroplasts. Proc Natl Acad Sci USA 82:5370–5374
Blum A, Ebercon A (1981) Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci 21:43–47
Bor M, Özdemir F, Türkan I (2003) The effects of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritima L. Plant Sci 164:77–84
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brennan T, Frenkel C (1977) Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol 59:411–416
Castelfranco PA, Jones OTG (1975) Protoheme turnover and chlorophyll synthesis in greening barley tissue. Plant Physiol 55:485–490
Chakrabarti N, Mukherjee S (2003) Effect of phytohormones pre-treatment on nitrogen metabolism in Vigna radiata L. under salt stress. Biol Plant 36:63–66
Cha-um S, Charoenpanich A, Roytrakul S, Kirdmanee C (2009) Sugar accumulation, photosynthesis and growth of two indica rice varieties in response to salt stress. Acta Physiol Plant 31:477–486
de Lacerda CF, Cambraia J, Oliva MA, Ruiz HA, Prisco JT (2003) Solute accumulation and distribution during shoot and leaf development in two sorghum genotypes under salt stress. Environ Exp Bot 49:107–120
Delauney AJ, Verma DPS (1993) Proline biosynthesis and osmoregulation in plants. Plant J 4:215–223
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxyl ammonium chloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
El-Tayeb MA (2005) Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul 45:215–224
Foyer CH, Noctor G (2000) Oxygen processing in photosynthesis: regulation and signalling. New Phytol 146:359–388
Glenn EP (1987) Relationship between cation accumulation and water content of salt-tolerant grasses and a sedge. Plant Cell Environ 10:205–212
Gong HJ, Chen KM, Zhao ZG, Chen GC, Zhou WJ (2008) Effects of silicon on defense of wheat against oxidative stress under drought at different developmental stages. Biol Plant 52:592–596
Gucci R, Xilyannis C, Flore JA (1991) Gas exchange parameters, water relations and carbohydrate partitioning in leaves of field-grown Prunus domestica following fruit removal. Physiol Plant 83:497–505
Hajlaoui H, Denden M, El Ayeb N (2009) Changes in fatty acids composition hydrogen peroxide generation and lipid peroxidation of salt-stressed corn (Zea mays L.) roots. Acta Physiol Plant 31:787–796
Hernandez JA, Almansa MS (2002) Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol Plant 115:251–257
Hernandez JA, Olmos E, Corpas FJ, Sevilla F, del Rio LA (1995) Salt-induced oxidative stress in chloroplasts of pea plants. Plant Sci 105:151–167
Hopkins WJ (1995) Introduction to plant physiology. Kluwer, Dordrecht, The Netherlands
Hotta Y, Tanaka T, Takaoka H, Takeuchi Y, Konnai M (1997a) Promotive effects of 5-aminolevulinic acid on the yield of several crops. Plant Growth Regul 22:109–114
Hotta Y, Tanaka T, Takaoka H, Takeuchi Y, Konnai M (1997b) New physiological effects of 5-aminolevulinic acid in plants: the increase of photosynthesis, chlorophyll content, and plant growth. Biosci Biotechnol Biochem 61:2025–2028
Hotta Y, Tanaka T, Bingshan L, Takeuchi Y, Konnai M (1998) Improvement of cold resistance in rice seedlings by 5-aminolevulinic acid. J Pesticide Sci 23:29–33
Hull HM, Morton HL, Wharrie JR (1975) Environmental influence on cuticle development and resultant foliar penetration. Bot Rev 41:421–451
Jbir N, Chaibi W, Ammar S, Jemmali A, Ayadi A (2001) Root growth and lignification of two wheat species differing in their sensitivity to NaCl, in response to salt stress. C.R. Hebd Seances Acad Sci 324:863–868
Jones MM, Turner TC (1978) Osmotic adjustment in leaves of sorghum on response to water deficits. Plant Physiol 25:591–597
Kanazawa S, Sano S, Koshiba T, Ushimaru T (2000) Changes in antioxidative enzymes in cucumber cotyledons during natural senescence: comparison with those during dark-induced senescence. Physiol Plant 109:211–216
Law MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. Biochem J 210:899–903
Leul M, Zhou WJ (1999) Alleviation of waterlogging damage in winter rape by uniconazole application: effects on enzyme activity, lipid peroxidation and membrane integrity. J Plant Growth Regul 18:9–14
Madan S, Nainawatee HS, Jain S, Jain RK, Malik MS, Chowdhury JB (1994) Leaf position-dependent changes in proline, pyrroline-5-carboxylate reductase activity and water relations under salt-stress in genetically stable salt-tolerant somaclones of Brassica juncea L. Plant Soil 163:151–156
Nakano Y, Asada K (1981) Hydrogen peroxide scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nayyar H, Walia DP (2003) Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid. Biol Plant 46:275–279
Netondo GW, Onyango JC, Beck E (2004) Sorghum and salinity: II. Gas exchange and chlorophyll fluorescence of sorghum under salt stress. Crop Sci 44:806–811
Nishihara E, Kondo K, Masud Parvez M, Takahashi K, Watanabe K, Tanaka K (2003) Role of 5-aminolevulinic acid (ALA) on active oxygen-scavenging system in NaCl-treated spinach (Spinacia oleracea). Plant Physiol 160:1085–1091
Niu X, Bressan RA, Hasegawa PM, Pardo JM (1995) Ion homeostasis in NaCl stress environments. Plant Physiol 109:735–742
Rhoades JD, Loveday J (1990) Salinity in irrigated agriculture. In: Steward BA, Neilsen DR (eds) Irrigation of agricultural crops. ASA, CSSA, SSSA, Madison, pp 1089–1142
Roy-Macauley H, Zuily-Fodil Y, Kidric M, Thi ATP, Da Silva JV (1992) Effect of drought stress on proteolytic activities in Phaseolus and Vigna leaves from sensitive and resistant plants. Physiol Plant 85:90–96
Sairan RK, Tyagi A (2004) Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci 86:407–421
Shalata A, Mittova V, Volokita M, Guy M, Tal M (2001) Response of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii to salt-dependent oxidative stress: the root antioxidative system. Physiol Plant 112:487–494
Singha S, Choudhuri MA (1990) Effect of salinity (NaCl) on H2O2 mechanism in Vigna and Oryza seedlings. Biochem Physiol Pflanz 186:69–74
Sreenivasulu N, Grimm B, Wobus U, Weschke W (2000) Differential response of antioxidant compounds to salinity stress in salt tolerant and salt-sensitive seedlings of foxtail millet (Setaria italica). Physiol Plant 109:435–444
Venus JC, Causton DR (1979) Plant growth analysis: a re-examination of the methods of calculation of relative growth rate and net assimilation rates without using fitted functions. Ann Bot 43:633–638
Watanabe K, Tanaka T, Kuramochi H, Takeuchi Y (2000) Improving salt tolerance of cotton seedling with 5-aminolevulinic acid. Plant Growth Regul 32:97–101
Yemm EW, Cocking EC (1955) Determination of amino acids with ninhydrin. Analyst 80:209–213
Yeo AR (1998) Molecular biology of salt tolerance in the context of whole plant physiology. J Exp Bot 49:915–929
Yusuf M, Hasan SA, Ali B, Hayat S, Fariduddin Q, Ahmad A (2008) Effect of salicylic acid on salinity induced changes in Brassica juncea L. J Integr Plant Biol 50:1096–1102
Zhang XZ (1992) The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. In: Zhang XZ (ed) Research methodology of crop physiology. Agriculture Press, Beijing, pp 208–211
Zhang ZJ, Li HZ, Zhou WJ, Takeuchi Y, Yoneyama K (2006) Effect of 5-aminolevulinic acid on development and salt tolerance of potato (Solanum tuberosum L.) microtubers in vitro. Plant Growth Regul 49:27–34
Zhang WF, Zhang F, Raziuddin R, Gong HJ, Yang ZM, Lu L, Ye QF, Zhou WJ (2008) Effects of 5-aminolevulinic acid on oilseed rape seedling growth under herbicide toxicity stress. J Plant Growth Regul 27:159–169
Zhou WJ, Leul M (1998) Uniconazole-induced alleviation of freezing injury in relation to changes in hormonal balance, enzyme activities and lipid peroxidation in winter rape. Plant Growth Regul 26:41–47
Zhou WJ, Leul M (1999) Uniconazole-induced tolerance of rape plants to heat stress in relation to changes in hormonal levels, enzyme activities and lipid peroxidation. Plant Growth Regul 27:99–104
Zhou WJ, Xi HF (1993) Effects of mixtalol and paclobutrazol on photosynthesis and yield of rape (Brassica napus). J Plant Growth Regul 12:157–161
Zhou W, Zhao D, Lin X (1997) Effects of waterlogging on nitrogen accumulation and alleviation of waterlogging damage by application of nitrogen fertilizer and mixtalol in winter rape (Brassica napus L.). J Plant Growth Regul 16:47–53
Acknowledgments
This work was supported by the National Natural Science Foundation of China (20632070, 30871652), the National High Technology Research and Development Program of China (2006AA10A214), the National Key Science and Technology Supporting Program of China (2010BAD01B04), the Industry Technology System of Rapeseed in China (nycytx-005), Zhejiang Provincial Natural Science Foundation (R307095), the Science and Technology Department of Zhejiang Province (2008C22078), and the Higher Education Commission of Pakistan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Rapacz.
Rights and permissions
About this article
Cite this article
Naeem, M.S., Rasheed, M., Liu, D. et al. 5-Aminolevulinic acid ameliorates salinity-induced metabolic, water-related and biochemical changes in Brassica napus L.. Acta Physiol Plant 33, 517–528 (2011). https://doi.org/10.1007/s11738-010-0575-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0575-x